Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
After the eighth week of pregnancy, the period of organogenesis (embryonic period) is largely completed, and the fetal period begins. By the end of the embryonic period, almost all the organs are present in a grossly recognizable form. The external contours of the embryo show a very large head in proportion to the rest of the body and greater development of the cranial than of the caudal part of the body ( Figures 18.1 and 18.2 ).
The fetal period has often been considered a time of growth and physiological maturation of organ systems, and it has not received much attention in traditional embryology courses. Advances in imaging and other diagnostic techniques, however, have provided considerable access to the fetus. Determining the fetus’ pattern of growth and state of well-being with remarkable accuracy is now possible. Improved surgical techniques and the realization that surgical wounds in the fetus heal without scarring have led to the field of fetal surgery.
This chapter emphasizes the functional development of the fetus and the adaptations that ensure a smooth transition to independent living after the fetus has passed through the birth canal and the umbilical cord is cut. Techniques that are used to monitor the functional state of the fetus are also described in Clinical Correlation 18.1 .
Despite the intense developmental activity that occurs during the embryonic period (3 to 8 weeks), the absolute growth of the embryo in length and mass is not great ( Figure 18.3 ). The fetal period (9 weeks to birth), however, is characterized by rapid growth. A major obstetrical problem is fetal growth restriction , a leading cause of stillbirths ( Clinical Correlation 18.2 ).
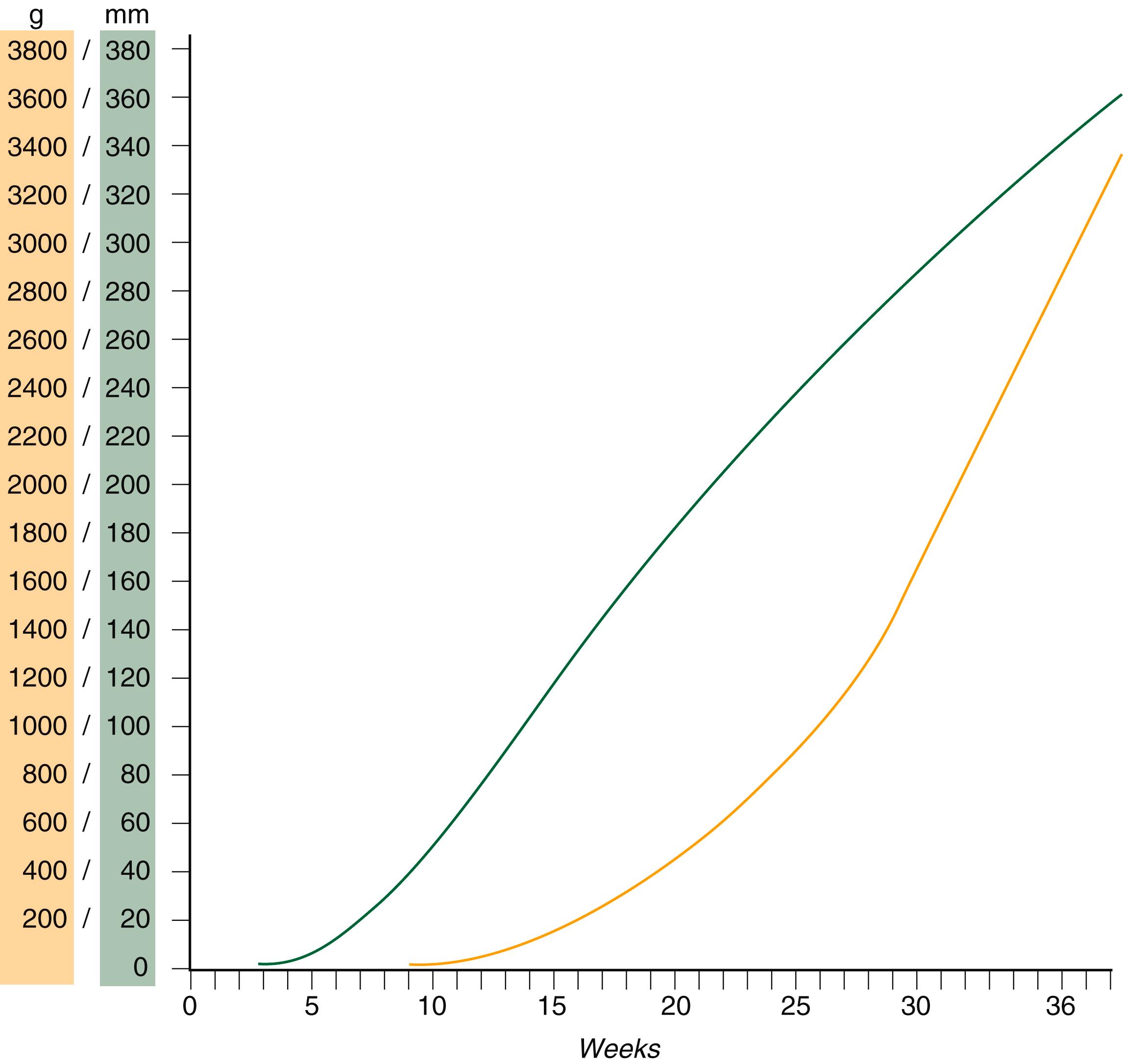
The change in proportions of the various regions of the body during the prenatal and postnatal growth periods is as striking as the absolute growth of the embryo. The early dominance of the head is reduced, whereas development of the trunk becomes a major factor in the growth of the early fetus. Even later, a relatively greater growth of the limbs changes the proportions of various regions of the body. During the early fetal period, the entire body is hairless and very thin because of the absence of subcutaneous fat ( Figure 18.4 ). By midpregnancy, the contours of the head and face approach those of the neonate, and the abdomen begins to fill out. Beginning at approximately week 27, the deposition of subcutaneous fat causes the body to round out. (Some major developmental landmarks during the fetal period are summarized in the Table on pp. x and xi in the 6/e.)
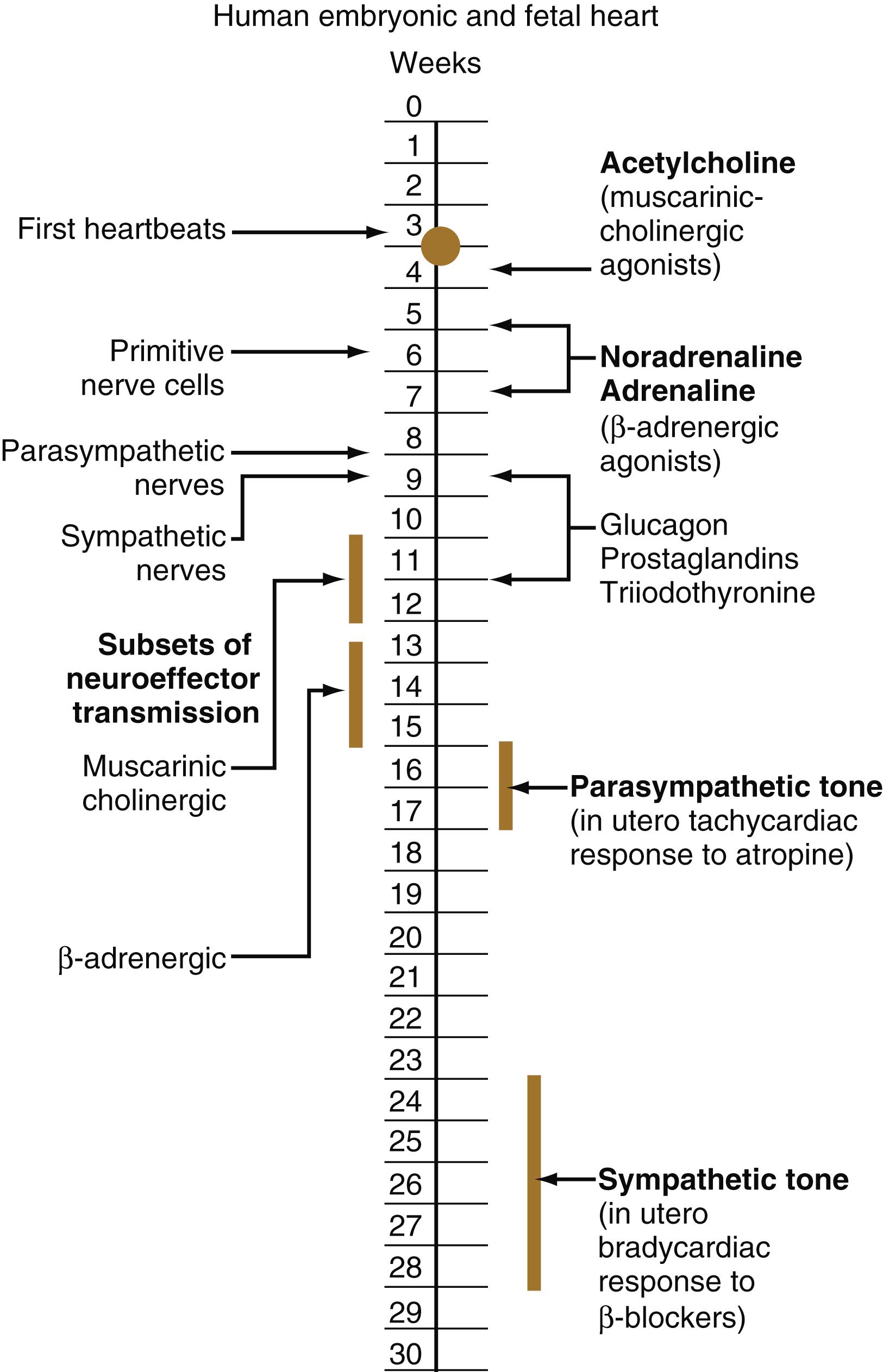
The circulation of the human embryo can be first studied at about 5 weeks by means of ultrasound. At that time, the heart beats at a rate of approximately 100 beats/min. This probably represents an inherent atrial rhythm. The pulse rate increases to approximately 160 beats/min by 8 weeks and then decreases to 150 beats/min by 15 weeks, with a further slight decline near term. The pulse rate in utero is remarkably constant, and embryos exhibiting bradycardia (slow pulse rate) often die before term. Near term, the pulse rate varies to some extent if conditions in the uterus change or if the embryo is stressed. This variation is related to the functional establishment of the autonomic innervation of the heart ( Figure 18.5 ).
The heart of the fetus has gross physiological properties quite different from those of the postnatal heart. The myocardial force, the velocity of shortening, and the extent of shortening all are less in the fetal heart. Some gross functional characteristics of the fetal heart are related to the presence of fetal isoforms of contractile proteins in the cardiac myocytes and a smaller proportion of contractile to noncontractile elements than in the postnatal heart. In fetal heart cells, the β-myosin heavy chain isoform predominates. This is advantageous because a lower oxygen requirement and less adenosine triphosphate are needed to develop the same amount of force as the α-myosin isoform in the adult heart.
The stroke volume (blood expelled with one heartbeat) of the early (18 to 19 weeks’ gestation) fetus is very small (<1 mL), but it increases rapidly with continued growth of the fetus. In a term human fetus, the combined ventricular output is approximately 450 mL/kg/min. The right ventricle of a human fetus has a greater stroke volume than the left ventricle. This is correlated with an 8% greater diameter of the pulmonary artery than of the fetal aorta. Approximately 55% of the ventricular output comes from the right ventricle and 45% from the left. Of the right ventricular output, only 25% goes to the lungs because of the underdeveloped capacity of the fetal pulmonary circulation. The remainder (75%) passes directly through the ductus arteriosus to join the aortic blood.
Quantitative studies have shown a good correlation between blood flow and functional needs of various regions of the embryo. Approximately 40% of the combined cardiac output goes to the head and upper body and supplies the relatively great needs of the developing brain. Another 30% of the combined cardiac output goes to the placenta via the umbilical arteries for replenishment. Figure 18.6 shows the relative amounts of blood that enter and leave the heart via various vascular channels. (The general qualitative pattern of blood flow in a human fetus is presented in Figure 17.34 .)
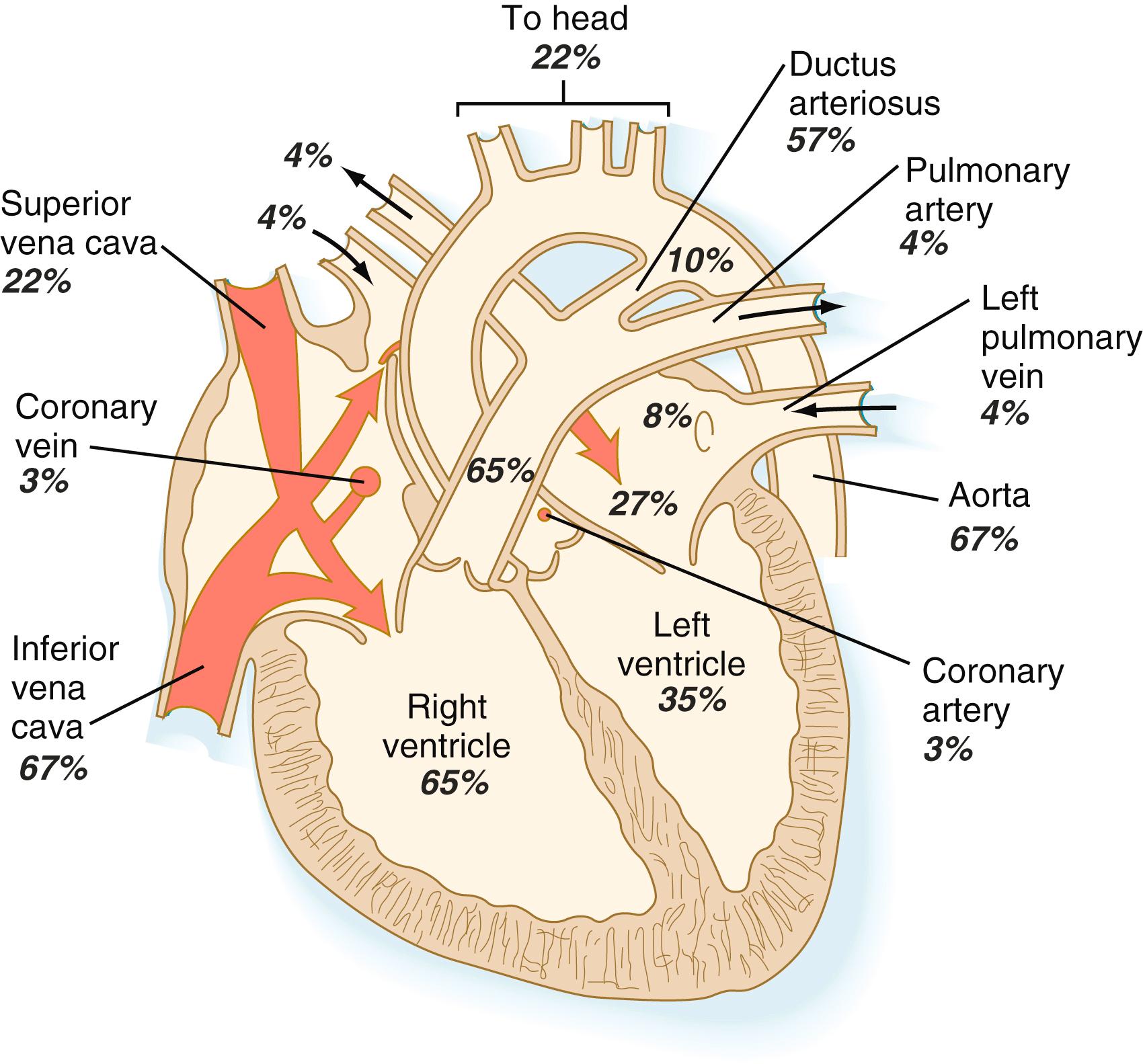
Differential streaming of blood within the heart results in different concentrations of oxygen in the chambers of the fetal heart. Blood in the left ventricle is 15% to 20% more saturated with oxygen than blood in the right ventricle. This increased oxygen saturation and the high volume of blood supplying the head via branches of the ascending aorta ensure that the developing heart and brain receive an adequate supply of oxygen. Overall, 70% to 80% of fetal hemoglobin is saturated with oxygen.
A key factor in the maintenance of the fetal pattern of circulation is the patency of the ductus arteriosus and the ductus venosus , which has a thicker wall than either the fetal aorta or pulmonary artery. The fetal ductus venosus is kept open through the actions of prostaglandins E 2 and I 2 , whereas only prostaglandin E 2 is involved in maintaining patency of the ductus arteriosus. Patency of the ductus arteriosus in the fetus and its closure after birth reflects its ability to sense oxygen concentrations, a capacity seen in only a small number of other sites in the body (e.g., the carotid body).
Myocardial cells of the developing atrium gradually produce and store granules containing atrial natriuretic peptide , a hormone that has pronounced vasodilatory, natriuretic, and diuretic properties. This hormone is released after the atrial walls are stretched, normally a sign of increased blood volume. It has been detected in atrial cardiomyocytes as early as 8 to 9 weeks’ gestation. After intrauterine blood transfusions during midgestation or later, blood levels of atrial natriuretic peptide increase significantly in response to the increased blood volume.
The lungs develop late in the embryo and are not involved in respiratory gas exchange during fetal life. They must be prepared, however, to assume the full burden of gas exchange as soon as the umbilical cord is cut.
The fetal lungs are filled with a chloride-rich acidic fluid, and the blood circulation to them is highly reduced. To perform normal postnatal breathing, the lungs must grow to an appropriate size, respiratory movements must occur continuously, and the air sacs ( alveoli ) must be appropriately configured for air exchange.
Normal growth of the fetal lungs depends on their containing an adequate amount of fluid. During the last trimester of pregnancy, fluid constitutes 90% to 95% of the total weight of the lung. The fluid filling the fetal lungs differs in composition from amniotic fluid, and it has been shown to be secreted by the pulmonary epithelial cells. Secretion begins with a net movement of chloride ions into the lumina of the pulmonary passages. Water movement follows the chloride ions. Studies have shown a relationship between total fluid volume in the lungs and fetal breathing movements, with dilation and constriction of the larynx serving a valvelike function. In vitro studies have shown that proliferation of lung epithelial cells is stimulated by mechanical stretching. In vivo, the internal pressure of the lung fluid serves as the stretching agent. A reduced volume of lung fluid is associated with pulmonary hypoplasia .
Ultrasound analysis has shown that the fetus begins to make gross breathing movements as early as 10 weeks. These movements are periodic rather than continuous, and they have two forms. One type of movement is rapid and irregular, with varying rate and amplitude. The other form is represented by isolated, slow movements, almost like gasps. The former type is more prominent and is associated with conditions of rapid eye movement (REM) sleep. Periods of rapid breathing (often for about 10 minutes) alternate with periods of apnea (cessation of breathing).
Breathing movements in the adult are controlled by two centers located in the medulla. One of these centers controls inspiration and the other controls expiration. In rodent fetuses, the center that controls expiration becomes functional in the midfetal period, slightly before functional maturation of the inspiratory center. Breathing movements are known to be responsive to maternal factors, many of which remain to be identified. The amount of breathing (minutes of breathing per hour) is highest in the evening and lowest in the early morning. The fetal breathing rate increases after the mother has eaten. This increase is related to the concentration of glucose in the maternal blood. Maternal smoking causes a rapid decrease in the rate of fetal breathing for as long as 1 hour and is linked to impaired lung development.
Fetal breathing movements are essential for postnatal survival. One function of fetal breathing is to condition the respiratory muscles so that they can perform regular postnatal contractions. Another important function is to stimulate the growth of the embryonic lungs. If intrauterine breathing movements are suppressed, lung growth and histological maturation are retarded. This is a result of a reduction in the production of platelet-derived growth factor, insulin-like growth factor , and thyroid transcription factor-1 , which stimulate cell proliferation and reduce apoptosis in the peripheral parts of the fetal lungs.
An important developmental adaptation of the fetal respiratory system is growth of the upper airway. Although a newborn is approximately 4% the weight of an adult, the diameter of its trachea is one-third that of the adult trachea. Other components of the airway are similarly proportioned. If the trachea were narrower, the physical resistance to airflow would be so great that movement of air would be almost impossible. Even with these adaptations, the resistance of a neonate’s airway is five to six times greater than that of an adult.
A functionally important aspect of fetal lung development is the secretion of pulmonary surfactant by the newly differentiating type II alveolar cells of the lung, starting around 24 weeks’ gestation. Surfactant is a mixture of phospholipids (about two-thirds phosphatidylcholine [lecithin]) and protein that lines the surface of the alveoli and reduces the surface tension. This reduction in surface tension reduces the inspiratory force required to inflate the alveoli and prevents the collapse of the alveoli during expiration.
Despite the early initiation of surfactant synthesis, large amounts are not synthesized until a few weeks before birth. At this time, the production of surfactant by the type II alveolar cells is higher than at any other period in an individual’s life, an adaptation that is an important preparation for the newborn’s first breath. Certain hormones and growth factors are involved in the synthesis of surfactant, and the effects of thyroid hormone and glucocorticoids are particularly strong.
Premature infants often have respiratory distress syndrome , which is manifested by rapid, labored breathing shortly after birth. This condition is related to a deficiency in pulmonary surfactant and can be ameliorated by the administration of glucocorticoids, which stimulate the production of surfactant by the alveolar epithelium. The risk for respiratory distress syndrome in infants born at 29 weeks is greater than 60% and decreases to 20% at 34 weeks and less than 5% at 37 weeks.
Ultrasonography has revolutionized the analysis of fetal movements and behavior because the fetus can be examined virtually undisturbed (except for an increase in vascular activity induced by the ultrasound) for extended periods. Earlier studies of fetal movements were principally concerned with the development of reflex responses, and the information was obtained largely by the analysis of newly aborted fetuses (see Chapter 11 ). Although valuable information on maturation of reflex arcs was obtained in this manner, many of the movements elicited were not those normally made by the fetus in utero.
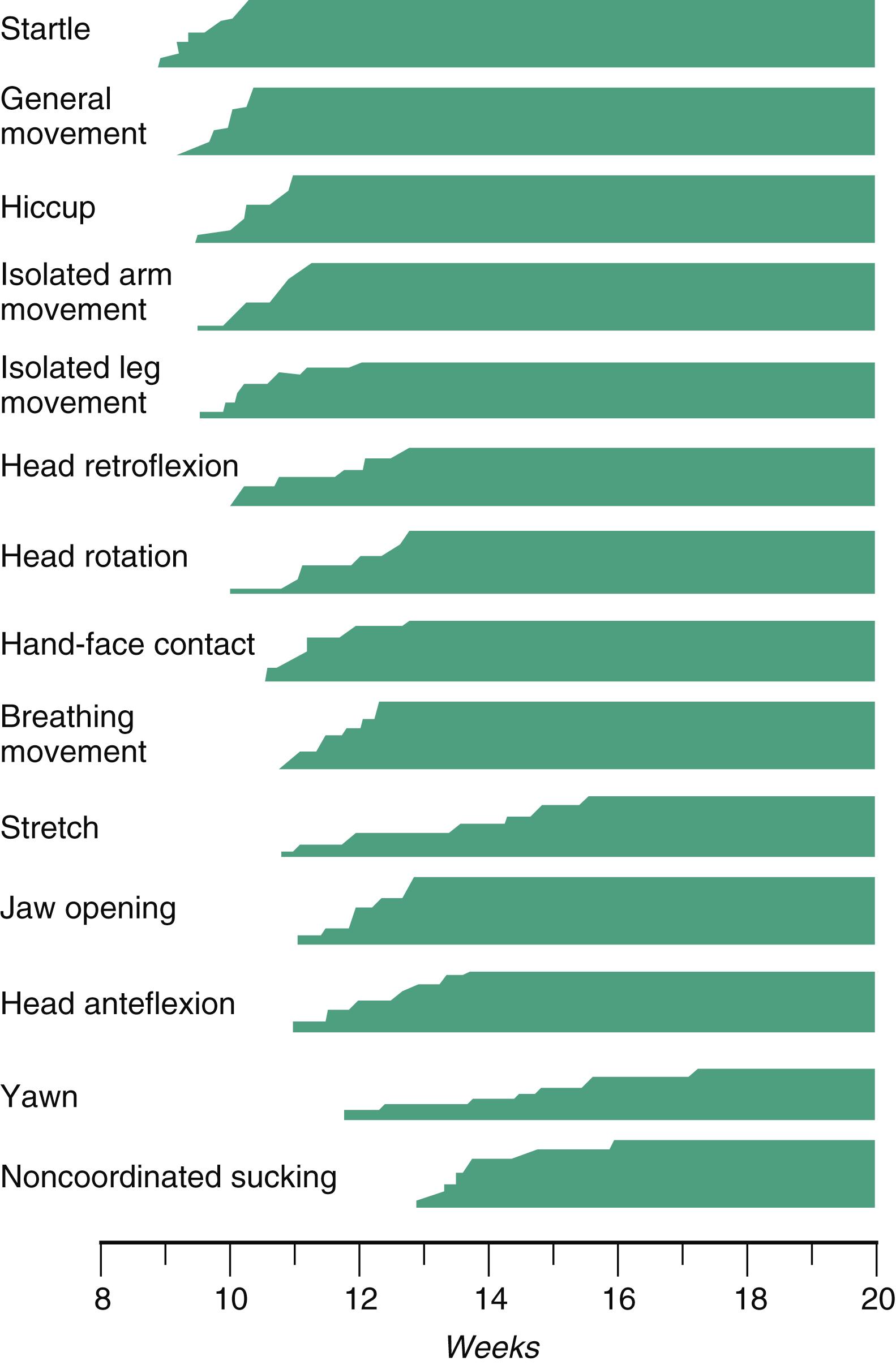
The undisturbed embryo does not show any indication of movement until approximately 71/2 weeks. The first spontaneous movements consist of slow flexion and extension of the vertebral column, with the limbs being passively displaced. The first movements occur once a central pattern generator has been established, and they can occur in the absence of sensory input. Within a short time, a large repertoire of fetal movements evolves. By 16 to 18 weeks, all movements seen in the newborn have already appeared in the fetus. After study by numerous investigators, a classification of fetal movements has been suggested ( Box 18.1 ). The first fetal movements are followed in a few days by startle and general movements. Shortly thereafter, isolated limb movements are added ( Figure 18.7 ). Movements associated with the head and jaw appear later. Toward the end of the fourth month, the fetus begins a pattern of periods of activity, followed by times of inactivity. Many women first become aware of fetal movements at this time. Between the fourth and fifth months, the fetus becomes capable of gripping firmly onto a glass rod. Although weak protorespiratory movements are possible, they cannot be sustained. Swallowing appears by 13 to 14 weeks, and finger sucking is occasionally seen shortly thereafter. By 16 to 18 weeks, eye movements begin. Their frequency increases with increasing gestational age, and their type varies from slow to rapid or even repetitive (nystagmus like). Hiccups are a prominent component of the fetal movement repertoire. These decrease during the last 10 weeks of fetal life through an inhibitory effect by the developing brain. Breathing movements, which begin 2 weeks after the onset of hiccups, are essential for normal lung development, including the differentiation of alveolar epithelial cells.
Anteflexion of the head: Normally slow, forward bending of the head
Fetal breathing movements: Paradoxical movements in which the thorax moves inward and the abdomen outward with each contraction of the diaphragm
General movements: Slow gross movements involving the whole body lasting several seconds to a minute
Hand-face contact: Contact that occurs any time the moving hand touches the face or mouth
Hiccups: Repetitive phasic contractions of the diaphragm (an episode may last several minutes)
Isolated arm or leg movements: Movements of extremities that occur without movement of the trunk
Lateral rotation of the head: Movement that involves isolated turning of the head from side to side
Opening of mouth: Isolated movement that may be accompanied by protrusion of the tongue
Retroflexion of the head: Slow to jerky backward bending of the head
Startle movements: Quick (1-second), generalized movements that always start in the limbs and may spread to the trunk and neck
Stretch: Complex movement that involves overextension of the spine, retroflexion of the head, and elevation of the arms
Sucking: Burst of rhythmical jaw movements that is sometimes followed by swallowing (with this movement, the fetus may be drinking amniotic fluid)
Yawn: Movement in which the mouth is slowly opened and rapidly closed after a few seconds
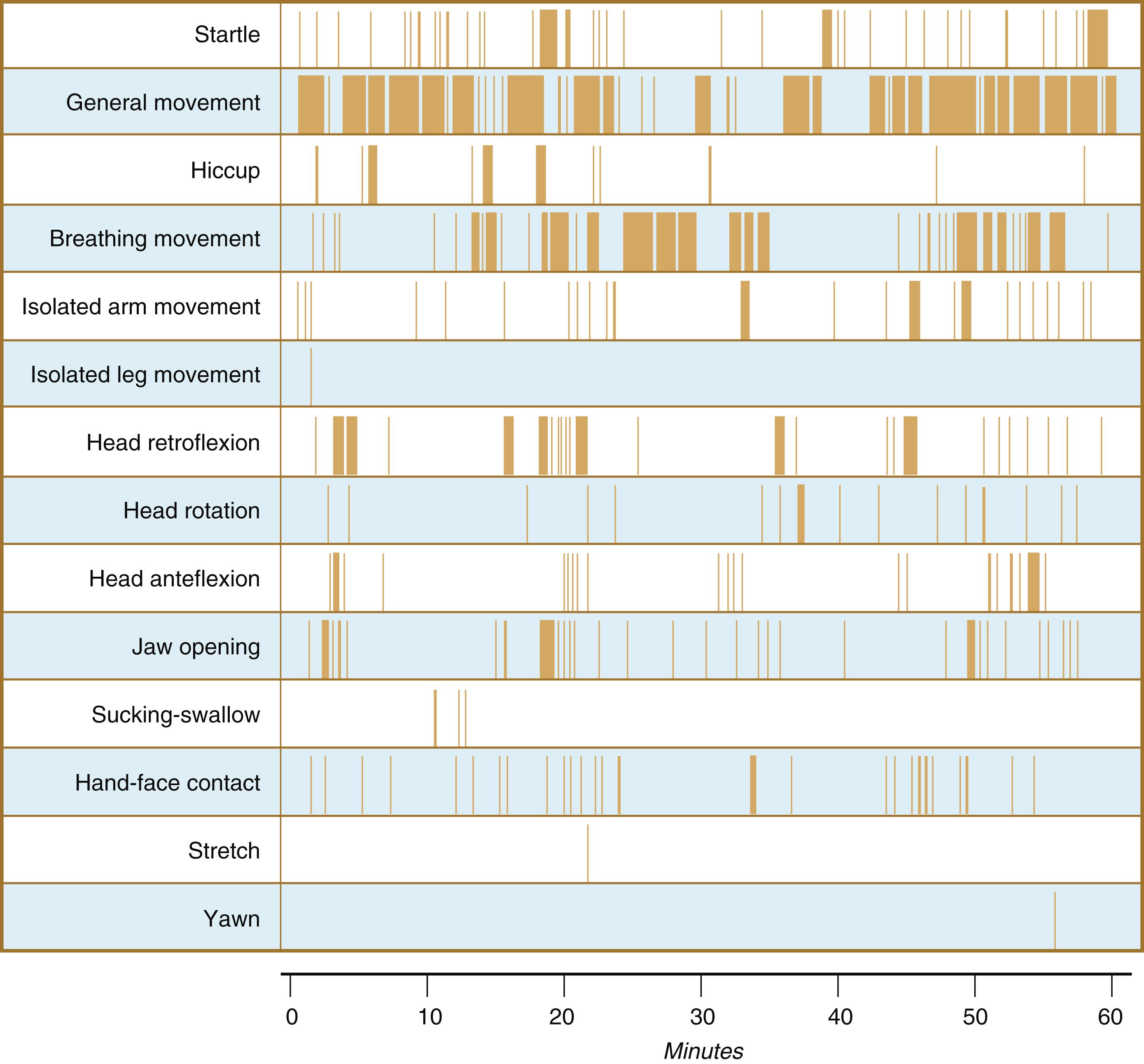
Continuous ultrasound monitoring for extended periods reveals patterns involving many types of movements ( Figure 18.8 ). At different weeks of pregnancy, some movements are predominant, whereas others are in decline or are just beginning to take shape. Analysis of anencephalic fetuses has shown that although many movements occur, they are poorly regulated. These movements start abruptly, are maintained at the same force, and then stop abruptly. These abnormal patterns of movements are considered evidence for strong supraspinal modulation of movement in the fetus.
Human fetal activities, as reflected in breathing or general activity level, show distinct diurnal rhythms beginning at approximately 20 to 22 weeks’ gestation. There is a strong negative correlation between maternal plasma glucocorticoid levels and fetal activity. Fetal activity, especially breathing, is highest in the early evening, when maternal blood glucocorticoid levels are lowest, and lowest in the early morning, when the concentration of maternal hormone peaks. Studies of women who have been given additional glucocorticoids or inhibitors have shown increased fetal activity when maternal corticoid levels are low. Usually, when the overall fetal activity is low, the fetus is in a state of REM sleep, but definitions of sleep and wakefulness in the fetus need further clarification.
Several sensory systems also begin to function during the fetal period. Near-term fetuses are responsive to 2000-Hz stimuli when in a state of wakefulness, but they are unresponsive during periods of sleep. Loud vibroacoustic stimuli applied to the maternal abdomen produce a fetal response consisting of an eye blink, a startle reaction, and an increase in heart rate. Although the fetus is constantly in the dark, the pupillary light reflex can usually be elicited by 30 weeks.
By the late fetal period, the capacity to taste and smell is well established. As early as 16 weeks, a fetus can respond to sweet or bitter tastes in the amniotic fluid by swallowing more or less. By 24 weeks, the mucous plugs that block the nasal passages dissolve, and amniotic fluid can bathe the olfactory receptors in the nose. Psychological experiments have shown that late fetuses can become habituated to dietary preferences of the mother, and newborns respond positively to flavors of food to which they have been exposed in utero. Similarly, newborns can recognize sounds to which they have been repetitively exposed before birth.
The issue of fetal pain has remained controversial with respect to both fetal surgery and abortion techniques. Key is whether fetal withdrawal reactions to noxious stimuli really represent an adultlike sensation of pain. Because of the late development of thalamocortical fibers, which are required for awareness of noxious stimuli, and the presence of other endogenous inhibitors, many fetal physiologists believe that functional perception of pain in utero is unlikely to exist before 29 or 30 weeks of gestation. Others suggest that the pain threshold is reached several weeks earlier. Over recent decades increasing evidence has suggested that a fetus can perceive pain earlier than that which had been previously recognized.
The fetal digestive tract is not functional in the conventional sense because the fetus obtains its nutrition from the maternal blood via the placenta. The digestive tract must be prepared, however, to assume the full responsibility for nutritional intake after birth. When the basic digestive tube and glands have formed in the early embryo, the remainder of the intrauterine period is devoted to cellular differentiation of the epithelia of the gut and preparation of the numerous cells involved for their specific roles in the digestive process. Beneath the epithelium, the walls of the digestive tube must become capable of propelling ingested food and liquid. Analysis of development of the fetal digestive tract has concentrated on (1) the biochemical adaptations of the epithelium of the various regions for digestive function and (2) the development of motility of the digestive tube.
The development and differentiation of epithelia or specific regional characteristics of the gut lining typically follow gradients along the length of the segment of the gut specifically involved. In the esophagus and stomach, differentiation of the mucosal epithelium is well underway starting around 4 months. Although parietal cells (hydrochloric acid–producing cells) and chief cells (pepsinogen-producing cells) are first seen at 11 and 12 weeks, there is little evidence of their secretions during fetal life. The contents of the stomach have a nearly neutral pH until after birth, but then gastric acid production increases greatly within a few hours.
In the small intestine, villi begin to form in the upper duodenum at the end of the second month, and crypts appear 1 to 2 weeks later. The formation of villi and crypts spreads along the length of the intestine in a spatiotemporal gradient. By approximately 16 weeks of gestation, villi have formed along the entire length of the intestine, and crypts appear in the lower ileum by 19 weeks. Villi even form in the colon during the third and fourth months, but they then regress and are gone by the seventh or eighth month.
Individual epithelial cell types, including Brunner’s glands , which protect the duodenal lining from gastric acid, appear in the small intestine early in the second trimester. Although the presence of most enzymes or proenzymes characteristic of the intestinal lining can be shown histochemically during the midfetal period, the amounts of these substances are generally quite small. Activity of some of the enzymes secreted by the exocrine pancreatic tissue can also be shown between 16 and 22 weeks’ gestation. Meconium , a greenish mixture of desquamated intestinal cells, swallowed lanugo hair, and various secretions, begins to fill the lower ileum and colon late in the fourth month ( Figure 18.9 ).
![Fig. 18.9, Meconium ileus (accumulation of fetal meconium [the greenish material]) in the fetal small intestine. Fig. 18.9, Meconium ileus (accumulation of fetal meconium [the greenish material]) in the fetal small intestine.](https://storage.googleapis.com/dl.dentistrykey.com/clinical/FetalPeriodandBirth/6_3s20B9780323881685000183.jpg)
Differentiation of the neuromuscular complex of the digestive tract also follows a gradient, with the circular layer of smooth muscle forming in the esophagus at 6 weeks. Myenteric plexuses (parasympathetic neurons) take shape after the inner circular muscle layer is present, but before the formation of the outer longitudinal layer of muscle a couple weeks later in any given region. Starting in the esophagus at 6 weeks, the final formation of myenteric plexuses throughout the length of the digestive tract is complete at 12 weeks. The first spontaneous rhythmical activity in the small intestine is seen in the seventh week, at approximately the time of formation of the inner circular muscular layer. Recognizable peristaltic movements do not begin until the fourth month, however. Fetuses older than 34 weeks are able to pass meconium in utero.
Another intrauterine preparation for feeding is the development of swallowing and the sucking reflex. Swallowing is first detected at 10 to 11 weeks of gestation, and then its incidence gradually increases. The function of fetal swallowing is unclear, but by term, fetuses swallow 200 to 750 mL or more of amniotic fluid per day. The amniotic fluid contains protein, and much of this is absorbed through the gut by a process of intracellular digestion, occurring by the uptake of macromolecules by fetal enterocytes. According to some estimates, 15% to 20% of total body protein deposition is derived from protein found in amniotic fluid. The swallowed amniotic fluid may contain growth factors that facilitate the differentiation of epithelial cells in the digestive tract. To a certain extent, taste seems to regulate fetal swallowing. Taste buds are seemingly mature by 12 weeks, and the amount of swallowing increases if saccharin is introduced into the amniotic fluid. Conversely, swallowing is reduced if noxious chemicals are added. Fetal swallowing is followed by gastric peristalsis and gastric emptying, which begin at 12 weeks. The gastric emptying cycles mature throughout the fetal period and are important in maintaining the overall balance of amniotic fluid.
Coordinated sucking movements do not appear until late in fetal development. Although noncoordinated precursors of sucking movements occur by 16 to 18 weeks, it is not until 32 to 36 weeks that the fetus undertakes short bursts of sucking. These short bursts are not associated with effective swallowing movements. Ineffective sucking is the main reason that premature infants of this age must be fed through a nasogastric tube. Mature sucking capability appears after 36 weeks.
The normal human adult intestinal tract houses an immense number of bacteria—three to four times as many as there are cells in the entire body. Collectively, these bacteria are known as the intestinal microbiome . Traditionally, the fetus has been assumed to be free of bacteria, but small numbers of nonpathogenic bacteria have been found in the human placenta. These bacteria are most similar to those found in the mouth, leading to the hypothesis that they are carried to the placenta via the maternal blood. Placental bacteria may condition the fetal immune system to respond to the immense number of bacteria that soon populate the intestinal tract of the newborn. In natural childbirth, these bacteria most resemble those resident in the maternal vaginal tract.
Although the placenta performs most excretory functions characteristic of the kidney during prenatal life, the developing kidneys also function by producing urine. As early as 5 weeks of gestation, the mesonephric kidneys produce small amounts of very dilute urine, but the mesonephros degenerates late in the third month, after the metanephric kidneys have taken shape.
Tubules of the metanephric kidneys begin to function between 9 and 12 weeks of gestation, and resorptive functions involving the loop of Henle occur by 14 weeks, even though new nephrons continue to form until birth. New nephrons are produced in a thin nephrogenic zone located just beneath the renal capsule.
The urine produced by the fetal kidney is hypotonic to plasma throughout most of pregnancy. This reflects immature resorptive mechanisms, which are manifested morphologically by short loops of Henle. The concentration of Na + is high in fetal urine than in the newborn, but the fetus is more efficient in resorbing glucose. While the neural lobe of the hypophysis produces antidiuretic hormone beginning at 11 weeks, another mechanism for the concentration of urine begins to be established.
Fetal kidneys receive only approximately 3% of the total blood flow, as opposed to 15% in the newborn. The total production of urine rises from 0.1 mL/min during midpregnancy to 1.0 mL/min at term. Intrauterine renal function is unnecessary for fetal life because embryos with bilateral renal agenesis survive in utero. Bilateral renal agenesis, however, is commonly associated with oligohydramnios (see Chapter 7 ), thus indicating that the overall balance of amniotic fluid requires a certain amount of fetal renal function.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here