Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The prevalence of skeletal dysplasias is about 2.4/10,000 births, and 9.1/1000 among perinatal deaths; the most common anomalies are thanatophoric dysplasia, achondroplasia, osteogenesis imperfecta, and achondrogenesis.
The new edition of Nosology and Classification of Genetic Skeletal Disorders includes 436 clinical conditions classified into 42 groups involving 364 affected genes; therefore, molecular diagnosis is possible in about 75% of skeletal disorders.
Molecular diagnosis can be offered to couples with a family history of skeletal dysplasia to estimate the risk in a future pregnancy, or to pregnant women with ultrasound signs suggestive of fetal skeletal dysplasia.
Two-dimensional (2D) and three-dimensional (3D) sonography and 3D helical computed tomography (3D-HCT) are the main imaging modalities used to evaluate a fetus with suspected skeletal dysplasia.
Thanatophoric dysplasia and achondrogenesis account for 60% of all lethal skeletal dysplasias and are characterized by extremely reduced thoracic dimensions and very short long bones.
A complete fetal ultrasound evaluation, including assessment of fetal movements, should be performed if a skeletal disorder is suspected.
Ultrasound signs suggestive of a skeletal dysplasia include bones with reduced length, abnormal shape, reduced mineralization, and fractures; abnormal fetal facial profile; polyhydramnios; and abnormal pattern of fetal movements.
The diagnosis of a possible skeletal dysplasia during pregnancy should be corroborated by molecular diagnosis and neonatal or pathologic evaluation.
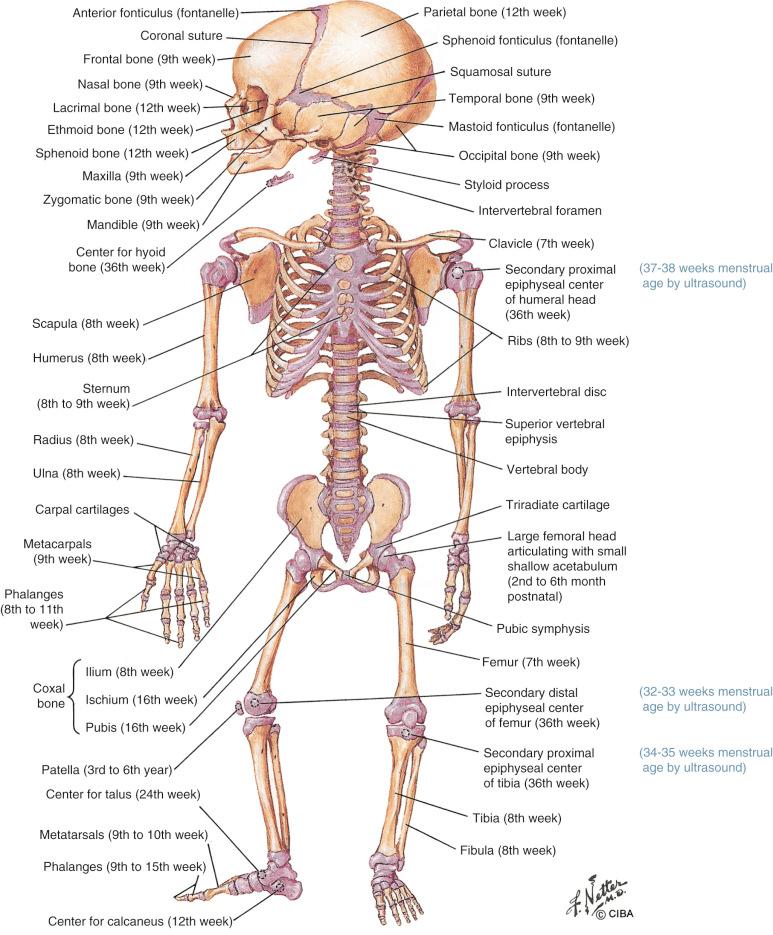
The skeleton is formed by 206 skeletal elements constituted by two tissues (bone and cartilage) and three cell types (osteoblasts, osteoclasts, and chondrocytes). Skeletal tissues derive from three embryonic cell lineages: (1) cranial neural crest cells, from which the craniofacial skeleton originates; (2) paraxial mesoderm cells or somites, which are the embryologic precursors of the axial skeleton; and (3) the lateral plate mesoderm, which is responsible for limb formation. Limb buds begin to develop during the 4th week of embryonic life (6th menstrual week) as clusters of mesenchymal cells covered by a layer of ectoderm. Mesenchymal models of bone (anlagen: templates for future bones) form around the 5th week of embryonic life (7th menstrual week) ( Fig. 11-1 ). Development of the upper limbs precedes the lower limbs in bud appearance, differentiation, and final relative limb size. Limbs develop in a proximodistal sequence, with the anlagen of the humerus and femur forming first, followed by the radius and ulna, the tibia and fibula, the metacarpal and metatarsal bones, and phalanges.

Skeletogenesis involves four steps: patterning, organogenesis, growth, and homeostasis. Patterning is the process by which the final size, shape, number, and arrangement of bones are determined. This process takes place long before skeletogenesis, and three signaling regions have been identified: (1) an apical ectodermal ridge; (2) an area consisting of ectoderm covering the sides of the bud; and (3) a zone of polarizing activity. The apical ectodermal ridge consists of densely packed ectodermal cells located at the tip of the limb bud, which express several fibroblast growth factors (FGFs) that initiate and control limb outgrowth. The ectoderm covering the sides of the bud regulates dorsoventral patterning. The zone of polarizing activity is located on the posterior limb bud margin. It is responsible for anteroposterior patterning and, thus, the formation of digits. Besides FGFs, other genes are involved in the control of limb patterning, including Sonic hedgehog (Shh), GLI-Kruppel family member GLI3 (Gli3), sal-like 1 (Sall1), Hoxd13, Hoxa13, bone morphogenetic/cartilage-derived morphogenetic protein (CDMP), growth differentiation factors (GDFs), Noggin (Nog), Wn7-a, engrailed (en), and LIM homeobox transcription factor 1 beta (Lmx1b).
Bone and cartilage are formed during the organogenesis period, which consists of three phases: condensation, cell differentiation, and histogenesis. Condensation is of great importance in skeletal development, because the templates for future bones are defined at this stage. Condensation initiation, boundary set, proliferation, adhesion, and growth are regulated by complex interactions between extracellular matrix molecules, cell surface receptors, cell adhesion molecules (e.g., fibronectin, tenascin, Noggin, syndecan, and neural cell adhesion molecule [N-CAM]), homeobox genes (e.g., Hoxa-2, Hoxa-13, Hoxd-11, and Hoxd-11-13), transcription factors (e.g., runt-related transcription factor 2 [RUNX2], winged-helix transcription factor [CFKH-1], mesenchyme forkhead 1 [MFH-1], paired box transcription factors 1 and 9 [PAX-1, PAX-9], periaxin 1 and 2 [PRX-1 and PRX-2], scleraxis, and mammalian SRY box 9 [SOX-9]), and growth factors (e.g., bone morphogenetic proteins, fibroblast growth factor 2 [FGF-2], transforming growth factor beta [TGF-β]). Osteogenesis initiates during the 7th week of embryonic development (9th menstrual week), with bones developing by either endochondral or membranous ossification.
The axial and appendicular skeletons are formed by endochondral ossification ( Fig. 11-2 ). The axial skeleton (e.g., vertebrae and the dorsal part of the ribs) originates from the somites. Cartilaginous models of the future bones differentiate within mesenchymal condensations during the 6th week of development (8th menstrual week), with primary ossification centers developing in the middle of the anlagen between the 7th and 12th weeks of development (9th to 14th menstrual weeks). SOX9 plays an important role in chondrogenesis. Mutations in this gene cause campomelic dysplasia, a severe skeletal disorder characterized by congenital bowing and angulation of the long bones (especially of the tibia), hypoplastic scapulae, sex reversal in male fetuses, and a high lethality rate due to respiratory insufficiency. Another gene involved in chondrogenesis is procollagen type II alpha 1 ( COL2A1 ), which encodes collagen type II.
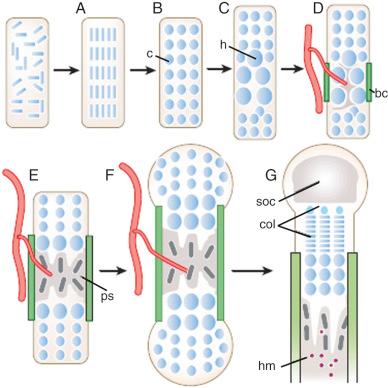
Within primary ossification centers, hypertrophic cartilage matrix is degraded, chondrocytes undergo apoptosis, osteoblasts replace the disappearing cartilage with trabecular bone, and bone marrow is formed. Simultaneously, osteoblasts in the perichondrium begin to deposit a collar of compact bone matrix along the diaphysis. Osteoblast differentiation is controlled by RUNX2 and Osterix, whereas proliferation is controlled by the low-density lipoprotein receptor–related protein 5 (LPR5) signaling pathway. Eventually, cartilage in the center of the anlagen degrades, mineralizes, and is removed by osteoclasts. Vascular ingrowth is stimulated by vascular endothelial growth factor, with the influx of osteoprogenitor cells occurring at the same time that the bone matrix is deposited along the periosteum of the midshaft. Some of these invading cells differentiate into hematopoietic stem cells, whereas others differentiate into osteoclasts or osteoblasts.
Secondary ossification centers begin to appear at the extremities of bones (epiphyses) later in pregnancy. The portion of cartilage trapped between the expanding primary and secondary ossification centers is known as the growth plate or physis. This structure is formed by a cartilaginous component (epiphyseal plate), a bony component (metaphysis), and fibrous tissue surrounding the periphery of the plate. The growth plate is responsible for the longitudinal growth of long bones until definitive fusion of epiphyses and diaphyses occurs at the end of puberty. Longitudinal growth is coordinated by Indian hedgehog (Ihh), a stimulator of chondrocyte proliferation at the growth plate. Bone mass, shape, and strength are maintained throughout development and adult life by balancing bone destruction and formation. Homeostasis is the process that controls the continuing remodeling of bones.
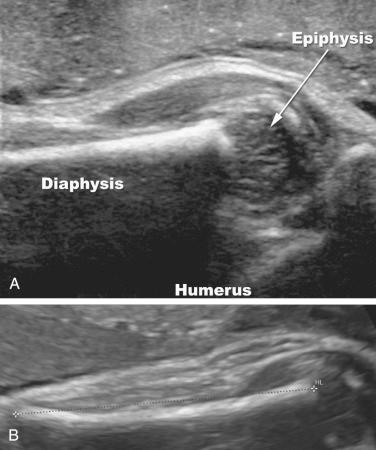
It is noteworthy that when a long bone is imaged by ultrasound, only the diaphyses are measured, because the epiphyses are hypoechoic and are not always clearly visualized. Under favorable conditions, good sonographic images of the epiphysis can be obtained, especially when high-frequency transducers are used ( Fig. 11-3 ). Secondary ossification centers may often be visualized by ultrasound in the third trimester. The distal femoral ossification center may be seen at approximately 32 to 33 weeks of gestation, the proximal tibial epiphyseal center at 34 to 35 weeks, and the proximal humeral epiphyseal ossification center at 37 to 38 weeks of gestation ( Fig. 11-4 ). When all three centers are identified, it is likely that the fetus is at least 37 weeks' gestational age. These ossification centers may be seen slightly earlier in female fetuses than in male fetuses. The timeline for radiographic and histologic appearance of primary and secondary ossification centers is illustrated in Figure 11-5 .
Formation and detachment of new somites in the axial skeleton from the paraxial mesoderm occur in craniocaudal direction following a molecular clock produced by oscillations of cycling genes (e.g., c-hairy-1, lunatic fringe [l-fng], and naked cuticle 1 [nkd1]) that stimulate Notch receptor signaling waves that sweep through the paraxial mesoderm. Spatial coordination is provided by a decreasing gradient of FGF8 from the posterior to the anterior pole of the embryo. Differential expression of delta-like (Dll) proteins determines the size and polarity of the somites, which mature as they move rostrally and differentiate into dermatomyotomes and sclerotomes. Dermatomyotomes give rise to the appendicular and axial musculatures, as well as the dorsal epithelium. The sclerotome is the precursor of the axial skeleton, and its formation is initiated and controlled by the Sonic hedgehog gene ( SHH ).
The craniofacial skeleton and clavicles develop by intramembranous ossification. This process differs from endochondral ossification by the direct differentiation of mesenchymal cells into osteoblasts, which produce a bone matrix rich in type I collagen. Bone remodeling is accomplished by continuous and concerted action of osteoblasts (cells that produce bone matrix) and osteoclasts (cells that remove bone).
Abnormal development, growth, or maintenance of cartilage and bone tissues results in skeletal dysplasias. Skeletal dysplasias are a heterogeneous group of disorders affecting the development of chondro-osseous tissues leading to abnormalities in the size, mineralization, and shape of various segments of the skeleton. Despite recent advances in imaging and molecular genetics, accurate prenatal diagnosis of skeletal dysplasias remains a clinical challenge. In the most recent revision of the International Nosology and Classification of Constitutional Disorders of Bone it is mentioned that in approximately 25% of all bone disorders the mutated gene has not been yet identified. It is important to acknowledge the contribution of the International Skeletal Dysplasia Registry in the identification and study of skeletal anomalies, assisting providers and patients in the diagnosis and clinical management of skeletal disorders.
In subsequent sections of this chapter, we will review the birth prevalence, classification, and molecular genetics of skeletal dysplasias that are identifiable at birth.
In a large multicenter study conducted by Camera and Mastroiacovo the birth prevalence of skeletal dysplasias (excluding limb amputations) was estimated as 2.4/10,000 births. Twenty-three percent of the affected infants were stillborn, whereas 32% died during the first week of life. The overall frequency of skeletal dysplasias among perinatal deaths was 9.1/1000. This study also reported on the birth prevalence of the different skeletal dysplasias and their relative frequency among perinatal deaths. The four most common skeletal dysplasias were thanatophoric dysplasia, achondroplasia, osteogenesis imperfecta (OI), and achondrogenesis. Thanatophoric dysplasia and achondrogenesis accounted for 62% of all lethal skeletal dysplasias, and the most common nonlethal skeletal dysplasia was achondroplasia. In another large series from western Scotland, the prevalence of skeletal dysplasias at birth was 1.1/10,000 births. The most frequent conditions were thanatophoric dysplasia (1/42,000), OI (1/56,000), chondrodysplasia punctata (1/84,000), campomelic syndrome (1/112,000), and achondrogenesis (1/112,000). Rasmussen and associates reported a prevalence of 2.1/10,000 deliveries in a longitudinal study, which included elective pregnancy termination, stillborn infants at more than 20 weeks of gestation, and liveborn infants diagnosed by the 5th day of life. The rate of lethal cases in this study was 0.95/10,000 deliveries. Of interest, the study reporting the highest prevalence of skeletal dysplasias at birth (9.5/10,000 births) was conducted in a population with a high rate of consanguineous unions. An overview of the prevalence of skeletal dysplasias in different populations is presented in Table 11-1 .
| Study * | Prevalence | Comment |
|---|---|---|
| Gustavson and Jorulf | 1/2117 | Newborns |
| Camera and Mastroiacovo | 1/4600 | Neonates |
| Connor et al | 1/8900 | Neonates |
| Weldner et al | 1/1333 | Achondroplasia |
| Orioli et al | 1/4347 | First 3 days of life |
| Stoll et al | 1/3125 | First 8 days of life |
| Andersen | 1/6667 | At birth |
| Kallen et al | 1/6250 | Multicenter study |
| Rasmussen et al | 1/4166 | First 5 days of life |
| Barbosa-Buck et al | 1/21,276 | Thanatophoric dysplasia |
| Donnelly et al | 1/8000 | Thanatophoric dysplasia |
| Stevenson et al | 1/7900 | Osteogenesis imperfecta |
* Studies are listed in the references at the end of the chapter.
The first classification of skeletal anomalies was proposed more than 40 years ago and was mainly based on clinical and radiographic findings. Since that time, the classification has continuously evolved owing to the contribution of imaging and molecular biology techniques. This classification is currently known as Nosology and Classification of Genetic Skeletal Disorders. Most skeletal anomalies are a phenotypic manifestation of a mutation in a gene and altered protein expression; therefore, they can be grouped according to the affected genes as they share similar clinical characteristics. The International Skeletal Dysplasia Society (ISDS) periodically reviews this classification, and the last version corresponding to the 9th edition was published in 2015. In this document, the authors acknowledge the contribution of next-generation sequencing technologies and the increasing availability of whole exome sequencing, allowing the discovery of more gene-related skeletal anomalies. For this review, 436 clinical conditions were classified into 42 groups involving 364 affected genes ( Table 11-2 ). The classification provides (1) the group/name of the skeletal disorder; (2) type of inheritance; (3) MIM (Mendelian Inheritance in Man) number; (4) locus of the mutation in the gene; (5) affected protein; and (6) associations/difference with other skeletal anomalies. Skeletal dysplasias, metabolic bone disorders, dysostosis, skeletal malformations, and reduction syndromes are included in this classification. However, the authors also clarify that in approximately 25% of skeletal disorders, the mutated gene has not yet been identified. The genetic basis for classification of very rare diseases was done by family pedigree, or was based on homogeneity or phenotype in unrelated families. The classification aims to provide more complete information for prenatal counseling and clinical management. The full document can be consulted at the ISDS website.
| GROUPS/NAME OF DISORDER |
|---|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
The molecular pathogenic classification of genetic disorders of the skeleton is very extensive and includes many conditions that may not be apparent at birth and therefore are not amenable to prenatal diagnosis by imaging methods. A more clinically relevant list of skeletal dysplasias that may be recognizable during pregnancy along with their mode of inheritance and causative genes has been published by Krakow ( Table 11-3 ). The authors acknowledge that for a full classification of skeletal anomalies the Nosology and Classification of Genetic Skeletal Disorders should be consulted.
| Group or Name of the Disorder | Mode of Inheritance | Gene Symbol |
|---|---|---|
| Fibroblast Growth Factor Receptor 3 (FGFR3) Disorders | ||
| Thanatophoric dysplasia | AD | FGFR3 |
| Achonodroplasia | AD | FGFR3 |
| Hypochondroplasia | AD | FGFR3 |
| Severe achondroplasia with developmental delay and acanthosis nigricans (SADDAN) | AD | FGFR3 |
| Type II Collagen Disorders | ||
| Achondrogenesis II | AD | COL2A1 |
| Hypochondrogenesis | AD | COL2A1 |
| Spondyloepiphyseal dysplasia congenita (SEDC) | AD | COL2A1 |
| Kniest dysplasia | AD | COL2A1 |
| Type XI Collagen Disorders | ||
| Fibrochondrogenesis | AR | COL11A1 |
| Fibrochondrogenesis | AD | COL11A1, COL11A2 |
| Otospondylomegaepiphyseal dysplasia (OSMED) | AR | COL11A2 |
| Sulfation Disorders | ||
| Achondrogenesis IB | AR | SLC26A2 |
| Atelosteogenesis II | AR | SLC26A2 |
| Diastrophic dysplasia | AR | SLC26A2 |
| Chondrodysplasia with congenital joint dislocations | AR | CHST3 |
| Perlecan Disorders | ||
| Dyssegmental dysplasia | AR | PLC |
| Dyssegmental dysplasia, Silverman-Handmaker type | AR | PLC |
| Dyssegmental dysplasia, Rolland-Desbuquois type | AR | PLC |
| Filamin Disorders and Similar Disorders | ||
| Otopalatodigital syndrome I and II | XLD | FLNA |
| Osteodysplasty, Melnick-Needles | XLD | FLNA |
| Atelosteogenesis types I and III | AD | FLNB |
| Larsen syndrome | AD | FLNB |
| Spondylo-carpal-tarsal dysplasia | AR | FLNB |
| Serpentine fibula–polycystic kidney syndrome | AD | NOTCH2 |
| TRPV4 Disorders | ||
| Metatropic dysplasia | AD | TRPV4 |
| Short Rib Dysplasias (With and Without Polydactyly) | ||
| Chondroectodermal dysplasia (Ellis–van Creveld) | AR | DYNC2H1 |
| Short rib–polydactyly syndrome I, II, III, and IV including asphyxiating thoracic dystrophy | AR | IFT80 NEK WDR35 |
| Thoracolaryngeal dysplasia | AD | Unknown |
| Metaphyseal Dysplasias | ||
| Cartilage-hair hypoplasia | AR | RMRP |
| Metaphyseal dysplasia, Jansen type | AD | PTHR1 |
| Spondylo-Epi-(Meta)-Physeal Dysplasia (SEMD) | ||
| SEMD, short limb abnormal calcification type | AR | DDR2 |
| Severe Spondylodysplastic Dysplasias | ||
| Achondrogenesis 1A | AR | GMAP210 |
| Schneckenbecken dysplasia | AR | SLC35D1 |
| Opsismodysplasia | AR | INPPL1 |
| Acromesomelic Disorders | ||
| Acromesomelic dysplasia, type Maroteaux | AR | NPR2 |
| Mesomelic and Rhizomesomelic Dysplasias | ||
| Langer type (homozygous dyschondrosteosis) | Pseudo-AR/XLD | SHOX |
| Omodysplasia | AR | GPC6 |
| Robinow syndrome, recessive | AR | ROR2 |
| Robinow syndrome, dominant | AD | WNT5 |
| Bent Bone Dysplasias | ||
| Campomelic dysplasia | AD | SOX9 |
| Stuve-Wiedemann dysplasia | AR | LIFR |
| Bent bone dysplasia FGFR2 type | AD | FGFR2 |
| Slender Bone Dysplasias | ||
| Microcephalic osteodysplastic primordial dwarfism (MOPD1) | AR | RNU4ATAC |
| Microcephalic osteodysplastic primordial dwarfism (MOPD2) | AR | PCNT |
| Dysplasias With Multiple Joint Dislocations | ||
| Desbuquois dysplasia | AR | CANT1, XYLT1 |
| Pseudodiatrophic dysplasia | AR | Unknown |
| Chondrodysplasia Punctata Group (CDP) | ||
| CDP, X-linked dominant | XLD | EBP |
| Conradi-Hunermann type (CDPX2) | XLR | ARSE |
| Brachytelephalangic type (CDPX1) | XLD | NSDHL |
| Congenital hemidysplasia with ichthyosiform erythroderma and limb defects (CHILD) syndrome | XLD | EBP |
| Greenberg dysplasia | AR | LBR |
| Rhizomelic CDP type 1 | AR | PEX7 |
| Rhizomelic CDP type 2 | AR | DHPAT |
| Rhizomelic CDP type 3 | AR | AGPS |
| Neonatal Osteosclerotic Dysplasias | ||
| Bloomstrand dysplasia | AR | PTHR1 |
| Desmosterolosis | AR | DHCR24 |
| Caffey disease (infantile) | AD | COL1A1 |
| Raine dysplasia | AR | FAM20C |
| Increased Bone Density Group | ||
| Osteopetrosis (severe neonatal or infantile forms) | AR | TCIRG1 |
| Osteopetrosis (severe neonatal or infantile forms) | AR | CLCN7 |
| Dysosteosclerosis | AR | SLC29A3 |
| Lenz-Majewski hyperostotic dysplasia | SP | PTDSS1 |
| Osteogenesis Imperfecta and Decreased Bone Density Group | ||
| Osteogenesis imperfecta (OI), moderate, severe, and perinatal lethal | AD | COL1A1, COL1A2, IFITM5, |
| OI, moderate, severe, and perinatal lethal | AR | CRTAP, P3H1, PPBI, FKBP10, HSP47, SP7, WNT1, TMEM33B, |
| Bruck syndrome | PLOD2, FKBP10, | |
| Osteoporosis-pseudoglioma syndrome | AR | LRP5 |
| Cole-Carpenter dysplasia | SP | LRP5, SEC24D, P4HB, CRTAP |
| Abnormal Mineralization Group | ||
| Hypophosphatasia, perinatal and infantile forms | AR | ALPL |
A skeletal anomaly is suspected prenatally either by family history or by ultrasound findings, while after birth, the diagnosis is suspected based on family history and on clinical and radiographic findings. When a previous family member is affected, a gene panel can be offered to the mother (and if necessary to the father) to evaluate the risk to the fetus. Direct testing can also be performed and genetic panels evaluated on amniotic fluid or chorionic villus samples when sonographic findings suggestive of a skeletal anomaly are identified. Krakow and colleagues compiled a practical list of abnormal ultrasonographic findings seen in skeletal dysplasias along with a list of differential diagnoses for each finding ( Table 11-4 ). Once the differential diagnoses have been narrowed down to the most likely disorder(s), definitive genetic testing can be offered. Limitations of genetic panels include study cost, time to obtain the results, and that the genetic basis of all skeletal anomalies are not known. A benefit of molecular testing is that identification of the gene mutation can provide information on the severity of the disease.
| Disorders | MIM No. | Gene Defect |
|---|---|---|
| Poor Mineralization of the Calvarium | ||
| Achondrogenesis IA | 200600 | Unknown |
| Cleidocranial dysplasia | 119600 | RUNX2 |
| Hypophosphatasia | 241500 | ALPL |
| Osteogenesis imperfecta type II | 166210 | COL1A1 |
| 166210 | COLIA2 | |
| 610854 | CRTAP | |
| Fractures of Long Bones (Particular Femora) | ||
| Hypophosphatasia | 241500 | ALPL |
| Neurofibromatosis | 162200 | NF1 |
| Osteogenesis imperfecta types II and III | 166210 | COL1A1 |
| 166210 | COLIA2 | |
| 610854 | CRTAP | |
| 259440 | P3H1 | |
| Bent/Bowed Bones by Ultrasound | ||
| Achondrogenesis IA | 200600 | Unknown |
| Achondrogenesis IB | 600972 | SLC26A2 |
| Antley-Bixler syndrome | 207410 | FGFR2 |
| Atelosteogenesis I | 108720 | FLNB |
| Atelosteogenesis II | 256050 | SLC26A2 |
| Atelosteogenesis III | 108721 | FLNB |
| Campomelic dysplasia | 114290 | SOX9 |
| Diastrophic dyplasia | 222600 | SLC26A2 |
| Hypophosphatasia | 241500 | ALPL |
| Osteogenesis types II and III | 166210 | COL1A1 |
| 166210 | COLIA2 | |
| 610854 | CRTAP | |
| 259440 | P3H1 | |
| Short-rib polydactyly syndromes (types I-IV) | 263530 | Unknown |
| 263520 | Unknown | |
| 263510 | Unknown | |
| 269860 | Unknown | |
| Stuve-Wiedemann syndrome | 601559 | LIFR |
| Thanatophoric dysplasia types I and II | 187600 | FGFR3 |
| 187601 | FGFR3 | |
| Poor Mineralization of the Vertebrae | ||
| Achondrogenesis IA | 200600 | Unknown |
| Achondrogenesis IB | 600972 | SLC26A2 |
| Achondrogenesis II | 200610 | COL2A1 |
| Atelosteogenesis I | 108720 | FLNB |
| Atelosteogenesis II | 256050 | SLC26A2 |
| Atelosteogenesis III | 108721 | FLNB |
| Opsismodysplasia | 258480 | Unknown |
| SMD—sedaghatian type | 250220 | Unknown |
| Thanatophoric dysplasia types I and II | 187600 | FGFR3 |
| 187601 | FGFR3 | |
| Absent/Hypoplastic Scapula | ||
| Campomelic dysplasia | 114290 | SOX9 |
| Equinovarus | ||
| Achondrogenesis IA | 200600 | Unknown |
| Achondrogenesis IB | 600972 | SLC26A2 |
| Achondrogenesis II | 200610 | COL2A1 |
| Atelosteogenesis I | 108720 | FLNB |
| Atelosteogenesis II | 256050 | SLC26A2 |
| Atelosteogenesis III | 108721 | FLNB |
| Campomelic dysplasia | 114290 | SOX9 |
| Desbuquois dysplasia | 251450 | Unknown |
| Diastrophic dyplasia | 222600 | SLC26A2 |
| Ehler-Danlos syndrome types VIIA and B | 130060 | COL1A1, COL1A2 |
| Hypophosphatasia | 241500 | ALPL |
| Larsen syndrome | 150250 | FLNB |
| Osteogenesis imperfecta types II and III | 166210 | COL1A1 |
| 166210 | COLIA2 | |
| 610854 | CRTAP | |
| 259440 | P3H1 | |
| Pseudodiastrophic dysplasia | 264180 | Unknown |
| Short-rib polydactyly syndromes (types I-IV) | 263530 | Unknown |
| 263520 | Unknown | |
| 263510 | Unknown | |
| 269860 | Unknown | |
| Thanatophoric dysplasia types I and II | 187600 | FGFR3 |
| 187601 | FGFR3 | |
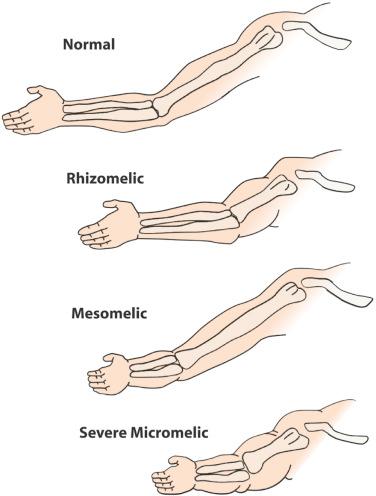
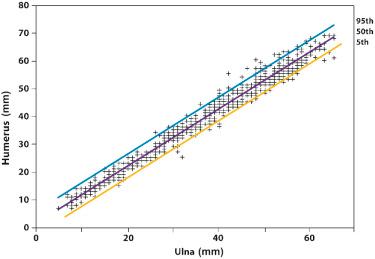
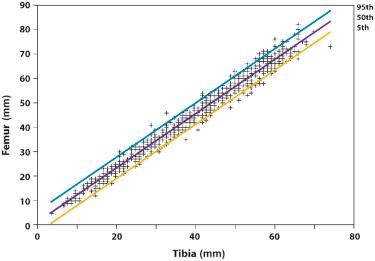
Shortening of the extremities can involve the entire limb (micromelia), the proximal segment (rhizomelia), the intermediate segment (mesomelia), or the distal segment (acromelia) ( Fig. 11-6 ). The diagnosis of rhizomelia or mesomelia requires comparing the bony dimensions of the lower legs and forearms with those of the thighs and arms. Figures 11-7 and 11-8 display the relationships between the humerus and ulna, as well as the femur and tibia, which can be used for the objective assessment of rhizomelia and mesomelia. Table 11-5 presents a list of skeletal dysplasias characterized by rhizomelia, mesomelia, and micromelia.
|
Several skeletal dysplasias feature alterations of the hands and feet. The term polydactyly refers to the presence of more than five digits. It is classified as postaxial if the extra digits are on the ulnar or fibular side, and preaxial if they are located on the radial or tibial side. Syndactyly refers to soft tissue or bony fusion of adjacent digits. Clinodactyly consists of deviation of a finger or fingers.
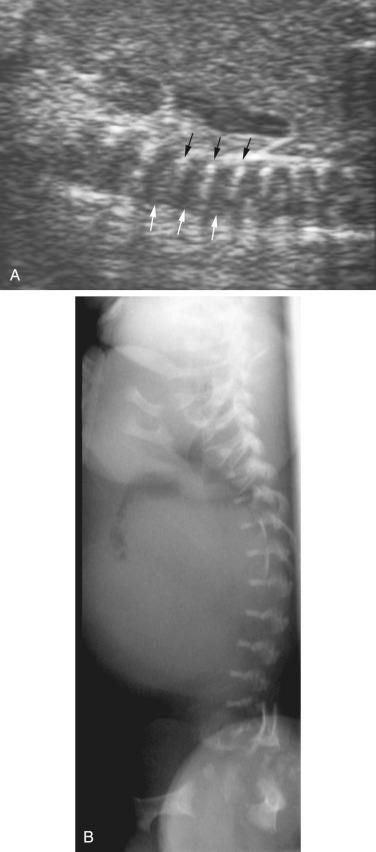
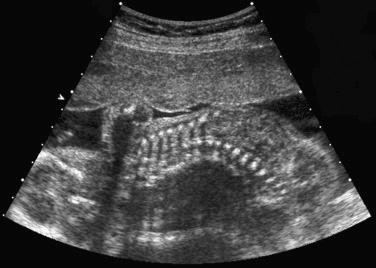
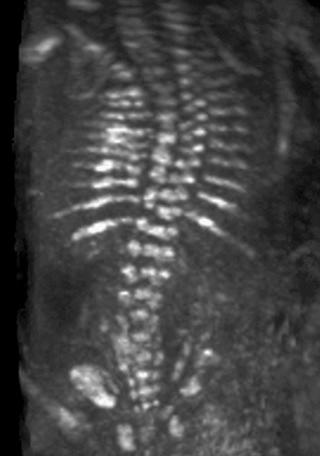
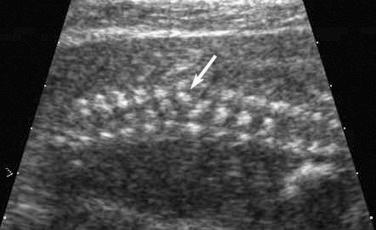
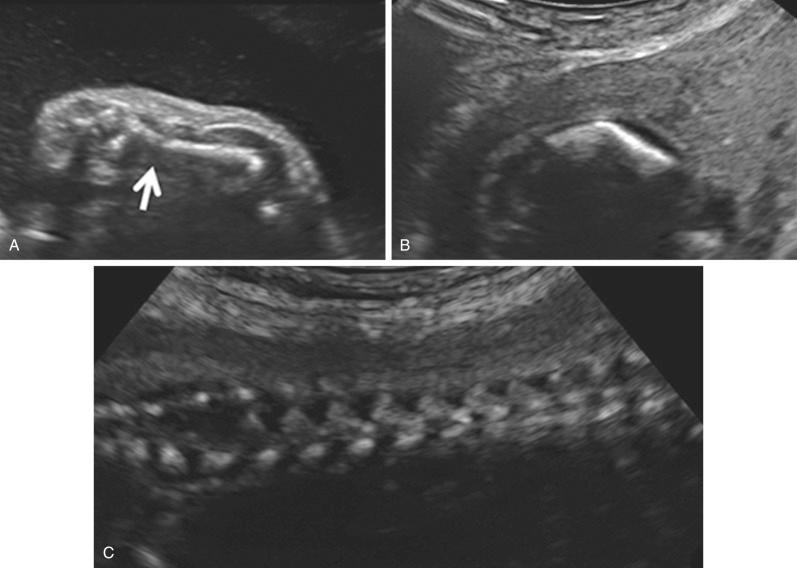
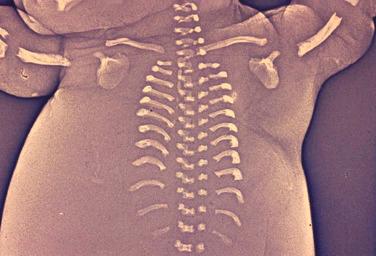
The most common spinal abnormality seen in skeletal dysplasias is platyspondyly, which consists of flattening of the vertebrae ( Fig. 11-9 ). Xyphosis, scoliosis ( Figs. 11-10 and 11-11 ), hemivertebra ( Fig. 11-12 ), and coronal clefting of vertebral bodies have been also reported.
Long bone biometry has been used extensively for the prediction of gestational age. Nomograms for this purpose display the distribution of bone lengths in relation to gestational weeks. For the proper use of these nomograms, the clinician must know the accurate gestational age of the fetus. Therefore, patients at risk for skeletal dysplasias are advised to seek prenatal care at an early gestational age in order to assess all clinical estimators of gestational age. Tables 11-6 and 11-7 present nomograms of the measurement of limb biometry for the upper and lower extremities, respectively. Comparisons between the limb dimensions and head circumference can be used for patients presenting with uncertain gestational age ( Figs. 11-13 and 11-14 ).
| FEMUR | TIBIA | FIBULA | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Week | 3rd | 50th | 97th | 3rd | 50th | 97th | 3rd | 50th | 97th |
| 12 | 4.4 | 7.7 | 11.1 | 4.4 | 7.6 | 10.8 | 3.6 | 6.8 | 10.0 |
| 13 | 7.5 | 10.9 | 14.4 | 5.8 | 9.2 | 12.5 | 5.2 | 8.5 | 11.8 |
| 14 | 10.6 | 14.1 | 17.6 | 8.0 | 11.4 | 14.8 | 7.4 | 10.8 | 14.2 |
| 15 | 13.6 | 17.2 | 20.8 | 10.6 | 14.1 | 17.6 | 10.0 | 13.5 | 17.0 |
| 16 | 16.5 | 20.3 | 24.0 | 13.3 | 16.9 | 20.5 | 12.8 | 16.4 | 20.0 |
| 17 | 19.4 | 23.3 | 27.2 | 16.2 | 19.9 | 23.5 | 15.6 | 19.3 | 23.0 |
| 18 | 22.3 | 26.3 | 30.2 | 19.0 | 22.8 | 26.6 | 18.4 | 22.2 | 26.0 |
| 19 | 25.1 | 29.2 | 33.3 | 21.8 | 25.7 | 29.6 | 21.2 | 25.1 | 29.0 |
| 20 | 27.9 | 32.1 | 36.3 | 24.5 | 28.5 | 32.5 | 23.9 | 27.9 | 31.8 |
| 21 | 30.6 | 34.9 | 39.2 | 27.2 | 31.2 | 35.3 | 26.4 | 30.5 | 34.6 |
| 22 | 33.2 | 37.6 | 42.0 | 29.7 | 33.8 | 38.0 | 28.9 | 33.1 | 37.3 |
| 23 | 35.8 | 40.3 | 44.8 | 32.1 | 36.4 | 40.6 | 31.2 | 35.5 | 39.8 |
| 24 | 38.3 | 42.9 | 47.6 | 34.4 | 38.8 | 43.1 | 33.5 | 37.9 | 42.3 |
| 25 | 40.8 | 45.5 | 50.2 | 36.6 | 41.0 | 45.5 | 35.6 | 40.1 | 44.6 |
| 26 | 43.1 | 48.0 | 52.8 | 38.7 | 43.2 | 47.8 | 37.6 | 42.2 | 46.8 |
| 27 | 45.4 | 50.4 | 55.3 | 40.7 | 45.3 | 49.9 | 39.6 | 44.3 | 49.0 |
| 28 | 47.6 | 52.7 | 57.8 | 42.6 | 47.3 | 52.0 | 41.4 | 46.2 | 51.0 |
| 29 | 49.8 | 55 | 60.1 | 44.4 | 49.2 | 54.0 | 43.1 | 48.0 | 52.9 |
| 30 | 51.8 | 57.1 | 62.4 | 46.1 | 51.0 | 55.9 | 44.8 | 49.8 | 54.8 |
| 31 | 53.8 | 59.2 | 64.6 | 47.7 | 52.7 | 57.7 | 46.4 | 51.5 | 56.6 |
| 32 | 55.7 | 61.2 | 66.7 | 49.3 | 54.4 | 59.5 | 47.9 | 53.1 | 58.3 |
| 33 | 57.5 | 63.1 | 68.7 | 50.8 | 55.9 | 61.1 | 49.3 | 54.6 | 59.9 |
| 34 | 59.2 | 64.9 | 70.6 | 52.2 | 57.5 | 62.7 | 50.7 | 56.1 | 61.5 |
| 35 | 60.8 | 66.6 | 72.4 | 53.5 | 58.9 | 64.3 | 52.0 | 57.5 | 63.0 |
| 36 | 62.3 | 68.2 | 74.1 | 54.8 | 60.3 | 65.7 | 53.2 | 58.8 | 64.4 |
| 37 | 63.7 | 69.7 | 75.8 | 56 | 61.6 | 67.2 | 54.4 | 60.1 | 65.8 |
| 38 | 64.9 | 71.1 | 77.3 | 57.2 | 62.9 | 68.5 | 55.5 | 61.3 | 67.1 |
| 39 | 66.1 | 72.4 | 78.7 | 58.3 | 64.1 | 69.8 | 56.6 | 62.5 | 68.4 |
| 40 | 67.2 | 73.6 | 79.9 | 59.4 | 65.2 | 71.1 | 57.6 | 63.6 | 69.6 |
| 41 | 68.1 | 74.6 | 81.1 | 60.4 | 66.4 | 72.3 | 58.6 | 64.7 | 70.8 |
| 42 | 69.0 | 75.6 | 82.2 | 61.4 | 67.4 | 73.5 | 59.5 | 65.8 | 72.0 |
| HUMERUS | ULNA | RADIUS | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Week | 3rd | 50th | 97th | 3rd | 50th | 97th | 3rd | 50th | 97th |
| 12 | 3.7 | 7.1 | 10.6 | 3.9 | 7.3 | 10.7 | 2.2 | 5.5 | 8.8 |
| 13 | 7.2 | 10.7 | 14.2 | 6.2 | 9.6 | 13.1 | 4.8 | 8.2 | 11.6 |
| 14 | 10.5 | 14.1 | 17.7 | 8.8 | 12.4 | 15.9 | 7.6 | 11.0 | 14.5 |
| 15 | 13.7 | 17.3 | 21.0 | 11.6 | 15.3 | 18.9 | 10.3 | 13.9 | 17.4 |
| 16 | 16.7 | 20.4 | 24.2 | 14.5 | 18.2 | 22.0 | 13.0 | 16.7 | 20.3 |
| 17 | 19.6 | 23.4 | 27.2 | 17.3 | 21.2 | 25.0 | 15.6 | 19.3 | 23.1 |
| 18 | 22.3 | 26.2 | 30.1 | 20.1 | 24.0 | 28.0 | 18.1 | 21.9 | 25.7 |
| 19 | 24.9 | 28.9 | 32.9 | 22.8 | 26.8 | 30.8 | 20.4 | 24.4 | 28.3 |
| 20 | 27.4 | 31.5 | 35.5 | 25.3 | 29.4 | 33.5 | 22.7 | 26.7 | 30.7 |
| 21 | 29.8 | 34.0 | 38.1 | 27.8 | 32.0 | 36.2 | 24.8 | 28.9 | 32.9 |
| 22 | 32.1 | 36.3 | 40.5 | 30.1 | 34.4 | 38.7 | 26.8 | 30.9 | 35.1 |
| 23 | 34.3 | 38.6 | 42.9 | 32.3 | 36.6 | 41.0 | 28.6 | 32.9 | 37.1 |
| 24 | 36.4 | 40.7 | 45.1 | 34.3 | 38.8 | 43.3 | 30.4 | 34.7 | 39.1 |
| 25 | 38.4 | 42.8 | 47.2 | 36.3 | 40.9 | 45.5 | 32.0 | 36.5 | 40.9 |
| 26 | 40.3 | 44.8 | 49.3 | 38.2 | 42.8 | 47.5 | 33.6 | 38.1 | 42.6 |
| 27 | 42.1 | 46.7 | 51.3 | 39.9 | 44.7 | 49.5 | 35.1 | 39.7 | 44.3 |
| 28 | 43.9 | 48.5 | 53.2 | 41.6 | 46.5 | 51.3 | 36.5 | 41.2 | 45.8 |
| 29 | 45.5 | 50.2 | 55.0 | 43.2 | 48.2 | 53.1 | 37.8 | 42.6 | 47.3 |
| 30 | 47.1 | 51.9 | 56.7 | 44.7 | 49.8 | 54.8 | 39.0 | 43.9 | 48.7 |
| 31 | 48.6 | 53.5 | 58.4 | 46.2 | 51.3 | 56.4 | 40.2 | 45.1 | 50.1 |
| 32 | 50.0 | 55.0 | 59.9 | 47.5 | 52.7 | 58.0 | 41.3 | 46.4 | 51.4 |
| 33 | 51.4 | 56.4 | 61.5 | 48.8 | 54.1 | 59.4 | 42.4 | 47.5 | 52.6 |
| 34 | 52.7 | 57.8 | 62.9 | 50.0 | 55.4 | 60.8 | 43.4 | 48.6 | 53.8 |
| 35 | 53.9 | 59.1 | 64.3 | 51.2 | 56.7 | 62.2 | 44.3 | 49.6 | 54.9 |
| 36 | 55.1 | 60.3 | 65.6 | 52.3 | 57.9 | 63.5 | 45.2 | 50.6 | 56.0 |
| 37 | 56.2 | 61.5 | 66.8 | 53.4 | 59.1 | 64.7 | 46.1 | 51.6 | 57.0 |
| 38 | 57.2 | 62.6 | 68.0 | 54.4 | 60.2 | 65.9 | 46.9 | 52.5 | 58.0 |
| 39 | 58.2 | 63.7 | 69.2 | 55.4 | 61.2 | 67.1 | 47.7 | 53.3 | 59.0 |
| 40 | 59.1 | 64.7 | 70.3 | 56.3 | 62.2 | 68.2 | 48.4 | 54.2 | 59.9 |
| 41 | 60.0 | 65.6 | 71.3 | 57.2 | 63.2 | 69.3 | 49.1 | 55.0 | 60.8 |
| 42 | 60.8 | 66.5 | 72.2 | 58.0 | 64.1 | 70.3 | 49.8 | 55.7 | 61.6 |
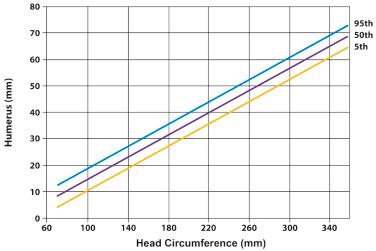
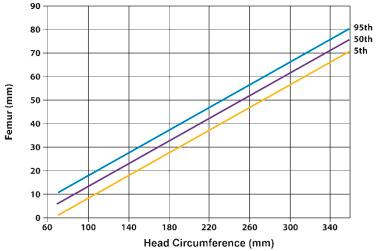
The nomograms and figures in this chapter provide the mean, 3rd, and the 97th percentiles of limb biometric parameters. The reader should be aware that approximately 6% of the general population will fall outside these boundaries. Ideally, a more stringent criterion, such as the 1st percentile of limb growth for gestational age, should be used for diagnosis. Unfortunately, none of the currently available nomograms has been based on a sufficient number of patients to provide an accurate discrimination between the 3rd and the 1st percentiles. However, most skeletal dysplasias diagnosed in utero or at birth are associated with dramatic long bone shortening, and under these circumstances, the precise boundary used (i.e., 1st or 3rd percentile) is not critical. An exception to this is achondroplasia, in which limb biometry is only mildly affected until the third trimester, when abnormal growth can be detected by examining the slope of growth of the femur length. In a study including 127 cases of 17 skeletal dysplasias, Gonçalves and Jeanty concluded, with the use of discriminant analysis, that the degree of femur length shortening can be used as the initial step in distinguishing among the five most common disorders: thanatophoric dysplasia, OI type II, achondrogenesis, achondroplasia, and hypochondroplasia. Gabrielli and coworkers reported the early diagnosis of skeletal dysplasias in eight women with previous pregnancies affected with skeletal dysplasias. Five recurrent cases were identified during the first trimester by the femur length/crown rump length ratio and the femur length/biparietal diameter ratio. The results of this study suggest that early evaluation of fetal structures might be helpful in the diagnosis of severe skeletal dysplasias. Nomograms for long bone measurements according to crown rump length in a large population of normal fetuses examined between 11 and 14 weeks of gestation have been published, but still their role in the early assessment of pregnancies at risk for skeletal dysplasias remains to be determined.
In general, the challenges of the prenatal diagnosis of skeletal dysplasias presents in one of two ways: (1) a patient who has delivered an infant with a skeletal dysplasia and desires antenatal assessment in a subsequent pregnancy or (2) the incidental finding of a shortened, bowed, or anomalous extremity during a routine sonographic examination. In patients at risk, the examination is easier when the particular phenotype of the skeletal dysplasia is known. The inability to obtain reliable information about skeletal mineralization and the effect of depth on sonography is a limiting factor in establishing an accurate diagnosis after identification of an incidental finding. Another limitation is the paucity of information about the in utero natural history of these disorders. Yet, despite these difficulties and limitations, good medical reasons justify attempting an accurate prenatal diagnosis of skeletal dysplasias. A number of these disorders are uniformly lethal, whereas others are associated with severe physical handicap and neurodevelopmental disabilities. In addition, there is a group of disorders associated with thrombocytopenia for which vaginal delivery may expose the infant to an increased risk of intracranial hemorrhage. Therefore, accurate diagnosis of skeletal dysplasias is important for prenatal counseling.
Despite increasing availability of molecular testing, in about one third of skeletal dysplasias their molecular basis has not been defined. The role of diagnostic imaging in the prenatal investigation of skeletal dysplasias is (1) to narrow the differential diagnosis of skeletal dysplasias, so that appropriate confirmatory molecular tests can be done; (2) to predict lethality; and (3) to identify the fetus with a skeletal dysplasia early enough in gestation so that the diagnostic workup can be completed before the limit of fetal viability.
Sonography is the primary imaging modality used for detection of an affected fetus. The prevalence of skeletal dysplasias identified by ultrasound examination during the second and third trimesters of pregnancy is about 7.5/10,000 pregnancies. In early pregnancy (11-14 weeks) the most frequently diagnosed skeletal dysplasias are thanatophoric dysplasia and achondrogenesis. However, despite ultrasound findings highly suggestive of skeletal dysplasia, the definitive diagnosis should only be determined by molecular testing and confirmation of the ultrasound findings later in pregnancy. Table 11-8 summarizes the sensitivity of 2D sonography for the prenatal diagnosis of skeletal dysplasias. Schramm and associates reported a 67.9% (110/162) correct diagnosis and a 30.9% (50/162) partially correct diagnosis of skeletal dysplasia by evaluating the following sonographic parameters: (1) measurements of all segments of the long bones; (2) examination of the hands, spine, and head; (3) assessment of mineralization and shape of the bones; and (4) a full anatomic survey. For lethal skeletal dysplasias, the accuracy of sonographic findings was 99% (113/114). The authors reported that an accurate diagnosis of skeletal dysplasias before 24 weeks of gestation was possible in 62% of cases, although achondroplasia was the only skeletal dysplasia that could not be diagnosed before 24 weeks of gestation. When there is a family history of skeletal dysplasias, the following should be carefully evaluated on ultrasound imaging: complete biometry including the biparietal diameter, head and abdominal circumferences, lengths of all long bones, femur-foot ratio, and measurements of the mandible, clavicle, scapula, skull, chest, and spine. Other ultrasound parameters that might also be helpful in differentiating skeletal dysplasias include the fetal facial profile (e.g., flattened nasal bridge), presence and shape of vertebral bodies, appearance of the hands and feet (e.g., extra, missing, or malformed digits), and fetal thorax to assess the risk for lethality. More recently, Nelson and colleagues reported an overall prevalence of skeletal dysplasias of 2/10,000 pregnancies. In their series, 60% of skeletal dysplasias survived to hospital discharge, although 20% died in the neonatal period, 4% were stillbirths, and 16% were terminated. The authors reported that a femur/abdominal circumference ratio less than 0.16 was the main discriminator among fetuses with lethal skeletal dysplasias, and that this measurement had better performance than femur shortening, thoracic circumference, and thoracic circumference/abdominal circumference ratio.
| Author | Year | Number of Cases | Sensitivity (%) ( n ) |
|---|---|---|---|
| Gordienko et al | 1996 | 26 | 73 (9) |
| Gaffney et al | 1998 | 35 | 31 (11) |
| Tretter et al | 1998 | 27 | 48 (13) |
| Hersh et al | 1998 | 23 | 48 (11) |
| Doray et al | 2000 | 47 | 60 (28) |
| Parilla et al | 2003 | 31 | 65 (20) |
| Witters et al | 2008 | 38 | 66 (25) |
| Schramm et al | 2009 | 162 | 68 (110) |
| Yeh et al | 2011 | 40 | 70 (28) |
| Khalil et al | 2011 | 15 | 40 (6) |
Several investigators have also proposed that 3D ultrasonography may improve the prenatal diagnostic accuracy for skeletal dysplasias and arthrogryposis. It allows observation of phenotypic features that can be difficult to detect by 2D sonography such as detailed evaluation of the face, scapular anomalies, and abnormal calcification patterns. As an example, Moeglin and Benoit applied the multiplanar view to demonstrate the pointed appearance of the upper femoral diaphysis in achondroplasia. 3D reconstruction of the fetal bones is best performed using the maximum intensity projection or skeletal mode, a rendering algorithm that prioritizes the display of the brightest voxels contained within a region of interest selected by the operator ( Fig. 11-15 ). It is noteworthy that if a fetus is examined early enough in pregnancy, the entire skeleton can be included within the region of interest, and therefore, panoramic imaging can be obtained. However, in some skeletal dysplasias the abnormal features may not be detectable until later in fetal development. Case reports and small series of skeletal dysplasias have described phenotypic characteristics or skeletal features for which 3D sonography may provide additional information ( Table 11-9 ).
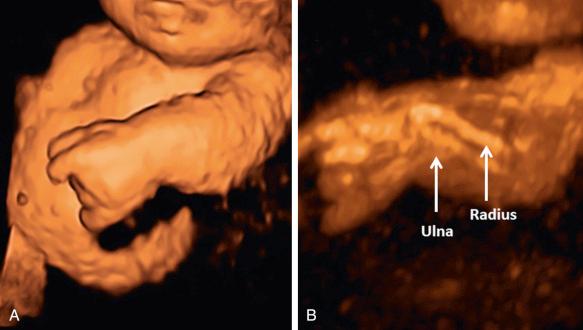
| Skeletal Dysplasia | Phenotypic Characteristics Identified Better by 3DUS than 2DUS |
|---|---|
| Platyspondylic lethal chondrodysplasia | Enhanced visualization of femoral and tibial bowing; Better characterization of the facial soft tissues with surface rendering |
| Campomelic dysplasia | Micrognathia, flat face, hypoplastic scapulae, bifid foot, fan-like position of the toes |
| Thanatophoric dysplasia | Improved characterization of frontal bossing and depressed nasal bridge; Demonstration of redundant skinfolds; Low-set dysmorphic ears |
| Achondroplasia | Improved characterization of frontal bossing and depressed nasal bridge; |
| Superior evaluation of the epiphyses and metaphyses of the long bones, with demonstration of a vertical metaphyseal slope; | |
| Caudal narrowing of the interpedicular distance; | |
| Clear visualization of trident hand; | |
| Better visualization of disproportion between limb segments | |
| Chondrodysplasia punctata, rhizomelic form | Improved characterization of the Binder facies (depressed nasal bridge, midface hypoplasia, small nose with upturned alae); |
| Identification of laryngeal stippling | |
| Achondrogenesis | Panoramic demonstration of short neck and severe shortening of all segments of the limbs |
| Jarcho-Levin syndrome | Vertebral defects with absence of ribs and transverse process |
| Spondylocostal dysostosis | Fan-like rib cage with rib fusion |
| Larsen syndrome | Genu recurvatum, midface hypoplasia, low-set ears |
| Cleidocranial dysplasia | Widened cranial sutures, poor mineralization of the occipital bones, pseudoarthrosis of the clavicle |
| Apert syndrome | Coronal craniosynostosis |
3D-HCT has been proposed as an adjunctive imaging modality for improving the prenatal diagnosis and clinical management of skeletal dysplasias ( Fig. 11-16 ). 3D-HCT overcomes the main limitation of 2D and 3D ultrasonography for assessment of bones (i.e., acoustic shadowing), allowing easy and exquisite 3D rendering of mineralized bones at the expense of a mild radiation exposure to the fetus. Long bone measurements in fetuses with suspected skeletal dysplasias obtained using ultrasound show a good correlation with measurements obtained by postmortem 3D-HCT within 24 hours of delivery. Measurements obtained from 3D-HCT are not distance dependent and therefore are more accurate. Excellent panoramic images of the fetal skeleton can be obtained without superimposition of the maternal skeleton (which occurs with radiography). Yet, because of the low radiation dose currently used in pregnancy, 3D-HCT provides limited visualization of bone density, of fetal metaphyseal deformities, and of the feet and hands at early gestational ages.
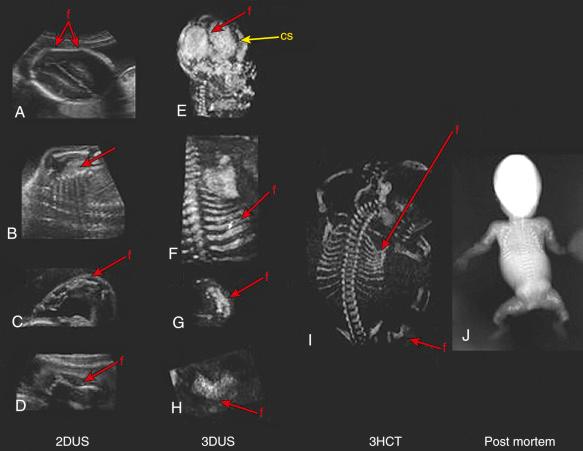
The contribution of 3D-HCT, 3D sonography, and 2D sonography in the evaluation of skeletal dysplasias was analyzed by Ruano and coworkers, who compared the phenotypic characteristics of fetuses with skeletal dysplasias (achondroplasia [ n = 3], OI [ n = 2], and chondrodysplasia punctata [ n = 1]) using these three imaging modalities. Deformation of the fetal pelvis and an increase in the intervertebral space of the lumbar vertebrae were diagnosed more often using 3D-HCT. In contrast, some phenotypic characteristics of fetuses with skeletal dysplasias were demonstrated only by ultrasound such as phalangeal hypoplasia, point-calcified epiphysis (by both 2D and 3D sonography), and facial dysmorphism (by 3D sonography only). Although the overall count of correct phenotypic characteristics detected prenatally favored 3D-HCT over 3D sonography (94.3% [33/35] vs. 77.1% [27/35]; P = 0.03), the diagnostic performance of 3D-HCT was not superior to that of 3D sonography, as the correct prenatal diagnosis was established by both modalities in all cases. However, 3D sonography has two important advantages over 3D-HCT, namely the lack of radiation exposure and wider availability in the clinical setting. It is also noteworthy that the overall experience with 3D sonography for the diagnosis of skeletal dysplasias is still limited. Nevertheless, even in the study of Ruano and coworkers 3D sonography performed better than 2D sonography in the identification of phenotypic characteristics (77.1% [27/35] vs. 51.4% [18/35]; P = 0.004) and in establishing an accurate diagnosis.
Fetal magnetic resonance imaging (MRI) is useful when the ultrasound examination is inconclusive because of oligohydramnios, maternal obesity, late gestational age, or fetal position. Both skeleton and muscle can be assessed by MRI using echoplanar imaging, with thick slab T2-weighted and dynamic sequences. Echoplanar imaging can demonstrate epimetaphyseal characteristics used in measurement of fetal long bones, increasing the accuracy of diagnosing short long bones. MRI can also be useful in differentiating between normal bone development and bone dysplasia caused by a disruption of endochondral ossification. Moreover, MRI can be applied to measure fetal thoracic and lung volumes, which are used to predict lethality in skeletal dysplasias.
A proposed systematic approach to the prenatal diagnosis of skeletal dysplasias is summarized in Table 11-10 and described in greater detail in the following section.
|
All long bones must be measured in all extremities. Comparisons with other segments should be performed to establish whether the limb shortening is predominantly rhizomelic, mesomelic, or acromelic, or whether it involves all segments (see Figs. 11-6 to 11-8 ). A detailed examination of each bone is necessary to exclude absence or hypoplasia of individual bones (fibula, tibia, ulna, radius, clavicles, and scapulae). From a group of fetuses less than 24 weeks of gestation with a short femur length, Papageorghiou and associates reported that 35% presented with skeletal dysplasias; all affected fetuses had associated anomalies. The authors suggested that in the presence of a short femur, a complete fetal anatomic evaluation is necessary. The most frequently associated skeletal dysplasias were asphyxiating thoracic dystrophy, osteogenesis imperfecta, thanatophoric dysplasia, and campomelic dysplasia.
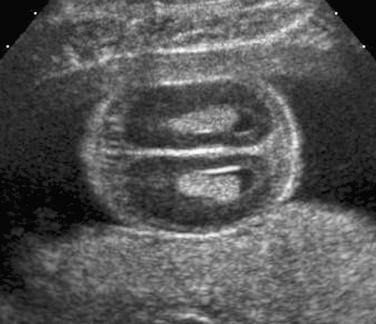
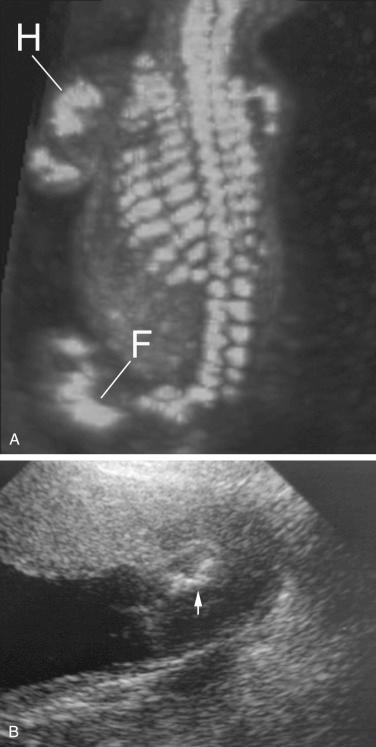
An attempt should be made to characterize the degree of mineralization, which can be assessed by examining the acoustic shadow behind the bone as well as the echogenicity of the bone itself. Signs of demineralization include visualization of an unusually prominent falx in the fetal brain and the absence of or decreased echogenicity of the spine. It should be emphasized that there are limitations to the sonographic evaluation of long bone mineralization and that other structures, such as the fetal skull, may be better suited for such assessment ( Fig. 11-17 ).
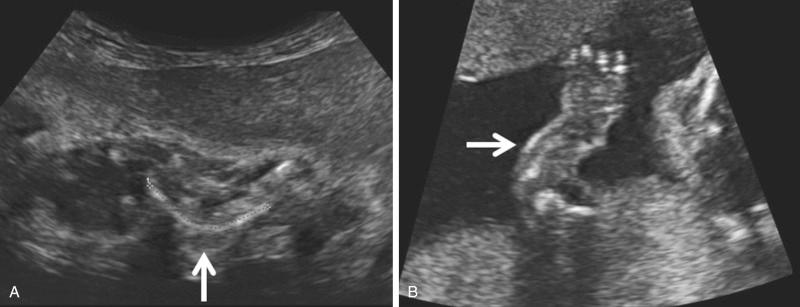
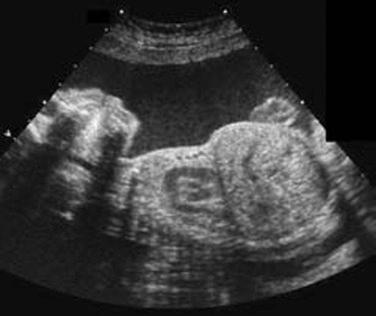
At present, there is no objective means to assess long bone curvature, and experience is necessary to assist the operator in discerning the boundary between normality and abnormality. Bowed or angulated femora (campomelia) ( Fig. 11-18 ) is characteristic of campomelic dysplasia, thanatophoric dysplasia, osteogenesis imperfecta, short rib dysplasia, and hypophosphatasia.
Metaphyseal flaring denotes widening at the level of the metaphyseal growth plate. It can be observed in many conditions, including achondroplasia, hypochondroplasia, hypochondrogenesis, asphyxiating thoracic dysplasia, chondrodysplasia punctata, diastrophic dysplasia, hypophosphatasia, Kniest dysplasia, kyphomelic dysplasia, metatropic dysplasia, and OI.
Khalil and colleagues reported an increased diaphysis-metaphysis femoral angle measured at 20 weeks to 23 weeks and 6 days of gestation in fetuses later diagnosed with achondroplasia. The femoral angle in affected fetuses was 125 degrees as compared to 95 degrees in normal fetuses. The authors conclude that estimation of the femoral angle might improve the early detection of fetuses with achondroplasia.
Fractures can be found in certain skeletal dysplasias such as OI and hypophosphatasia ( Fig. 11-19 ). The fractures may be extremely subtle or may lead to angulation and separation of segments of the affected bone ( Fig. 11-20 ).
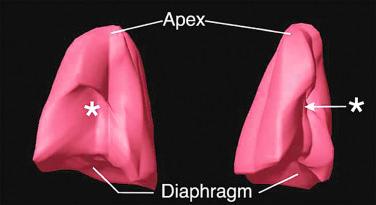
Skeletal dysplasias associated with a hypoplastic thorax frequently have lung hypoplasia, which is the most frequent cause of death for these conditions. When a severe skeletal dysplasia is diagnosed, the presence of marked thoracic involvement and pulmonary hypoplasia will allow the clinician to counsel the parents regarding prognosis, even though the specific type of skeletal dysplasia may be unknown. A number of sonographic parameters have been investigated for the prediction of pulmonary hypoplasia. They include measurements of the thorax and lungs, ratios between thoracic measurements and other biometric parameters, Doppler velocimetry of the pulmonary arteries, Doppler evaluation of tracheal fluid flow, and more recently, three-dimensional volumetric measurements of the fetal lungs by either sonography or MRI.
A detailed evaluation of the thorax in order to answer the following questions can assist in the diagnosis of the particular skeletal dysplasia :
Is the thorax extremely small? (thanatophoric dysplasia)
Is the thorax long and narrow? (Jeune syndrome)
Are the ribs extremely short? (short rib–polydactyly syndromes)
Are there rib fractures? (osteogenesis imperfecta type II)
Are there gaps within the ribs? (cerebra-costo-mandibular syndrome)
Are there fused ribs? (spondylocostal dysplasia)
Is there clavicular aplasia, hypoplasia, or pseudoarthrosis? (cleidocranial dysplasia)
Are the scapulae abnormal? (hypoplasia or absence in campomelic dysplasia)
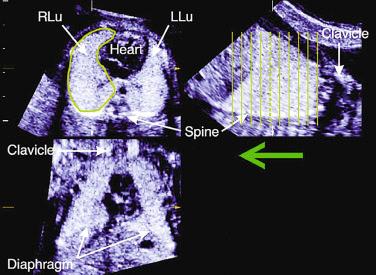
Thoracic and lung biometry has been extensively studied to identify fetuses at high risk for pulmonary hypoplasia. Table 11-11 lists the skeletal dysplasias associated with altered thoracic dimensions, and Figures 11-21 and 11-22 illustrate features associated with a hypoplastic thorax. Methods used to measure the thorax, lungs, and heart by 2D sonography are illustrated in Figure 11-23 . Thoracic areas and volumes in fetuses with a known gestational age can be evaluated using the nomograms reproduced in Tables 11-12 and 11-13 . When gestational age is uncertain, age-independent ratios, such as the thoracic/abdominal circumference ratio (normal value: 0.77 to 1.01) and the thoracic/head circumference ratio (normal value: 0.56 to 1.04) can be used.
| Long, Narrow Thorax |
| Asphyxiating thoracic dysplasia (Jeune) |
| Chondroectodermal dysplasia (Ellis–van Creveld) |
| Metatropic dysplasia |
| Fibrochondrogenesis |
| Atelosteogenesis |
| Camptomelic dysplasia |
| Jarcho-Levin syndrome |
| Achondrogenesis |
| Osteogenesis imperfecta type II |
| Hypophosphatasia |
| Dyssegmental dysplasia |
| Cleidocranial dysplasia |
| Short Thorax |
| Osteogenesis imperfecta (type II) |
| Kniest dysplasia (metatropic dysplasia type II) |
| Pena-Shokeir syndrome |
| Hypoplastic Thorax |
| Short rib–polydactyly syndrome (type I, type II) |
| Thanatophoric dysplasia |
| Cerebrocostomandibular syndrome |
| Cleidocranial dysplasia syndrome |
| Homozygous achondroplasia |
| Melnick-Needles syndrome (osteodysplasty) |
| Fibrochondrogenesis |
| Otopalatodigital syndrome type II |
| Weeks | LEFT LUNG AREA | RIGHT LUNG AREA | LEFT LUNG VOLUME | RIGHT LUNG VOLUME | TOTAL LUNG VOLUME | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2.5th | 50th | 97.5th | 2.5th | 50th | 97.5th | 2.5th | 50th | 97.5th | 2.5th | 50th | 97.5th | 2.5th | 50th | 97.5th | |
| 12 | 20 | 36 | 51 | 44 | 58 | 71 | 0.63 | 0.64 | 0.65 | 0.59 | 0.6 | 0.62 | 1.37 | 1.56 | 1.75 |
| 13 | 26 | 47 | 68 | 42 | 69 | 96 | 0.37 | 0.57 | 0.77 | 0.5 | 0.75 | 1 | 0.85 | 1.4 | 1.94 |
| 14 | 36 | 62 | 89 | 48 | 88 | 129 | 0.26 | 0.69 | 1.11 | 0.54 | 1.06 | 1.58 | 0.7 | 1.65 | 2.61 |
| 15 | 49 | 82 | 114 | 61 | 115 | 169 | 0.31 | 0.97 | 1.64 | 0.7 | 1.53 | 2.36 | 0.9 | 2.32 | 3.74 |
| 16 | 65 | 104 | 144 | 80 | 148 | 215 | 0.49 | 1.42 | 2.35 | 1 | 2.16 | 3.33 | 1.42 | 3.36 | 5.31 |
| 17 | 83 | 130 | 177 | 105 | 186 | 267 | 0.79 | 2.02 | 3.24 | 1.42 | 2.96 | 4.51 | 2.25 | 4.77 | 7.29 |
| 18 | 103 | 158 | 213 | 134 | 229 | 323 | 1.22 | 2.76 | 4.3 | 1.97 | 3.92 | 5.87 | 3.36 | 6.52 | 9.67 |
| 19 | 125 | 188 | 252 | 168 | 275 | 383 | 1.75 | 3.63 | 5.51 | 2.64 | 5.04 | 7.44 | 4.72 | 8.57 | 12.4 |
| 20 | 148 | 220 | 293 | 204 | 325 | 447 | 2.38 | 4.62 | 6.85 | 3.43 | 6.31 | 9.19 | 6.31 | 10.9 | 15.5 |
| 21 | 172 | 254 | 335 | 243 | 378 | 512 | 3.09 | 5.71 | 8.33 | 4.33 | 7.73 | 11.1 | 8.1 | 13.5 | 18.9 |
| 22 | 196 | 288 | 380 | 283 | 432 | 580 | 3.87 | 6.9 | 9.92 | 5.34 | 9.28 | 13.2 | 10.1 | 16.3 | 22.6 |
| 23 | 220 | 323 | 425 | 325 | 486 | 648 | 4.71 | 8.16 | 11.6 | 6.45 | 11 | 15.5 | 12.2 | 19.3 | 26.5 |
| 24 | 244 | 358 | 471 | 366 | 541 | 716 | 5.58 | 9.49 | 13.4 | 7.64 | 12.8 | 17.9 | 14.4 | 22.5 | 30.7 |
| 25 | 268 | 392 | 517 | 406 | 595 | 783 | 6.49 | 10.9 | 15.3 | 8.9 | 14.7 | 20.5 | 16.6 | 25.8 | 35 |
| 26 | 290 | 426 | 563 | 445 | 647 | 849 | 7.4 | 12.3 | 17.2 | 10.2 | 16.7 | 23.2 | 18.9 | 29.2 | 39.5 |
| 27 | 310 | 459 | 609 | 482 | 697 | 913 | 8.31 | 13.7 | 19.1 | 11.6 | 18.8 | 26 | 21.2 | 32.7 | 44.1 |
| 28 | 328 | 491 | 653 | 515 | 744 | 973 | 9.19 | 15.1 | 21.1 | 13 | 21 | 29 | 23.5 | 36.1 | 48.7 |
| 29 | 344 | 521 | 697 | 545 | 787 | 1029 | 10 | 16.6 | 23.1 | 14.4 | 23.2 | 32 | 25.7 | 39.6 | 53.4 |
| 30 | 358 | 548 | 738 | 569 | 825 | 1081 | 10.8 | 17.9 | 25 | 15.9 | 25.5 | 35.1 | 27.7 | 42.9 | 58.1 |
| 31 | 368 | 573 | 777 | 589 | 858 | 1127 | 11.5 | 19.3 | 27 | 17.2 | 27.7 | 38.2 | 29.6 | 46.2 | 62.7 |
| 32 | 374 | 594 | 814 | 602 | 885 | 1167 | 12.1 | 20.5 | 28.9 | 18.6 | 30 | 41.3 | 31.3 | 49.3 | 67.3 |
| THORACIC ANTEROPOSTERIOR DIAMETER | THORACIC TRANSVERSE DIAMETER | THORACIC CIRCUMFERENCE | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Weeks | 10th | 50th | 90th | 10th | 50th | 90th | 10th | 50th | 90th |
| 12 | 11.7 | 14.2 | 16.5 | 11.7 | 14.2 | 16.5 | 11.7 | 14.2 | 16.5 |
| 13 | 14.3 | 17.2 | 19.8 | 14.3 | 17.2 | 19.8 | 14.3 | 17.2 | 19.8 |
| 14 | 17.1 | 20.3 | 23.3 | 17.1 | 20.3 | 23.3 | 17.1 | 20.3 | 23.3 |
| 15 | 19.9 | 23.4 | 26.7 | 19.9 | 23.4 | 26.7 | 19.9 | 23.4 | 26.7 |
| 16 | 22.8 | 26.5 | 30.2 | 22.8 | 26.5 | 30.2 | 22.8 | 26.5 | 30.2 |
| 17 | 25.8 | 29.6 | 33.6 | 25.8 | 29.6 | 33.6 | 25.8 | 29.6 | 33.6 |
| 18 | 28.7 | 32.6 | 37.0 | 28.7 | 32.6 | 37.0 | 28.7 | 32.6 | 37.0 |
| 19 | 31.6 | 35.6 | 40.5 | 31.6 | 35.6 | 40.5 | 31.6 | 35.6 | 40.5 |
| 20 | 34.2 | 38.5 | 43.9 | 34.2 | 38.5 | 43.9 | 34.2 | 38.5 | 43.9 |
| 21 | 36.8 | 41.4 | 47.2 | 36.8 | 41.4 | 47.2 | 36.8 | 41.4 | 47.2 |
| 22 | 39.2 | 44.3 | 50.4 | 39.2 | 44.3 | 50.4 | 39.2 | 44.3 | 50.4 |
| 23 | 41.5 | 47.2 | 53.6 | 41.5 | 47.2 | 53.6 | 41.5 | 47.2 | 53.6 |
| 24 | 43.9 | 50.0 | 56.6 | 43.9 | 50.0 | 56.6 | 43.9 | 50.0 | 56.6 |
| 25 | 46.5 | 52.8 | 59.5 | 46.5 | 52.8 | 59.5 | 46.5 | 52.8 | 59.5 |
| 26 | 49.1 | 55.5 | 62.3 | 49.1 | 55.5 | 62.3 | 49.1 | 55.5 | 62.3 |
| 27 | 51.7 | 58.2 | 64.9 | 51.7 | 58.2 | 64.9 | 51.7 | 58.2 | 64.9 |
| 28 | 54.2 | 60.8 | 67.4 | 54.2 | 60.8 | 67.4 | 54.2 | 60.8 | 67.4 |
| 29 | 56.6 | 63.4 | 69.8 | 56.6 | 63.4 | 69.8 | 56.6 | 63.4 | 69.8 |
| 30 | 58.9 | 65.9 | 72.3 | 58.9 | 65.9 | 72.3 | 58.9 | 65.9 | 72.3 |
| 31 | 61.1 | 68.4 | 74.9 | 61.1 | 68.4 | 74.9 | 61.1 | 68.4 | 74.9 |
| 32 | 63.2 | 70.9 | 77.7 | 63.2 | 70.9 | 77.7 | 63.2 | 70.9 | 77.7 |
| 33 | 65.1 | 73.2 | 80.6 | 65.1 | 73.2 | 80.6 | 65.1 | 73.2 | 80.6 |
| 34 | 67.0 | 75.4 | 83.4 | 67.0 | 75.4 | 83.4 | 67.0 | 75.4 | 83.4 |
| 35 | 68.8 | 77.3 | 85.8 | 68.8 | 77.3 | 85.8 | 68.8 | 77.3 | 85.8 |
| 36 | 70.6 | 79.0 | 88.0 | 70.6 | 79.0 | 88.0 | 70.6 | 79.0 | 88.0 |
| 37 | 72.5 | 80.5 | 90.0 | 72.5 | 80.5 | 90.0 | 72.5 | 80.5 | 90.0 |
| 38 | 74.3 | 81.9 | 91.8 | 74.3 | 81.9 | 91.8 | 74.3 | 81.9 | 91.8 |
| 39 | 75.9 | 83.1 | 93.5 | 75.9 | 83.1 | 93.5 | 75.9 | 83.1 | 93.5 |
| 40 | 77.5 | 84.1 | 94.9 | 77.5 | 84.1 | 94.9 | 77.5 | 84.1 | 94.9 |
| 41 | 78.8 | 84.9 | 95.8 | 78.8 | 84.9 | 95.8 | 78.8 | 84.9 | 95.8 |
A summary of the diagnostic accuracy of biometric parameters for the diagnosis of pulmonary hypoplasia is presented in Table 11-14 . Of particular interest are measurements of the right lung diameter, or the ratio between right lung diameter and the bony thoracic circumference proposed by Merz and associates, who reported that fetuses with pulmonary hypoplasia all had a right lung diameter below the 5th percentile for gestational age, regardless of the primary disorder (skeletal dysplasias [ n = 7], renal agenesis [ n = 11], diaphragmatic hernia [ n = 7], and hydrothorax [ n = 2]). In another study of 19 fetuses with congenital diaphragmatic hernia, Bahlmann and colleagues demonstrated that the ratio of right lung diameter/bony thoracic circumference detected all fetuses with pulmonary hypoplasia with 100% sensitivity and 100% specificity.
| Author | Parameter | Fetuses at Risk | Prevalence (%) | Sensitivity (%) | Specificity (%) | Accuracy (%) | Population |
|---|---|---|---|---|---|---|---|
| Nimrod et al | TC | 45 | 38 | 88 | 96 | 93 | PROM; oligohydramnios; pleural effusion; other conditions affecting lung growth |
| Heling et al | TTD | 29 | 55 | 44 | 50 | 46 | Bilateral renal agenesis; bilateral multicystic kidneys; chronic PROM < 25 weeks of gestational age; hydrothorax |
| APTD | 57 | 42 | 52 | ||||
| LL | 29 | 66 | 42 | ||||
| Laudy et al | TC | 40 | 43 | 94 | 38 | 61 | Prolonged oligohydramnios due to PROM or congenital renal disease |
| CC/TC | 76 | 50 | 61 | ||||
| TC/AC | 69 | 71 | 70 | ||||
| LD | 71 | 93 | 82 | ||||
| TC/AC | 86 | 85 | 85 | ||||
| FL/AC | 86 | 85 | 85 | ||||
| TC | 71 | 89 | 83 | ||||
| Gerards et al | 3D LV | 33 | 48.5 | 62 | 100 | 81 | 2DUS and 3DUS in 33 fetuses at risk of pulmonary hypoplasia |
| TC vs FL | 25 | 100 | 64 | ||||
| TC/AC | 81 | 59 | 70 | ||||
| TA/HA | 94 | 47 | 70 | ||||
| Vergani et al | 3D LV | 32 | 41 | 85 | 95 | 93 | 35 fetuses at risk of pulmonary hypoplasia including skeletal malformations, rupture of membranes, hydrothorax and bilatreral renal dysplasia |
| TC | 23 | 89 | 62 | ||||
| TC/AC | 46 | 74 | 63 | ||||
| TA/HA | 23 | 95 | 65 | ||||
| Weaver et al | FL/AC < 0.124 | 23 | 52 | 75 | 91 | 83 | 23 fetuses with confirmed skeletal dysplasia |
| FL/AC < 0.16 | 83.3 | 41 | 63 | ||||
| O/E TLV < 47.9 | 75 | 81 | 78 |
Rahemtullah and coworkers studied 18 fetuses with skeletal dysplasias in which all lethal cases were associated with a femur length/abdominal circumference ratio of 0.16. Although the test detected lethal cases with 100% sensitivity, two cases of achondroplasia were erroneously identified as lethal using this method. A different approach has been proposed by Hersh and associates, who predicted lethality in 23 out of 25 cases of skeletal dysplasias with a femur length below the 1st percentile for gestational age and presence of bell-shaped thorax or decreased bone echogenicity.
Fetal lung volumetry by 3D sonography has been performed using two techniques: multiplanar ( Fig. 11-24 ) and VOCAL (Virtual Organ Computer-Aided AnaLysis, GE Medical Systems, Milwaukee, WI) ( Fig. 11-25 ). Nomograms for lung volumetry using 3D sonography are available in the literature (see Table 11-12 ). Kalache and colleagues demonstrated that both the 3D multiplanar and 3D VOCAL modes can be used to measure fetal lung volumes, an observation that was subsequently confirmed by Moeglin and associates. A potential advantage of the VOCAL technique is the possibility of obtaining fine contours of the lungs, which may be particularly valuable when the outline of the organ is irregular, such as in cases of congenital diaphragmatic hernia. In contrast, obtaining lung volume measurements using the 3D multiplanar technique is faster, taking usually less than 5 minutes to perform. Volumes are best estimated when datasets are acquired using a transverse view of the fetal thorax. Ruano and coworkers compared volumetric measurements of the fetal lungs obtained using the VOCAL method with lung volumes calculated at the time of autopsy in 8 cases of congenital diaphragmatic hernia and in 25 control fetuses without pulmonary malformations. The mean relative error of 3D sonography to estimate the actual lung volume was −7.19% (range: −42.70% to +18.11%) in cases of congenital diaphragmatic hernia and −0.72% (range: −30.25% to +19.22%) in normal fetuses. Barros and colleagues studied 24 fetuses with skeletal dysplasia and measured the total lung volume using VOCAL. An abnormally reduced lung volume was defined as below the 5th percentile for gestational age. From 18 fetuses diagnosed at birth with lethal pulmonary hypoplasia, 83% had a total lung volume below the 5th percentile for gestational age. Lung volume more accurately predicted pulmonary hypoplasia than thoracic circumference, thoracic circumference/abdominal circumference ratio, and thoracic area/cardiac area ratio.
Parameters proposed to evaluate lung volume by MRI include the relative lung volume (observed/expected lung volume ratio), lung volume/estimated fetal weight (LV/EFW) ratio, and the lung/spinal fluid signal intensity (L/SF) ratio. Fetal lung volume shows a linear increase across gestational age with the right lung volume accounting for 56% of the total fetal lung volume. Lung volume evaluated by MRI is significantly reduced in fetuses at risk of pulmonary hypoplasia, and MRI might perform better than sonography. In addition, reduced signal intensity and altered L/SF ratio on T2-weighted imaging have been reported in fetuses with pulmonary hypoplasia.
Kalache and coworkers proposed that the volume of lung fluid displaced in the trachea could be useful for the analysis of fetal lung function. The investigators tested this hypothesis in a case-control study that included six cases of congenital diaphragmatic hernia and in a control group of five healthy fetuses matched for gestational age. Parameters that were analyzed included the (1) length of the inspiratory phase; (2) length of the expiratory phase; (3) peak velocities during inspiration and expiration; and (4) volume estimation of the displaced fluid in the trachea during breathing, calculated as
where VTI = velocity time interval and d = distance. The estimated breathing-related tracheal volume flow in uncomplicated pregnancies increased with gestational age (from 0.21 ± 0.10 mL/breath at 26 weeks, to 1.37 ± 0.48 mL/breath at 36 weeks of gestation) and was significantly lower in fetuses with diaphragmatic hernia who died of pulmonary hypoplasia. Tracheal volume flow in survivors was comparable to that in control subjects.
Underdevelopment and structural changes of the pulmonary vascular bed in cases of pulmonary hypoplasia may result in increased pulmonary vascular resistance and reduced pulmonary arterial compliance. Therefore, several investigators have attempted to use Doppler measurements of the pulmonary arteries and its branches in an attempt to identify fetuses at risk for pulmonary hypoplasia. However the observed increment in the resistance or pulsatilty indices in fetuses at risk of pulmonary hypoplasia has not been consistently documented, and when present it has not been found superior to thoracic measurements. Other Doppler parameters, such as acceleration time, have been also proposed for identification of fetuses at risk of pulmonary hypoplasia.
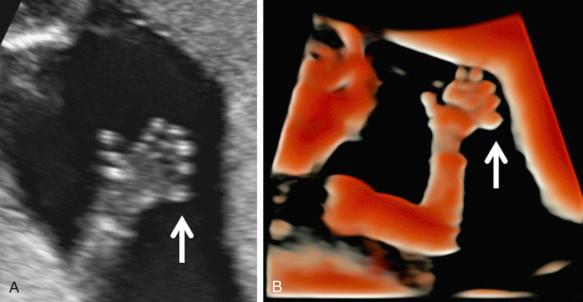
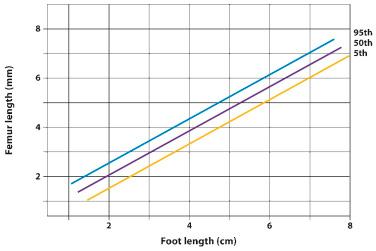
The fetal hands and feet should be examined to exclude polydactyly ( Fig. 11-26 ), brachydactyly, and extreme postural deformities, such as those seen in diastrophic dysplasia. Table 11-15 shows a nomogram of fetal foot size throughout gestation. Table 11-16 displays disorders associated with hand and foot deformities. Disproportion between the hands and feet and other parts of the extremity may also be a sign of skeletal dysplasias. Figure 11-27 illustrates the relationship between femur length and foot length. The femur length/foot length ratio is nearly constant from 14 to 40 weeks of gestation, with a mean value of 0.99 (standard deviation ±0.06). A ratio below 0.87 is considered abnormal. Although fetuses with skeletal dysplasias have been reported to have abnormally low ratios, more experience is required to establish the diagnostic value of this method. It is expected that a small proportion of normal fetuses may have an abnormal ratio. As in the case of other limb biometric parameters, large deviations from the lower limit of normal are likely to be significant.
| FOOT LENGTH | |||
|---|---|---|---|
| Weeks | 3rd | 50th | 97th |
| 12 | 5.9 | 8.9 | 11.8 |
| 13 | 8.5 | 11.7 | 14.9 |
| 14 | 11.3 | 14.6 | 18.0 |
| 15 | 14.0 | 17.6 | 21.2 |
| 16 | 16.8 | 20.6 | 24.4 |
| 17 | 19.6 | 23.6 | 27.6 |
| 18 | 22.4 | 26.6 | 30.8 |
| 19 | 25.2 | 29.6 | 34.0 |
| 20 | 28.0 | 32.6 | 37.2 |
| 21 | 30.8 | 35.6 | 40.4 |
| 22 | 33.5 | 38.6 | 43.6 |
| 23 | 36.2 | 41.5 | 46.7 |
| 24 | 38.9 | 44.4 | 49.8 |
| 25 | 41.5 | 47.2 | 52.8 |
| 26 | 44.1 | 50.0 | 55.8 |
| 27 | 46.6 | 52.7 | 58.7 |
| 28 | 49.1 | 55.3 | 61.6 |
| 29 | 51.4 | 57.9 | 64.3 |
| 30 | 53.7 | 60.4 | 67.0 |
| 31 | 55.9 | 62.8 | 69.6 |
| 32 | 58.0 | 65.1 | 72.1 |
| 33 | 60.0 | 67.3 | 74.5 |
| 34 | 61.9 | 69.4 | 76.8 |
| 35 | 63.7 | 71.4 | 79.0 |
| 36 | 65.4 | 73.3 | 81.1 |
| 37 | 66.9 | 75.0 | 83.1 |
| 38 | 68.4 | 76.7 | 85.0 |
| 39 | 69.7 | 78.2 | 86.7 |
| 40 | 70.9 | 79.6 | 88.3 |
| 41 | 71.9 | 80.8 | 89.7 |
| 42 | 72.8 | 81.9 | 91.0 |
| Postaxial Polydacytly |
| Chondroectodermal dysplasia |
| Short rib–polydactyly syndrome (type I, type II) |
| Asphyxiating thoracic dysplasia |
| Otopalatodigital syndrome |
| Mesomelic dysplasia, Werner type (associated with absence of thumbs) |
| Preaxial Polydactyly |
| Chondroectodermal dysplasia |
| Short rib–polydactyly syndrome type II |
| Carpenter syndrome |
| Syndactyly |
| Poland syndrome |
| Acrocephalosyndactyly (Carpenter syndrome, Apert syndrome) |
| Otopalatodigital syndrome type II |
| Mesomelic dysplasia, Werner type |
| Thrombocytopenia with absent radius (TAR) syndrome |
| Brachydactyly |
| Mesomelic dysplasia, Robinow type |
| Otopalatodigital syndrome |
| Hitchhiker Thumbs |
| Diastrophic dysplasia |
| Clubfoot Deformity |
| Diastrophic dysplasia |
| Osteogenesis imperfecta |
| Kniest dysplasia |
| Spondyloepiphyseal dysplasia |
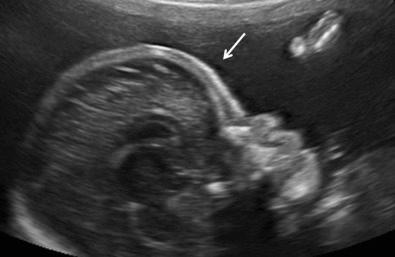
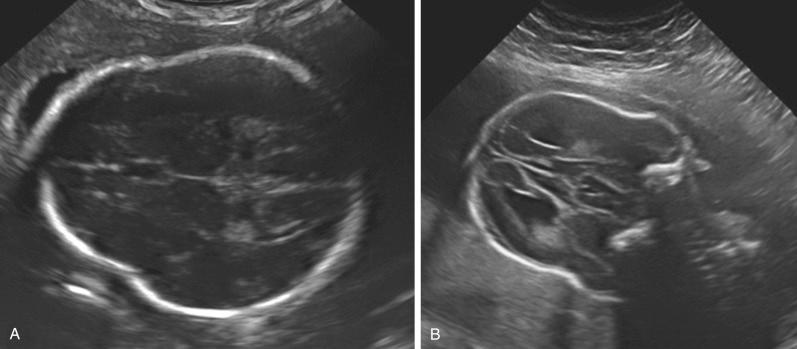
Several skeletal dysplasias are associated with defects of membranous ossification, and, therefore, skull bones are affected. Examination of the skull bones may reveal poor ossification (see Fig. 11-17 ), frontal bossing ( Fig. 11-28 ), or cloverleaf deformity ( Fig. 11-29 ). Table 11-17 presents abnormalities of the skull and face in various skeletal dysplasias.
| Large Head |
| Achondroplasia |
| Achondrogenesis |
| Thanatophoric dysplasia |
| Osteogenesis imperfecta |
| Cleidocranial dysplasia |
| Hypophosphatasia |
| Campomelic dysplasia |
| Short rib–polydactyly syndrome, type III |
| Robinow mesomelic dysplasia |
| Otopalatodigital syndrome |
| Cloverleaf Skull |
| Thanatophoric dysplasia |
| Campomelic dysplasia |
| Other Craniostenosis |
| Apert syndrome |
| Carpenter syndrome |
| Congenital Cataracts |
| Condrodysplasia punctata |
| Cleft Palate |
| Asphyxiating thoracic dysplasia |
| Kniest dysplasia |
| Diastrophic dysplasia |
| Spondyloepiphyseal dysplasia |
| Campomelic dysplasia |
| Jarcho-Levin syndrome |
| Ellis–van Creveld syndrome |
| Short rib–polydactyly syndrome, type II |
| Metatropic dysplasia |
| Otopalatodigital syndrome, type II |
| Dyssegmental dysplasia |
| Robert syndrome |
| Short Upper Lip |
| Chondroectodermal dysplasia |
| Micrognathia |
| Campomelic dysplasia |
| Diastrophic dysplasia |
| Weissenbacher-Zweymüller syndrome |
| Otopalatodigital syndrome |
| Pena-Shokeir syndrome |
| Thrombocytopenia–absent radius (TAR) syndrome |
| Langer syndrome |
Sonographic examination of the fetal face is of major importance in the assessment and diagnosis of skeletal dysplasias, because many of these disorders are associated with characteristic abnormalities. Sonographic evaluation of the fetal face is easily performed in a high percentage of patients from 16 to 20 weeks of gestation onward. The single most reliable view in detecting facial abnormalities is the sagittal plane. This view permits determination of midface hypoplasia, which occurs in several skeletal dysplasias, such as thanatophoric dysplasia, achondroplasia, campomelic dysplasia, osteogenesis imperfecta, and spondyloepiphyseal dysplasia congenita.
In cases of median cleft lip, the central portion of the upper lip is absent, and on the midline sagittal view, no upper lip will be demonstrated. In bilateral cleft lip, the midline view will have a variable appearance, depending upon the amount of residual premaxillary tissue present in the midline. In unilateral cleft lip, the midline sagittal scan may be relatively normal but the parasagittal view will reveal the cleft. Clefts should be confirmed by imaging the lips in the coronal plane.
Cleft palate occurs in 66% of patients with cleft lip. Isolated cleft palate is more difficult to diagnose with ultrasound due to the shadowing from the facial bones. 3D sonography has been shown to be superior to 2D sonography for prenatal diagnosis of cleft lip and palate. Potential advantages of 3D sonography over 2D sonography include the following: (1) a true coronal view of the lips can be displayed, even when the original scanning plane was obtained from a different orientation; (2) 3D standardized multiplanar imaging makes it easier to successfully demonstrate the maxillary tooth-bearing alveolar ridge in suspected cases; and (3) the maxillary tooth-bearing alveolar ridge can be more accurately localized by the multiplanar technique because this region can easily be mistaken for the mandibular ridge. Rendered views of the cleft lip/palate have also been particularly useful for patient counseling. A novel technique for visualization of the fetal palate, called the 3D reverse face view, has been proposed for the antenatal characterization of facial clefting; specifically, clefting of the hard palate. This technique consists of rotating the volume dataset 180 degrees around the vertical axis in order to examine the secondary palate. Campbell and associates reported that a cleft in the soft palate was missed in only one of eight cases of suspected orofacial clefting with the use of this rendering technique. Recent studies recommend the use of MRI in the evaluation of fetal cleft lip and palate identified by sonography. MRI appears to provide a better assessment of the degree of involvement of the secondary palate and of cleft extent since shadowing artifacts from adjacent osseous structures do not affect fetal MR images.
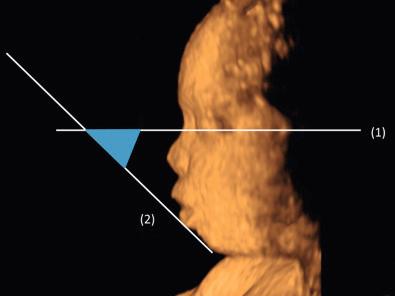
Micrognathia is frequently observed in cases of skeletal dysplasia ( Table 11-18 ). In an attempt to provide an objective tool to diagnose micrognathia prenatally, Paladini and colleagues proposed the jaw index, which is computed as the ratio between the anteroposterior mandibular diameter and the biparietal diameter. In a population of 198 fetuses with congenital anomalies (11 of which had micrognathia at necropsy or at birth), a jaw index below 23 correctly identified all cases of micrognathia with a false positive rate of 2%. Rotten and coworkers proposed two parameters to differentiate between retrognathia and micrognathia: the inferior facial angle ( Fig. 11-30 ) and the mandible width/maxilla width (MD/MX) ratio ( Fig. 11-31 ). The inferior facial angle is defined as the angle between two lines traced on a sagittal profile view of the fetal face: (1) a reference line, orthogonal to the vertical part of the forehead, at the level of the synostosis of the nasal bones, and (2) the profile line, joining the tip of the mentum to the anterior border of the more protruding lip. In a population of fetuses at high risk for facial anomalies, an inferior facial angle less than 50 degrees identified retrognathia with a sensitivity of 100% and specificity of 98.9%. The MD/MX ratio was computed using transverse sections of the mandible and maxilla, with the actual measurements performed 10 mm posteriorly to the anterior osseous border; an MD/MX ratio less than 0.8 (between 18 and 28 weeks of gestation) correctly identified micrognathia in three cases of Treacher Collins syndrome.
| Campomelic dysplasia |
| Diastrophic dysplasia |
| Otopalatodigital syndrome |
| Achondrogenesis |
| Mesomelic dysplasia |
| Pena-Shokeir syndrome |
| Treacher Collins syndrome |
| Nager acrofacial dysostosis |
| Oromandibular limb hypogenesis |
| Goldenhar syndrome |
| Atelosteogenesis |
| Hydrolethalus syndrome |
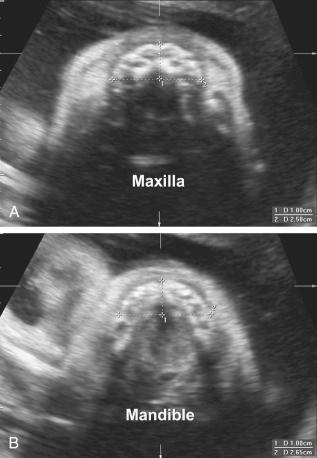
Intraorbital and interorbital diameters should also be measured, because hypertelorism may occur in cases of skeletal dysplasia ( Table 11-19 ).
| Otopalatodigital syndrome |
| Arthrogryposis multiplex congenita |
| Larsen syndrome |
| Roberts syndrome |
| Cleidocranial dysplasia |
| Achondroplasia |
| Campomelic dysplasia |
| Coffin syndrome |
| Klippel-Feil syndrome |
| Apert syndrome |
| Sprengel deformity |
| Mesomelic dysplasia |
| Holt-Oram syndrome |
Sonographic assessment of the fetal spine must be performed in all fetuses with suspected skeletal dysplasias. The following parameters should be assessed:
Fetal vertebral bodies are composed of three ossification centers representing the vertebral body and two laminae. Abnormalities of the ossification centers of the fetal vertebral bodies may result in hemivertebra (see Fig. 11-12 ), butterfly vertebrae, spinal dysgenesis ( Fig. 11-32 ), or block vertebrae causing congenital scoliosis (see Figs. 11-10 and 11-11 ). A study of the associated anomalies in 27 cases of prenatally detected hemivertebra noted that 16 (59%) have additional associated anomalies, including gastrointestinal, renal, facial, limb, and cranial anomalies; only five of the fetuses with additional anomalies survived. Usually these anomalies are not a risk factor for aneuploidy. Rib defects are often associated with thoracic vertebral body anomalies.
Platyspondyly can be diagnosed using high-resolution sonography (see Fig. 11-9 ). An objective evaluation of platyspondyly is obtained by estimating the ratio between measurements of the vertebral interspace and of vertebral body height.
Clefting of the vertebrae may be longitudinal or transverse and complete or incomplete. Coronal vertebral clefts are a result of missed fusion between the anterior and posterior primary ossification centers beyond 16 weeks of gestation and can be observed by prenatal sonography. Sagittal clefts in the vertebral bodies are believed to represent localized splitting of the notochord due to adhesions between ectoderm and endoderm during the embryonic period. The role of vertebral clefting in the diagnosis of skeletal dysplasias was assessed by Westvik and Lachman based on the International Skeletal Dysplasia Registry. The authors reported coronal and sagittal clefts in 40 different skeletal anomalies. Coronal clefts were more common than sagittal clefts and were mainly located in the thoracolumbar region. Clefts were most frequently observed in atelosteogenesis (88%), followed by chondrodysplasia punctata (79%), dyssegmental dysplasia (73%), Kniest dysplasia (63%), and short rib–polydactyly syndrome (SRPS) (53%). The authors concluded that vertebral clefts are of major diagnostic value in these groups of skeletal dysplasias.
The most common osseous anomaly causing scoliosis is unilateral unsegmented bar with contralateral hemivertebra. Spinal dysraphism may also occur with congenital scoliosis. An apparent etiologic relationship exists between neural tube defects and other vertebral anomalies. Siblings of infants with congenital scoliosis have a 4% risk of neural tube defects. This increased risk is present in siblings of children with a single hemivertebra, as well as multiple vertebral anomalies (with or without neural arch defects). The differential diagnosis of fetal scoliosis includes neural tube defects, large abdominal wall defects, amniotic band syndrome, caudal regression, and hemivertebra. Nonossification of the lumbar vertebral bodies has been detected in achondrogenesis and other diseases.
Hoopmann and associates performed detailed sonographic evaluation of the fetal spine and reported that the distance from the tip of the conus medullaris to the sacrum was useful in identifying fetuses with skeletal dysplasias with a shortened trunk ( Fig. 11-33 ). In their study, all fetuses with a shortened trunk at birth had a considerably shorter distance from the tip of the conus medularis to the sacrum as compared with normal fetuses. The authors suggest that evaluation of the conus medullaris might be useful in the evaluation of skeletal dysplasias.
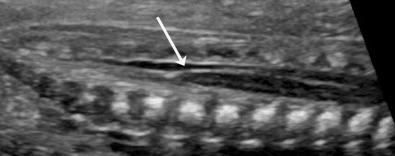
A detailed examination of the cardiovascular, genitourinary, gastrointestinal, and central nervous system (CNS) organs should be performed for differential diagnosis among syndromes presenting with fetal skeletal anomalies. For example, congenital heart disease is a prominent feature of Ellis–van Creveld and Holt-Oram syndromes.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here