Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Fetal development is a highly organized process. The most rapid phase of growth transpires in at a degree of hypoxemia that mimics ascent to Mount Everest, while nutrients reach the fetus indirectly, from the maternal circulation. The transition to the extrauterine environment occurs abruptly and is likewise extraordinarily well orchestrated. Indeed, within a matter of seconds, the breathing infant can supply sufficient oxygen to the tissues because of gas exchange in the previously fluid-filled newborn lung and rapid redirection of blood flow from fetal to adult pathways. Finally, the newborn must acclimate to its new milieu, where numerous homeostatic challenges confront the newly autonomous organs. Accordingly, the subchapters here address the growth of the fetus, the development of the cardiopulmonary system, the transition to the extrauterine environment, and early neonatal functions.
Growth of the fetus begins soon after fertilization, when the first cell division occurs. Cell division, hypertrophy, and differentiation are well-coordinated events that result in the growth and development of specialized organ systems. The fetus, fetal membranes, and placenta develop and function as a unit throughout pregnancy, and their development is interdependent. The growth trajectory of fetal mass is relatively flat during the first trimester, increases linearly at the beginning of the second trimester, and rises rapidly during the third trimester. Before addressing the details of growth, we describe the physiological factors that govern transport of nutrients and waste between the fetus and the placenta.
The fundamental difference between the circulatory system of the fetus and that of the infant/adult is the presence of the placenta, which produces essential hormones (e.g., progestins, estrogens, chorionic gonadotropins) and performs a number of vital functions (e.g., gas exchange, nutrient transport, fluid balance, and waste removal) that other systems provide in extrauterine life. The fetus accomplishes these tasks by directing a large fraction of fetal blood flow from the aorta via the two umbilical arteries to the fetoplacental circulation within chorionic villi (see p. 1137 ). There the umbilical arteries branch into small tufts of vessels (villus capillaries) that come into apposition with the maternal uteroplacental circulation, which has a high blood flow (see pp. 1136–1137 ). The placental membranes (the syncytiotrophoblast) that separate these two distinct circulations permit the efficient exchange of respiratory gases, fluid, and larger molecules that are critical to sustain growth and maintain homeostasis. Accordingly, disturbances in the placental circulation or delivery of nutritive substrates can have serious consequences for the growth and development of the fetus.
The transfer of respiratory gases occurs by diffusion, driven by the partial pressure differences of these gases in various compartments, as is the case in the lungs after birth (see pp. 660–661 ). The uterine arterial blood enters the placenta with a  of 80 to 100 mm Hg, whereas umbilical arterial blood enters with a
of 80 to 100 mm Hg, whereas umbilical arterial blood enters with a  of ~20 mm Hg, so that O 2 diffuses from the uterine to the umbilical circulation. The result is that the effluent umbilical venous blood has a
of ~20 mm Hg, so that O 2 diffuses from the uterine to the umbilical circulation. The result is that the effluent umbilical venous blood has a  of about 35 to 50 mm Hg, whereas the uterine venous
of about 35 to 50 mm Hg, whereas the uterine venous  is consistently higher by 4 to 15 mm Hg. This difference is analogous to the alveolar-arterial
is consistently higher by 4 to 15 mm Hg. This difference is analogous to the alveolar-arterial  gradient (see p. 698 ). However, with some substances, such as creatinine, the concentration is the same in fetal and maternal blood. Amino acids are transported across the syncytiotrophoblast and then diffuse into the fetal circulation. Transfer of immunoglobin G, a protein that provides passive immunity from the mother, is by receptor-mediated endocytosis (see p. 42 ). Fluid movement obeys the principles of Starling forces (see pp. 467–468 ).
gradient (see p. 698 ). However, with some substances, such as creatinine, the concentration is the same in fetal and maternal blood. Amino acids are transported across the syncytiotrophoblast and then diffuse into the fetal circulation. Transfer of immunoglobin G, a protein that provides passive immunity from the mother, is by receptor-mediated endocytosis (see p. 42 ). Fluid movement obeys the principles of Starling forces (see pp. 467–468 ).
The growth of an organ occurs as a result of an increase in cell number (hyperplasia), an increase in cell size (hypertrophy), or both. Growth follows three sequential phases that are organ specific: (1) pure hyperplasia, (2) hyperplasia and concomitant hypertrophy, and (3) hypertrophy alone. For example, the placenta goes through all three phases of growth, but these phases are compressed because the placental life span is relatively short. Moreover, simple hypertrophy is the primary form of placental growth. Thus, the weight, RNA content, and protein content of the human placenta increase linearly until term, but cell number does not increase during the third trimester. Disturbances of placental growth have secondary consequences for the fetus. Perhaps the most important factor that currently can be modified is maternal smoking, which contributes to a reduction of birth weight and raises the risk of chronic disease in the offspring, as both a child and an adult.
In contrast to placental growth and development, growth of the fetus occurs almost entirely by hyperplasia. Thus, DNA content increases linearly in all fetal organs beginning early in the second trimester. Stimuli that either increase or decrease cell number, cell size, or both may accelerate or retard the growth of the whole fetus or of individual organs. The phase of growth during which the stimulus acts determines the response of the organ. For example, chromosomal aneuploidy is likely to have an effect on growth throughout gestation, whereas malnutrition or substance abuse will impair growth only when the exposure is present. Although some effects—such as those of malnutrition on growth—are reversible, there may also be long-term consequences for health.
The fertilized egg contains the genetic material that directs cell multiplication and differentiation and guides development of the human phenotype. For specific developmental events to occur at precise times, a programmed sequence of gene activation and suppression is necessary. ![]() N57-1 Ignoring apoptosis, the fertilized egg must undergo an average of ~42 divisions before newborn size is reached. A fertilized ovum weighing less than 1 ng gives rise to a newborn weighing slightly more than 3 kg (an increase of >10 12 fold). Not only must the total cell number in a term fetus lie within relatively narrow limits, but also the developmental program must trigger cell differentiation after a specified number of cell divisions. After birth, only approximately five additional divisions are necessary for the net increase in mass that is necessary to achieve adult size. However, many tissues (e.g., gastrointestinal tract, skin, blood cells) must continually undergo cell division to replenish cells lost by apoptosis.
N57-1 Ignoring apoptosis, the fertilized egg must undergo an average of ~42 divisions before newborn size is reached. A fertilized ovum weighing less than 1 ng gives rise to a newborn weighing slightly more than 3 kg (an increase of >10 12 fold). Not only must the total cell number in a term fetus lie within relatively narrow limits, but also the developmental program must trigger cell differentiation after a specified number of cell divisions. After birth, only approximately five additional divisions are necessary for the net increase in mass that is necessary to achieve adult size. However, many tissues (e.g., gastrointestinal tract, skin, blood cells) must continually undergo cell division to replenish cells lost by apoptosis.
eTable 57-1 summarizes major developmental events during the first 7 weeks of intrauterine life.
| ORGAN | CHRONOLOGY OF DEVELOPMENT |
|---|---|
| Bronchial apparatus and pharyngeal pouches | 4th week, ridges and grooves appear over the future neck region |
| Thyroid gland | 4th week, endoderm appears over the floor of the pharynx |
| Tongue | 4th week, primordia appear in the floor of the pharynx |
| Face | End of 4th week, primordia appear |
| Palate | Begins in the 5th week |
| Upper respiratory system | 4th week, laryngotracheal groove appears |
| Digestive system | 4th week, primitive gut forms |
| Foregut derivatives | 4th week, formation of midgut derivatives begins |
| Pharynx and its derivatives | |
| Lower respiratory tract | |
| Esophagus, stomach, proximal duodenum (from stomach to entry of the common bile duct) | |
| Liver, biliary tract, gallbladder, pancreas | |
| Midgut derivatives | 6th week, primordia appear, midgut elongates |
| Small intestine, except proximal duodenum | |
| Cecum, appendix | |
| Ascending colon and proximal half of transverse colon | |
| Hindgut derivatives | End of 7th week, anal canal has formed |
| Distal half of transverse colon | |
| Descending and sigmoid colon | |
| Rectum and upper portion of anal canal | |
| Part of the urogenital system | |
| Kidneys, urinary bladder, urethra | 5th week, permanent adult kidney begins to develop |
| Adrenal glands | 5th week, primordia of adrenal glands develop |
| Gonads, genital ducts, external genitalia | 5th week, gonadal ridges form |
| Heart | 3rd week, development of the heart begins |
| Atria | 5th week, the atria are formed |
| Ventricles | 5th week, the ventricles form |
| Fetal circulation | 3rd week, embryonic blood vessels develop |
| Brain and spinal cord | End of 4th week, primary vesicles form and walls of the neural tube thicken to form the spinal cord |
| Pituitary | 6th week, connection of Rathke's pouch with oral cavity disappears |
| Limbs | End of 4th week, limb buds appear |
| Skull | 7th week, paired cartilages begin to fuse to form the cranium |
Although the genetic makeup of the fetus principally determines its growth and development, other influences—both stimulatory and inhibitory—are superimposed on the genetic program. During the first half of pregnancy, the fetus's own genetic program is the primary determinant of growth, and thus constrains patterns of growth. During the second half of pregnancy, the patterns of growth and development are more variable. The four primary epigenetic factors at work during the second half of pregnancy are placental, hormonal, environmental (e.g., maternal nutrition), and metabolic (e.g., diabetes). We discuss the first two factors (placental and hormonal) in the next two sections.
Studies in both healthy and growth-restricted infants have identified the important determinants of newborn body mass ( Fig. 57-1 ). The use of fetal ultrasonography now permits repetitive determination of measures of fetal growth, such as femur length, distance between the two parietal eminences of the head (biparietal diameter), head circumference, and abdominal circumference. For example, a healthy male infant gains 25 to 30 g/day during the last trimester of pregnancy. The ultrasound measurements provide valuable information about growth during gestation, whereas previously we made assumptions based solely on birth weight.
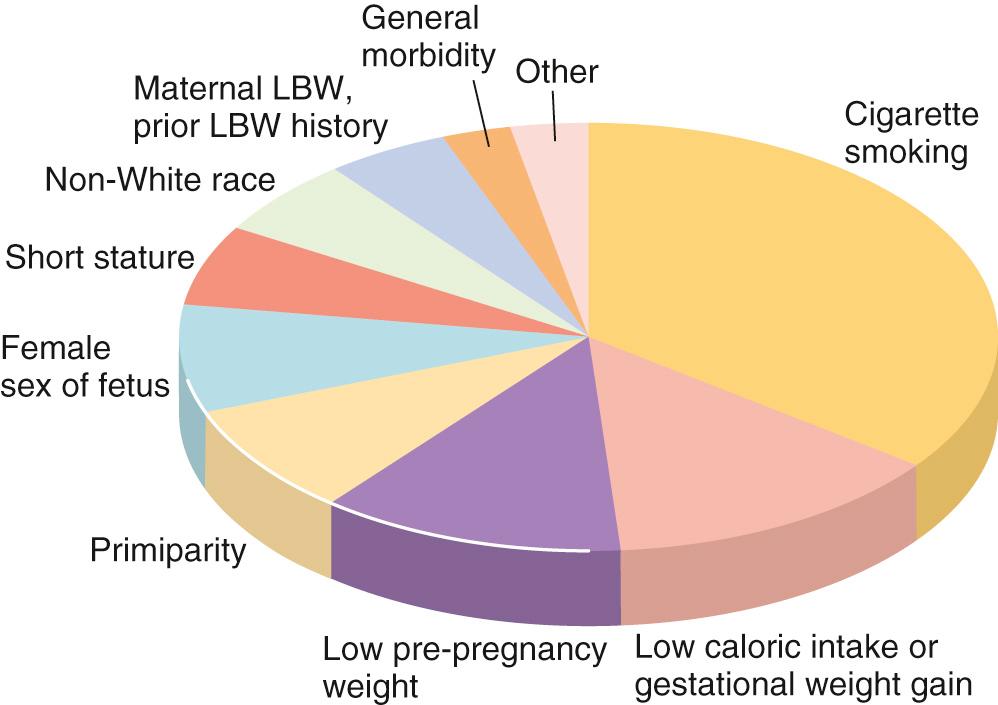
The mother's contribution to the fetal environment includes maternal health and nutritional status, age (e.g., adolescents and older women have infants with lower birth weight), parity, prepregnancy weight and prenatal weight gain, height, and cigarette smoking, although factors vary in importance depending on the environment (e.g., maternal infection with malaria is a significant factor in endemic areas). The father contributes to birth weight only via the genes that he passes on to his child, with the correlation between father and infant being ~12%. The correlation with maternal weight is ~22%. From observations in cases of oocyte donation, it is apparent that newborn weight is related more to anthropometric measures of the mother than of the father. The paternal body size has a small influence on fetal lean mass, and fetal sex also has a small effect (males have somewhat higher lean body mass). Multiparity and premature birth both correlate with low birth weight, and both have become more common with artificial fertilization techniques.
The placenta plays several important roles in fetal growth and development. In addition to serving transport and storage functions, the placenta is involved in numerous biosynthetic activities. These include the synthesis of steroids, such as estrogen and progesterone, and of protein hormones, such as human chorionic gonadotropin (hCG) and the human chorionic somatomammotropins (hCSs; see p. 1139 ).
Fetal growth closely correlates with placental weight and trophoblast surface area. During periods of rapid fetal growth, placental weight increases. As the placental mass increases, the total surface area of the placental villi (see p. 1136 ) increases to sustain gas transport and fetal nutrition. Moreover, maternal blood flow to the uterus and fetal blood flow to the placenta also increase in parallel with the increase in placental mass. Placental growth increases linearly until ~4 weeks before birth, and adequate placental reserve is particularly important during the third trimester, when fetal growth is very rapid. Fetal growth restriction ( Box 57-1 ) can occur as a result of decreased placental reserve caused by any insult. For example, mothers who smoke during pregnancy tend to have small placentas and are at high risk of delivering a low-birth-weight baby.
Growth restriction is an important common pathway for many conditions that significantly increase infant morbidity and mortality, and it is also associated with many short- or long-term consequences. Some of the most important morbidities associated with growth restriction are birth asphyxia, neonatal hypoglycemia, necrotizing enterocolitis, hypocalcemia, meconium aspiration, persistent pulmonary hypertension of the newborn, pulmonary hemorrhage, thrombocytopenia, polycythemia, delayed neurological development, and hypothermia.
Two general terms describe an abnormality of fetal growth: (1) small for gestational age, which is based on birth weight; and (2) intrauterine growth restriction (IUGR), which is based on the rate of fetal growth as detected by ultrasonography. Both terms describe infants who, for a variety of reasons, weigh much less than population-based norms (based on fetal growth curves); we will use the term IUGR.
IUGR has been variously defined as a birth weight lower than the 3rd, 5th, or 10th percentile for gestational age, or a birth weight that is more than two standard deviations lower than the mean for gestational age. Similar norms for body length and head circumference permit us to characterize growth restriction and determine whether it is symmetrical (i.e., anthropometric factors are uniformly decreased) or asymmetrical (e.g., weight is affected more than length, which is affected more than head growth). These distinctions are made by obtaining careful measurements at birth and then plotting length, weight, and head circumference on standard curves for the appropriate sex, gestational age, and comparable population.
The three general categories of IUGR are related to the time of onset of the pathological process as follows.
Symmetrical IUGR applies to infants whose length, weight, and abdominal and head circumferences are all less than the 10th percentile for gestational age. This type usually results from long-standing growth limitation that arises during early stages of fetal development (4 to 20 weeks' gestation). The result is fewer cells in the fetus. Causes include intrauterine infections (e.g., rubella, cytomegalovirus infection), chromosomal disorders, and congenital malformations. Maternal drug ingestion, excessive alcohol consumption, and smoking can also produce this pattern depending upon the length of exposure. Of fetuses with severe, early onset of growth restriction, ~25% have aneuploidy (i.e., abnormal number of chromosomes). The symmetrically diminished growth of these fetuses may result from inhibition of mitosis during early development.
Asymmetrical IUGR, which accounts for 70% to 80% of growth restriction in fetuses, is most frequently caused by uteroplacental insufficiency. Compared to symmetrical IUGR, this type of growth restriction results from an insult that occurs either later in gestation (usually after 28 weeks) or more briefly. Late in the second trimester, hypertrophy dominates as the means for growth and there is a rapid increase in cell size and increases in the formation of fat, muscle, bone, and other tissues. For fetuses with asymmetrical growth restriction, head growth is less impaired than weight gain. This form of IUGR is most often associated with small placentas and maternal conditions such as kidney disease, chronic hypertension, severe diabetes mellitus, or multiple gestation.
Intermediate IUGR is a combination of symmetrical and asymmetrical IUGR, accounting for 5% to 10% of all cases of fetal growth restriction. It probably occurs during the middle phase of fetal growth (20 to 28 weeks), between the hyperplastic and hypertrophic phases. During this middle period, mitotic rate decreases and overall cell size increases progressively. Chronic hypertension, lupus nephritis, or other vascular diseases in the mother that are severe and begin early in the second trimester may result in intermediate IUGR in the fetus, with symmetrical growth and no significant brain-sparing effect.
In Chapter 48 , we discussed several hormones—including glucocorticoids, insulin, growth hormone (GH), the insulin-like growth factors (IGFs), and thyroid hormones—that are important for achieving final adult mass.
As its major energy source, the growing fetus uses glucose, which moves across the placenta by facilitated diffusion via the glucose transporter GLUT1 (see p. 114 ). Unlike the adult, who uses sophisticated hormonal systems to control blood glucose levels ( pp. 1035–1050 , 1050–1053 , 1018–1026 , and 1033 ), the fetus is passive: the exchange of glucose across the placenta controls fetal blood glucose levels. The fetus normally has little need for gluconeogenesis, and the levels of gluconeogenic enzymes in the fetal liver are low. Glucocorticoids in the fetus promote the storage of glucose as glycogen in the fetal liver, a process that increases greatly during the final month of gestation in preparation for the increased glycolytic activity required during and immediately after delivery. Near term, when fetal glucose metabolism becomes sensitive to insulin, this hormone contributes to the storage of glucose as glycogen, as well as to the uptake and utilization of amino acids and lipogenesis (see pp. 1035–1050 ). In mice, knockout of the insulin receptor causes slight growth restriction in the fetus, whereas the additional knockout of the insulin gene severely reduces birth weight. Transient increases in maternal blood glucose levels after meals are closely mirrored by increases in fetal blood glucose levels. However, maternal insulin cannot cross the placenta, and this transient fetal hyperglycemia leads to increased fetal production of insulin.
Sustained hyperglycemia in mothers with poorly controlled diabetes (see Box 51-5 ) causes sustained fetal hyperglycemia, and therefore fetal hyperinsulinemia. The resulting high levels of fetal insulin, which is a growth factor (see pp. 999–1000 ), increase both the size of fetal organs (organomegaly) and fetal body mass (macrosomia; see p. 999 ). During the last half of the third trimester, fetal weight in pregnant women with poorly controlled diabetes generally exceeds that in normal pregnant women. In some cases, large fetal size leads to problems at delivery, prompting cesarean section. However, in mothers with severe diabetes, the placenta shows evidence of vascular insufficiency and the infants may be growth restricted.
Postnatally, GH acts by binding to the GH receptor (GHR; see p. 994 ), primarily in the liver, and triggering the production of somatomedin or IGF-1. IGF-2 is not so much under the control of GH. The IGF-1 receptor is similar, but not identical, to the insulin receptor and can bind both IGF-1 and IGF-2, as well as insulin (see p. 996 ). In the fetus, both IGF-1 and IGF-2, which are mitogenic peptides, are extremely important for growth. IGF-1 and IGF-2 are present in the fetal circulation from the end of the first trimester, and their levels increase thereafter in both mother and fetus. Birth weight correlates positively with IGF levels. However, both relative levels of the IGFs and control of the IGFs are very different in the fetal stage than they are postnatally. First, fetal IGF-2 levels are much higher than IGF-1 levels; soon after birth, IGF-1 and IGF-2 levels resemble those in adults. Second, in the fetus, both IGF-1 and IGF-2 levels correlate poorly with GH levels. GH levels, which are much higher than postnatally, may have only a limited influence on fetal growth because GHRs are not as abundant (e.g., in liver) as they are after birth. This observation may explain why anencephalic fetuses (see Box 10-2 ), which have low GH levels, generally grow normally in utero. On the other hand, GH-deficient infants are often shorter than normal infants.
The fetus has abundant epidermal growth factor (EGF) receptors (see p. 68 ), and EGF is well known for its mitogenic properties, especially with regard to development of ectodermal and mesodermal structures. However, the fetus has no detectable mRNA encoding EGF. On the other hand, transforming growth factor-α (TGF-α), another potent mitogen that binds to EGF receptors on target cells, may act as a ligand for the EGF receptor; its mRNA is present widely in human fetal tissues.
The thyroid hormones are obligatory for normal growth and development (see pp. 1013–1014 ). Before the second trimester, most of the thyroxine (T 4 ) in the fetus is maternal. Fetal production of thyrotropin (or thyroid-stimulating hormone [TSH]) and T 4 begin to increase in the second trimester, concurrent with development of the hypothalamic-pituitary portal system. Hypothyroidism has adverse effects on fetal growth, generally reflected as a reduction in the size of organs such as the heart, kidney, liver, muscle, and spleen.
Peptide hormones secreted by the placenta (see Table 56-4 ) can act via endocrine, paracrine, and autocrine mechanisms to stimulate growth and differentiation in several organ systems.
During early gestation, islands of cells form within the yolk sac, some of which become primitive blood cells or hemocytoblasts. This phase of hematopoiesis ends by 6 weeks' gestation. Later, red blood cell (RBC) production (erythropoiesis; see pp. 431–433 ) occurs in many tissues not normally thought of as erythropoietic in the adult. At about the fourth week of gestation, the endothelium of blood vessels and the mesenchyme also begin to contribute to the pool of RBCs, followed shortly by the liver. The bone marrow, spleen, and other lymphoid tissues begin to produce RBCs only near the end of the first trimester. All these organ systems except bone marrow gradually lose their ability to manufacture blood cells, and by the third trimester, the bone marrow becomes the dominant source of blood cells.
RBCs formed early in gestation are nucleated, but as fetal development progresses, more and more of the circulatory RBCs are non-nucleated. The blood volume in the common circulation of the fetoplacental unit increases as the fetus grows. The fraction of total RBCs that are reticulocytes ![]() N57-2 (immature, non-nucleated erythrocytes with residual polyribosomes) is high in the young fetus, but it decreases to only ~5% at term. In the adult, the reticulocyte count is normally <1%. The life span of fetal RBCs depends on the age of the fetus; in a term fetus, it is ~80 days, or two thirds that in an adult (see pp. 431–433 ); it is shorter in the less mature fetus.
N57-2 (immature, non-nucleated erythrocytes with residual polyribosomes) is high in the young fetus, but it decreases to only ~5% at term. In the adult, the reticulocyte count is normally <1%. The life span of fetal RBCs depends on the age of the fetus; in a term fetus, it is ~80 days, or two thirds that in an adult (see pp. 431–433 ); it is shorter in the less mature fetus.
As discussed on pages 431–433 , reticulocytes are immature erythrocytes (also known as red blood cells, or RBCs). These cells are called reticulocytes—or “retics” in the slang—because certain stains (e.g., new methylene blue) reveal a mesh-like “reticular” network due to the presence of ribosomal RNA.
In the adult, retics make up about 1% of all RBCs.
Embryonic hemoglobin (Hb) with different combinations of α-type chains (α and ζ) and β-type chains (ε and γ; see Table 29-1 ) is present very early in gestation. Production of ζ and ε chains ceases by 8 weeks, and programmed development ![]() N4-5 governs increased synthesis of fetal Hb (HbF, α 2 γ 2 ), which predominates at birth. Adult Hb (HbA, α 2 β 2 ) and a small amount of HbA 2 (α 2 δ 2 ) gradually replace HbF during the first 12 months of life until eventually the adult pattern of Hb expression is established (see Table 29-2 ). An exception is in certain genetic abnormalities of α- or β-chain production (e.g., thalassemia), in which HbF persists.
N4-5 governs increased synthesis of fetal Hb (HbF, α 2 γ 2 ), which predominates at birth. Adult Hb (HbA, α 2 β 2 ) and a small amount of HbA 2 (α 2 δ 2 ) gradually replace HbF during the first 12 months of life until eventually the adult pattern of Hb expression is established (see Table 29-2 ). An exception is in certain genetic abnormalities of α- or β-chain production (e.g., thalassemia), in which HbF persists.
The Hb content ([Hb]) of fetal blood rises to ~15 g/dL by midgestation, equivalent to the level in normal men (see p. 434 ) and higher than [Hb] in maternal blood, which may be only ~12 g/dL. Fetal [Hb] increases near term and is ~17 g/dL in full-term infants and slightly less in premature infants. Postnatally, a substantial decrease in [Hb], with a nadir of ~11 g/dL near 2 months of age, is termed the physiological anemia of infancy because it does not respond to administration of iron or other hematinic agents. This level of anemia does not affect the well-being of an otherwise healthy infant. However, infants who are born prematurely (and therefore with a lower nadir) or who have other disturbances in O 2 transport (e.g., hypoxemia, impaired cardiac output) can exhibit irritability, failure to grow normally, or tissue hypoxia.
The fetus imbibes considerable quantities of amniotic fluid by 20 weeks' gestation. However, not until the final 12 weeks of gestation is fetal gastrointestinal function similar to that of the normal infant at term. The fetal gastrointestinal tract continuously excretes small amounts of meconium into the amniotic fluid. Meconium consists of excretory products from the gastrointestinal mucosa and glands, along with unabsorbed residua from the imbibed amniotic fluid.
By the beginning of the second trimester, the fetus also begins to urinate. Fetal urine constitutes ~75% of amniotic-fluid production (see p. 1137 ). The fetal renal system does not acquire the capacity to regulate fluid, electrolyte, and acid-base balance until the beginning of the third trimester. Although the fetus has its final number of nephrons by 34 to 36 weeks' gestation, full development of renal function does not occur until several months following delivery, owing primarily to continued development of renal tubules and increased renal blood flow.
Fetal tissues constantly synthesize and break down proteins. Protein synthesis predominates throughout gestation, especially during the third trimester, when fetal protein synthesis—primarily in muscle and liver—increases 3- to 4-fold. The number of ribosomes per cell increases throughout gestation and early postnatal life. The efficiency of ribosomes at translating mRNA may also improve during gestation. Substrate availability (i.e., amino acids) and modulation of the synthetic apparatus by endocrine and other factors play important roles in regulating protein synthesis during gestation.
The formation of each peptide bond requires four molecules of ATP, so the energy cost of protein synthesis is 0.86 kcal/g. Protein synthesis comprises 15% to 20% of fetal metabolic expenditure in the third trimester. At equivalent phases of development, fetuses across several species invest similar fractions of total energy in protein synthesis. Because glucose is the major metabolic fuel, a shortfall of oxidized metabolic substrates (e.g., glucose and lactate) directly limits protein synthesis.
Increases in skeletal muscle mass account for 25% to 50% of fetal weight gain during the second half of gestation, when the number of muscle cells increases 8-fold and cell volume increases ~2.6-fold. Although skeletal muscle fibers are not differentiated in the first half of gestation, distinct type I and type II muscle fibers (see pp. 249–250 ) appear in equal amounts between 20 and 26 weeks of gestation.
Fetal fat stores account for only 1% of fetal body weight during the first trimester. By the third trimester, as much as 15% of fetal body weight is fat. At birth, humans have more fat than other warm-blooded animals (e.g., the newborn cat has 2%; the guinea pig, 9.5%; the rat, 11%), with the exception of hibernating mammals and migratory birds.
Approximately half the increase in body fat reflects increased lipid transport across the placenta, and the other half reflects increased fatty acid (FA) synthesis in the fetal liver. Blood levels of fetal lipids (i.e., triacylglycerols, FAs, and ketone bodies) remain low before 32 weeks' gestation. In the last 2 months, the fetus increases its lipid storage as triacylglycerols in white and brown adipose tissue as well as in liver. During this period, both subcutaneous fat (i.e., white fat) and deep fat (i.e., white and brown fat) increase exponentially. The stored fat ensures adequate fuel stores for postnatal survival, and it also provides thermal insulation to the newborn. In addition, brown fat is important for thermogenesis in the postnatal period.
Several factors are responsible for increased lipid stores in the near-term fetus. Increases in fetal albumin facilitate FA transfer across the placenta. Insulin acts on fetal hepatocytes to stimulate lipogenesis. Insulin also promotes the availability of substrates, including glucose and lactate, which in turn increase the synthesis of fat (see pp. 1047–1050 ).
Two critical factors make gas exchange in the infant lung as effective as that in the adult lung: (1) the structural growth and coincident branching of lung segments and blood vessels that creates an extensive alveolar-capillary interface for efficient diffusion of gases, and (2) the production of surfactant (see pp. 613–615 ), which permits lung expansion without excessive inspiratory effort. The fetal lung begins as an outpouching of the foregut at ~24 days' gestation. Several days later, this lung bud branches into two tubular structures, the precursors of the main bronchi. At 4 to 6 weeks' gestation, the bronchial tree begins to branch repetitively. The further maturation of the lungs occurs in four overlapping phases:
Pseudoglandular period (5 to 17 weeks). The lung “airways” resemble branching exocrine glands, with acinar buds forming in the peripheral lung.
Canalicular period (16 to 26 weeks). The creation of channels (canalization) within the airways is complete when ~17 generations of airways have formed, including the respiratory bronchioles. Each respiratory bronchiole gives rise to as many as six alveolar ducts, which give rise to the primitive alveoli during the second trimester. The branching of the pulmonary arterial tree, which begins during the pseudoglandular period, parallels both temporally and spatially the branching of the bronchial tree. However, at ~24 weeks' gestation, considerable interstitial tissue separates the capillaries from the respiratory epithelium. ![]() N57-3
N57-3
Saccular period (24 to 38 weeks). The respiratory epithelium thins greatly, with loss of connective tissue, and the capillaries push into the alveolar sacs. The potential for gas exchange improves after ~24 weeks' gestation, when capillaries proliferate and come into closer proximity to the thin type I alveolar pneumocytes (see p. 599 ). During this period, surfactant synthesis and storage begin (although not extensively) in the differentiated type II cells.
Alveolar period (late fetal life through early childhood). Alveoli-like structures are present at ~32 weeks' gestation, and at 34 to 36 weeks' gestation, 10% to 15% of the adult number of alveoli are present. Alveolar number continues to increase until as late as 8 years of age.
If the fetus were born at 24 weeks of development, the premature infant would have a very low diffusing capacity (see pp. 663–664 ) owing to the great distance between the edge of the alveolar lumen and the edge of the capillary lumen.
Hormones play a major role in controlling fetal lung growth and development in preparation for ex utero function. A key target is surfactant (see pp. 613–615 ), which increases lung compliance (see pp. 615–616 ) and thereby reduces the effort of inspiration. Numerous hormones stimulate surfactant biosynthesis, including glucocorticoids, thyroid hormones, thyrotropin-releasing hormone, and prolactin, as well as growth factors such as EGF. Glucocorticoids, in particular, play an essential role in stimulating fetal lung maturation by increasing the number of both type II pneumocytes and lamellar bodies (see pp. 614–615 ) within these cells. Glucocorticoid receptors are probably present in lung tissue at midterm. Fetal cortisol levels rise steadily during the third trimester and surge just before birth. Two thirds of this cortisol is of fetal origin; the rest crosses the placenta from the mother.
The predominant phospholipid in surfactant is dipalmitoylphosphatidylcholine ( DPPC; see pp. 613–614 ). At ~32 weeks' gestation, increases in cortisol and the other hormones mentioned previously stimulate several regulatory enzymes. ![]() N57-4 Thus, the net effect is vastly increased production of pulmonary surfactant late in gestation. Coincident with increased surfactant synthesis is a large increase in lung distensibility and stability on inflation. However, in infants born prematurely with insufficient surfactant and lungs that are not structurally mature, severe respiratory distress ( Box 57-2 ) can result. Because of the surfactant deficiency, the infant must invest excessive work to create an adequate tidal volume with each breath and to maintain a normal functional residual capacity following expiration.
N57-4 Thus, the net effect is vastly increased production of pulmonary surfactant late in gestation. Coincident with increased surfactant synthesis is a large increase in lung distensibility and stability on inflation. However, in infants born prematurely with insufficient surfactant and lungs that are not structurally mature, severe respiratory distress ( Box 57-2 ) can result. Because of the surfactant deficiency, the infant must invest excessive work to create an adequate tidal volume with each breath and to maintain a normal functional residual capacity following expiration.
Infant respiratory distress syndrome (IRDS), which affects ~30,000 newborns annually in the United States, is characterized by increased work of breathing (nasal flaring, the use of accessory musculature, intercostal and subcostal retractions, tachypnea, and grunting) and impaired gas exchange (cyanosis). Retractions occur because the lungs—with collapsed, fluid-filled, and poorly expanded alveoli—are less compliant than the chest wall, with nonossified ribs. The increased inspiratory effort to expand the noncompliant lungs creates a very negative intrapleural pressure. As a result, the chest wall becomes distorted, caving in between the ribs or beneath or above the rib cage, so that the increased inspiratory work does not much improve tidal volume. The more immature the infant, the more severe and life-threatening the lung disease and the more likely that signs of respiratory distress become apparent immediately after birth or within a few minutes. ![]() N57-10
N57-10
In more mature preterm infants, the signs of IRDS may evolve over several hours. A chest radiograph reveals atelectasis with air bronchograms (i.e., air-filled bronchi standing out against the white background of collapsed lung tissue) and pulmonary edema. The edema and consolidation or collapse of alveoli can produce severe restrictive lung disease (see p. 611 ) and subsequent fatigue from the increased effort needed to breathe, contributing to respiratory failure that requires mechanical ventilation. Milder cases usually resolve spontaneously. IRDS occurs most commonly in premature infants, and the course is often confounded by the coexistence of a patent ductus arteriosus (see p. 1158 ), poor nutrition, and risk of infection. This combination of problems also raises the risk of short- and long-term complications, such as disruption of lung development (bronchopulmonary dysplasia), necrotizing enterocolitis, and intraventricular hemorrhage.
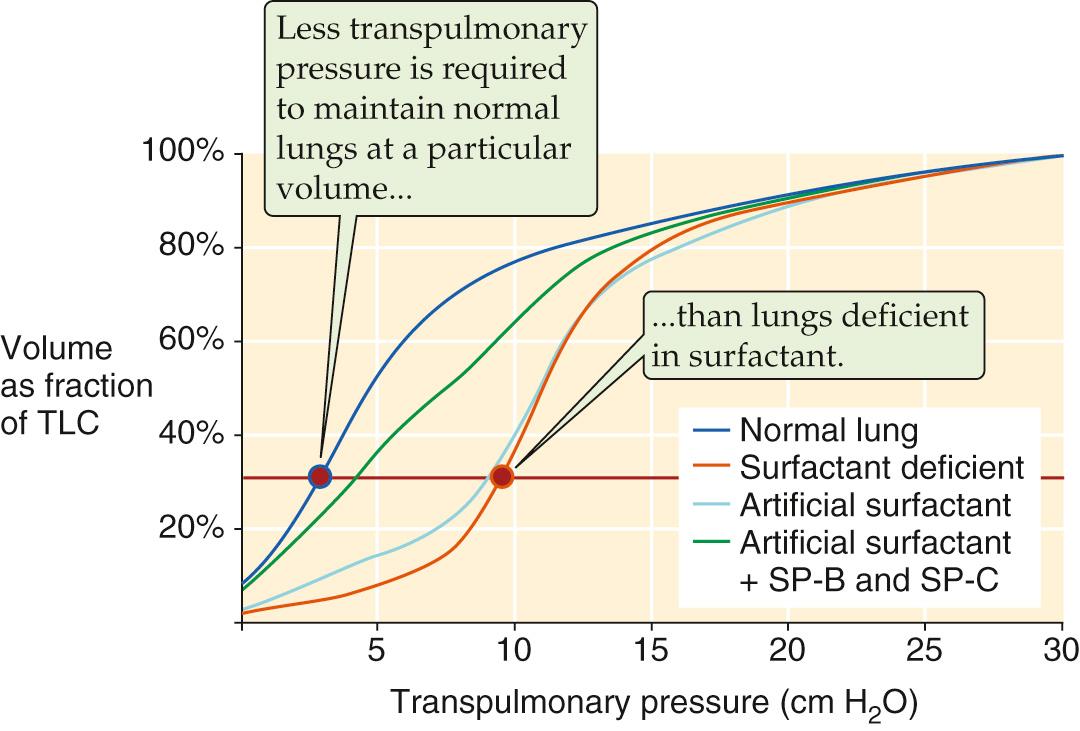
IRDS is usually caused by a deficiency of pulmonary surfactant owing to prematurity. Prematurity is by far the single most important risk factor for development of IRDS; others include male sex, delivery by cesarean section, perinatal asphyxia, second twin pregnancy, maternal diabetes, and deficiency of surfactant protein B or the ATP-binding cassette (ABC) protein ABCA3 (see p. 119 ). Surfactant insufficiency can result from abnormalities of surfactant synthesis, secretion, or recycling. Reduced surfactant lowers compliance directly (see pp. 615–616 ) and further lowers compliance indirectly because alveoli—with their propensity to collapse—tend to be on the lowest, flat part of the lung pressure-volume curve (stage 1 in Fig. 27-4 B ). Because of the tendency of alveoli to collapse, blood perfuses poorly ventilated or nonventilated alveoli, which results in hypoxemia (see pp. 692–693 ). In the extreme, when the alveoli are nonventilated, the result is venous admixture or shunt (see p. 693 ). In addition, capillary damage allows leakage into the alveolar space of plasma proteins, which may inactivate surfactant and thereby exacerbate the underlying condition.
The discovery that a deficiency of surfactant is the underlying problem in infants with IRDS prompted investigators to (1) develop means to assess fetal lung maturity and adequacy of surfactant production before delivery, (2) stimulate surfactant production in the fetus (by administration of corticosteroids to the mother prior to delivery), and (3) provide exogenous surfactant to the lungs until native synthesis occurs. As indicated in the following three paragraphs, each of these strategies has been a successful step in dramatically reducing the incidence and severity of IRDS.
Knowledge of lung maturity helps reduce complications in infants who are born prematurely or need to be delivered prematurely for specific medical indications related to the health of the mother or the infant. Clinical tests for assessing lung maturity exploit the knowledge that the major surfactant lipids are phosphatidylcholines (i.e., lecithins) and that phosphatidylglycerol (see pp. 613–614 ) is also overrepresented. A ratio of lecithin to sphingomyelin (L/S ratio) > 2.0 in the amniotic fluid is consistent with mature lungs, as is a positive result on phosphatidylglycerol assay.
Regarding surfactant production, the 2000 National Institutes of Health Consensus Development Conference Antenatal Corticosteroids Revisited recommended antenatal steroid therapy for pregnant women with fetuses between 24 and 34 weeks' gestational age who are at risk of preterm delivery within 7 days. This treatment accelerates lung maturation and surfactant production and diminishes the incidence of IRDS.
Finally, the postnatal administration of surfactant has dramatically reduced the mortality and improved the clinical course of IRDS, as illustrated for an experimental animal in Figure 57-2 .
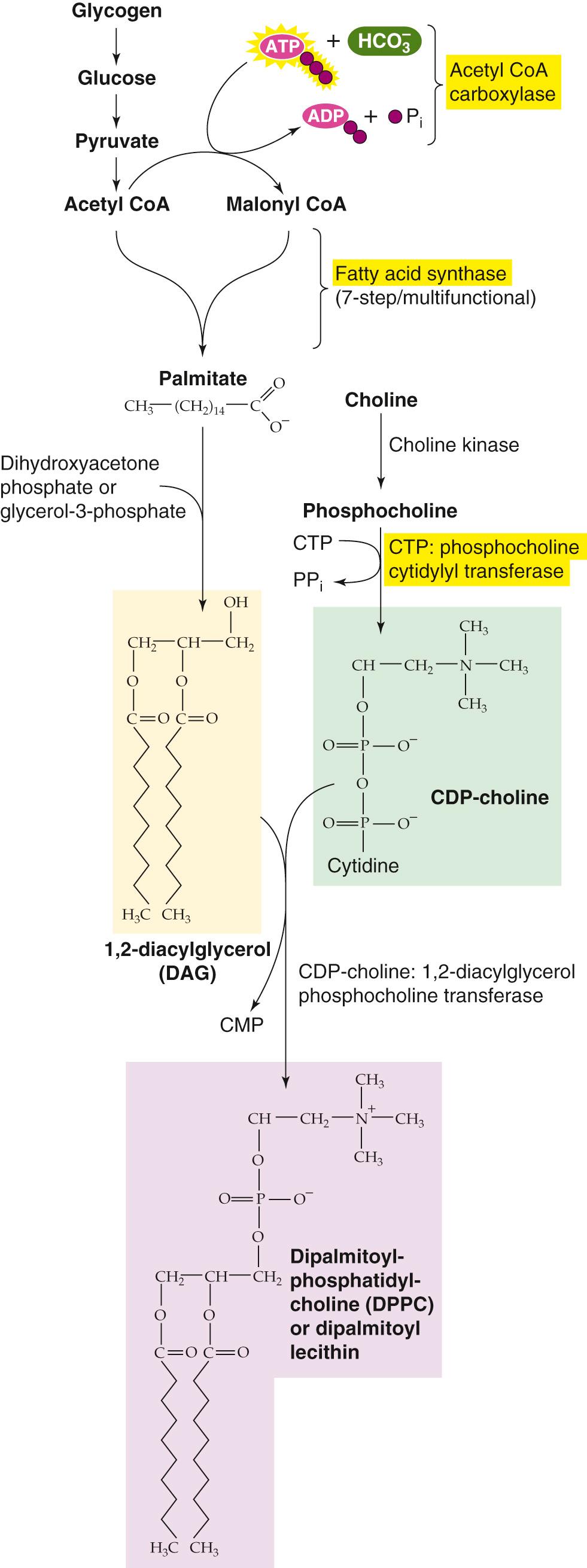
The predominant phospholipid in surfactant is DPPC (see pp. 613–614 ), which is synthesized as outlined in eFigure 57-1 . Abundant glycogen stores in fetal type II pneumocytes serve as a primary energy and carbon source for the FAs involved in phospholipid synthesis. The condensation of diacylglycerol with cytosine diphosphate choline ultimately leads to the production of DPPC. At ~32 weeks' gestation, increases in cortisol and other hormones stimulate several regulatory enzymes, including acetyl coenzyme A carboxylase, FA synthase, and CTP: phosphocholine cytidylyl transferase. The net effect is vastly increased production of pulmonary surfactant late in gestation. Coincident with increased surfactant synthesis is a large increase in lung distensibility and stability on inflation.
Two of the signs of infant respiratory distress described in Box 57-2 —cyanosis and tachypnea—reflect central hypoxemia, that is, reduced oxygen content of the arterial blood. In this case, the hypoxemia is the result of poor alveolar ventilation. As described on page 652 , cyanosis is the purplish color of poorly saturated Hb. As described in Box 32-2 , tachypnea is an increase in respiratory rate, in this case probably caused by stimulation of both the central and peripheral chemoreceptors due to respiratory acidosis and hypoxia.
Several of the other signs described in Box 57-2 —nasal flaring, intercostal and subcostal retractions, the use of accessory musculature, and grunting—are indications of low lung compliance. Nasal flaring (produced by cranial nerve VII; see Fig. 32-4 ) is a reflection of the increased inspiratory drive and is produced by one of the secondary muscles of inspiration (see point 4 —upper respiratory tract muscles—on page 607 ). Other accessory or secondary muscles of inspiration also come into play. The intercostal and subcostal retractions are the result of the very negative intrapleural pressure (P IP ) generated by the young patient in an attempt to inflate its poorly compliant alveoli. Grunting is the sound made by closure of the glottis as the infant halts expiration at a lung volume (V L ) that is considerably higher than the true functional residual capacity (FRC) to which the lungs would deflate without grunting. This true FRC would be quite low, owing to the extremely high elastic recoil of these low-surfactant lungs. One might think of the relatively high V L at the point of grunting as a “pseudo-FRC” from which the infant can begin the next inspiration with much less effort than if V L had been allowed to fall all the way to the true FRC. The reason that the effort is so much less at the V L of grunting is that the P TP -V L relationship (see Fig. 27-4 B ) is highly nonlinear, such that the compliance (i.e., the slope of the P TP -V L curve) is much higher at the V L of grunting than at the V L of the true FRC.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here