Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The editors wish to thank the authors for their valuable contributions to this chapter in the 5th edition. The chapter has been republished here virtually unchanged.
The adrenal cortex undergoes remarkable growth and unique metamorphosis during the fetal and neonatal period. Its morphology, functions, and secretory product profiles are distinctly different during fetal and postnatal life. The major products of the fetal adrenal cortex, the weak androgens dehydroepiandrosterone (DHEA) and its sulfate (DHEAS), disappear almost completely after birth, reappearing years later at the onset of adrenarche. The functions of the adrenal cortex during fetal life—participation in the maintenance of pregnancy and initiation of parturition, as well as secretion of cortisol to promote organ maturation before birth—also change postnatally for the maintenance of homeostasis, particularly in the presence of stress, and modulation of inflammatory challenges.
The cumulative work of many investigators has demonstrated that the structural and functional organization of the fetal adrenal cortex of humans and higher primates is unique. In addition, a major regulator of fetal adrenal development, placental corticotropin-releasing hormone (CRH), is present only in humans and anthropoid primates. Thus, although insights can be obtained from other animal models, caution must be exercised in applying such findings to human physiology. Wherever possible, this chapter will focus on studies of humans and other primates.
The primordial adrenal gland is first evident in humans as a distinct cluster of cells at 33 days postconception. By 50 to 52 days postconception, steroidogenic activity can be detected within the nascent fetal zone. The gland then grows exponentially, increasing 10-fold between 8 and 10 weeks postconception, equaling the kidney in weight by 20 weeks of gestation, then doubling in size between 20 and 30 weeks, and doubling again by term, reaching a combined weight of approximately 8 g ( Fig. 144.1 ). After birth, the gland quickly involutes, shrinking to a combined weight of approximately 2 g by 1 year of age before beginning to grow again.
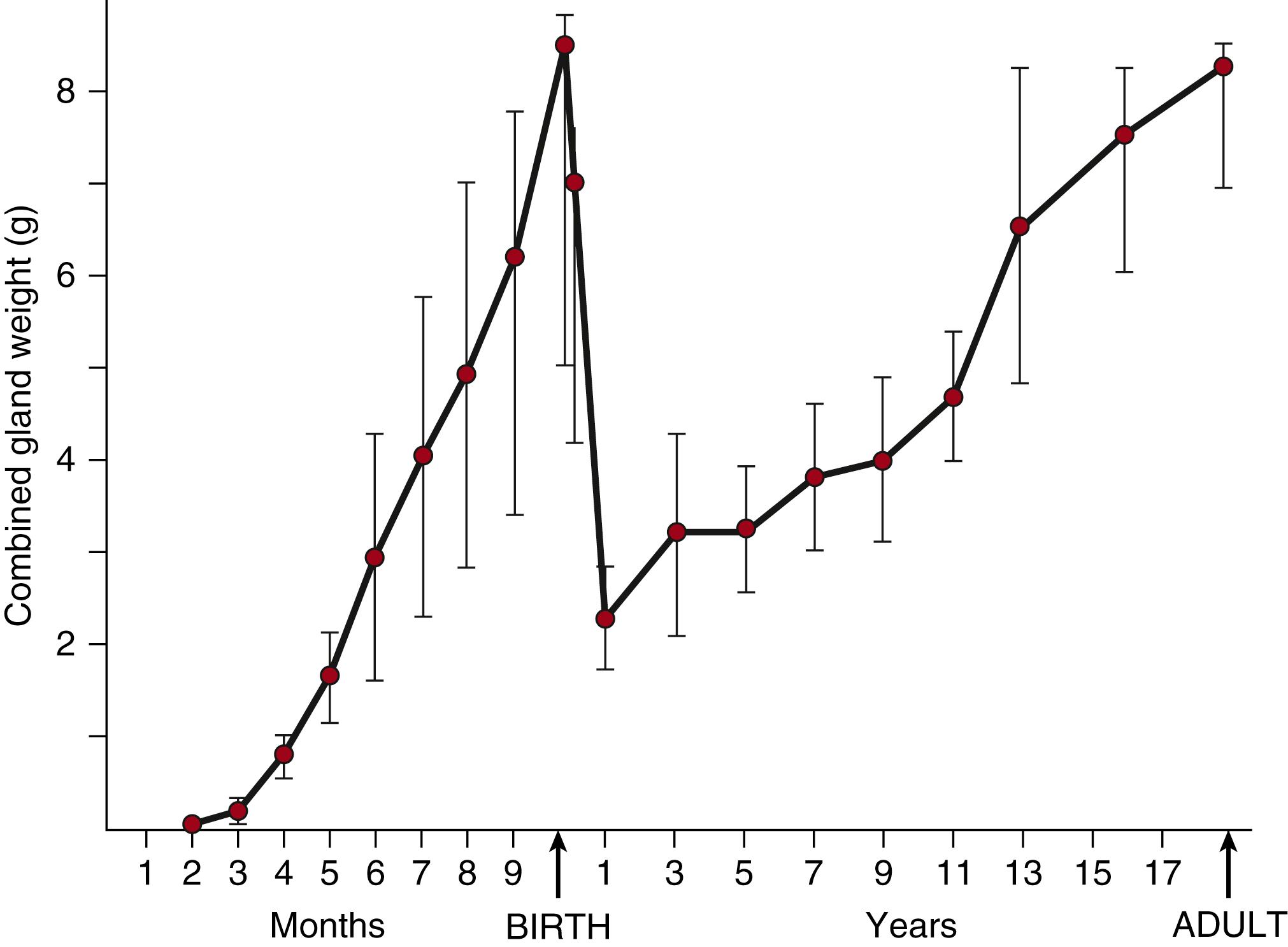
This remarkable growth during fetal life occurs through a combination of hyperplasia, hypertrophy, and reduced apoptosis. During the embryonic period, mitotic activity has been observed throughout the fetal adrenal cortex. The cortex then quickly differentiates into two distinct zones: an outer definitive zone, composed of a thin layer of tightly packed small, basophilic cells with ultrastructural characteristics consistent with cellular proliferation, and a much larger inner fetal zone, containing large, eosinophilic cells with a steroid secreting phenotype. ,
The ultrastructural appearance of these two zones led investigators to conclude that cells in the fetal zone did not divide. Using newer techniques, however, investigators have identified mitotic figures throughout the fetal zone, suggesting that it grows by hyperplasia as well as hypertrophy. Because the mitotic index in the definitive zone exceeds that in the fetal zone, however, proliferation appears to occur predominantly in the definitive zone, with new cells migrating toward the center, where they undergo hypertrophy and terminally differentiate into steroid producing cells. At the intersection of these two zones, a transitional zone appears and begins to secrete cortisol at week 22 to 24 of pregnancy. , This transitional zone expands through the latter part of gestation and becomes the cortisol-secreting zona fasciculata by term gestation; the outer, definitive zone becomes the zona glomerulosa, which secretes the mineralocorticoid aldosterone. ,
Apoptotic nuclei have been identified primarily at the center of the cortex. These nuclei are present in low but increasing numbers during gestation. After birth, maximal apoptosis is observed in the fetal zone, which subsequently disappears. Several years later, the zona reticularis will appear at the site of the earlier fetal zone and begin to secrete DHEA and DHEAS, thus regulating the onset of adrenarche.
The major secretory products of the adrenal cortex include weak androgens (DHEA and DHEAS), cortisol, and aldosterone. As shown in Fig. 144.2 , steroidogenesis requires a series of enzymatic steps, including four cytochrome P450 oxidase enzymes. Two of these are located in the mitochondria (cholesterol side-chain cleavage enzyme [P450scc] and 11β-hydroxylase [P450C11]) and two are found in the endoplasmic reticulum (17α-hydroxylase/17,20-lyase [P450C17] and 21-hydroxylase [P450C21]). The first, rate-limiting step in the synthesis of steroid hormones is the conversion of cholesterol to pregnenolone by P450scc, which is dependent on the delivery of cholesterol to the mitochondrion by the steroidogenic acute regulatory protein. Absence of this regulatory protein impairs adrenal and gonadal steroidogenesis and is clinically manifested as congenital lipoid adrenal hyperplasia. The critical branch point enzymes regulating steroidogenesis are 3β-hydroxysteroid dehydrogenase (type 2 isozyme, 3βHSD2) and P450C17. These two enzymes compete for substrate. In tissues in which 3βHSD2 is highly expressed, cortisol is produced preferentially; low 3βHSD activity in conjunction with high P450C17 activity promotes DHEA production. ,
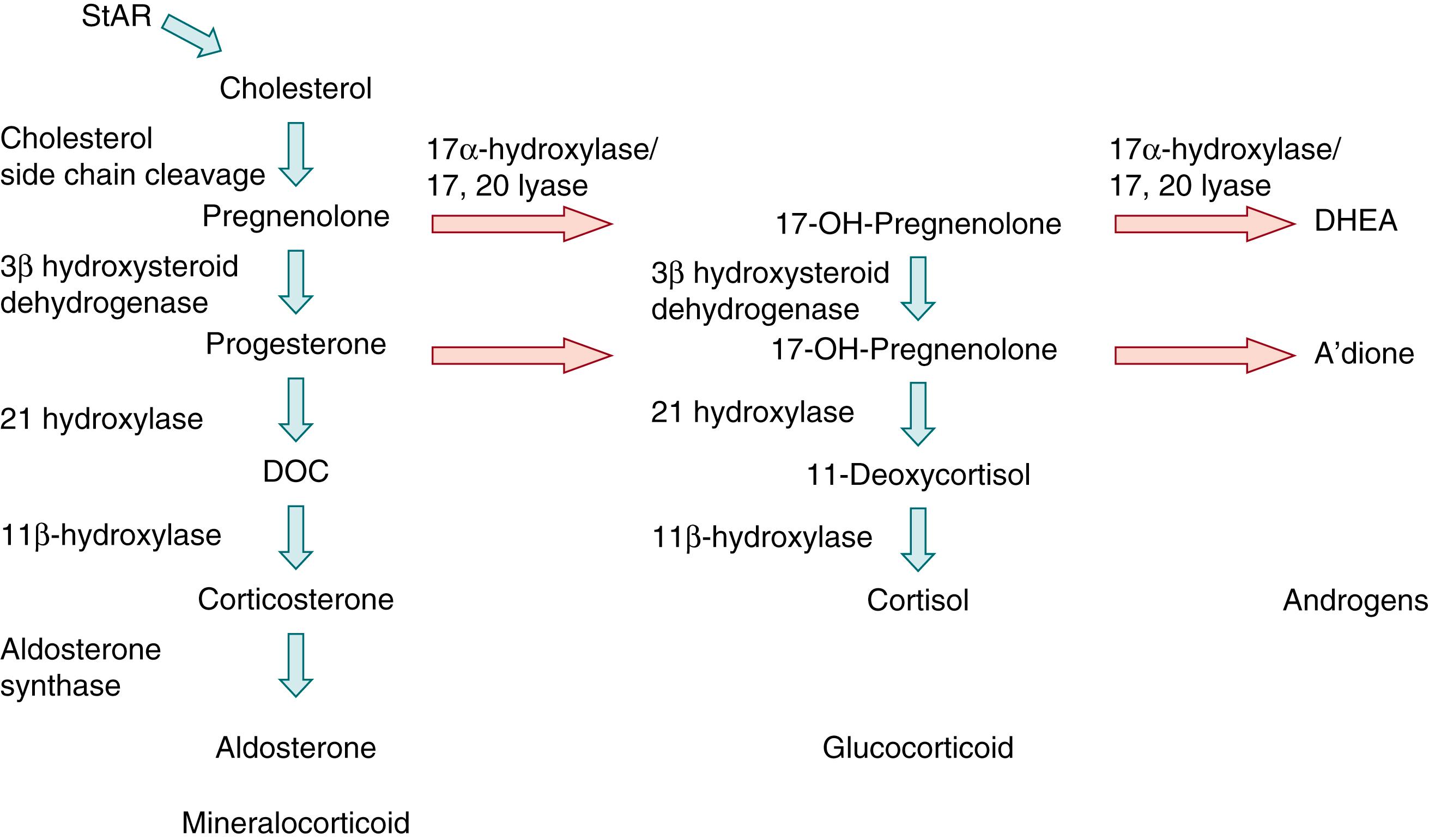
After the embryonic period, the enzymes and secretory patterns of each zone within the adrenal cortex are distinct, and the specific steroidogenic enzymes within each zone determine the type of steroid produced in that zone. , The fetal zone contains P450C17 but lacks 3βHSD2, and thus secretes DHEA and DHEAS in massive amounts—up to 200 mg/day during the third trimester. These adrenal androgens serve as substrates for placental aromatases, which convert them to the estrogens estriol, estradiol, and estrone ( Fig. 144.3 ). , This process appears to be fundamental to the normal maintenance of pregnancy, as reviewed later in this chapter.
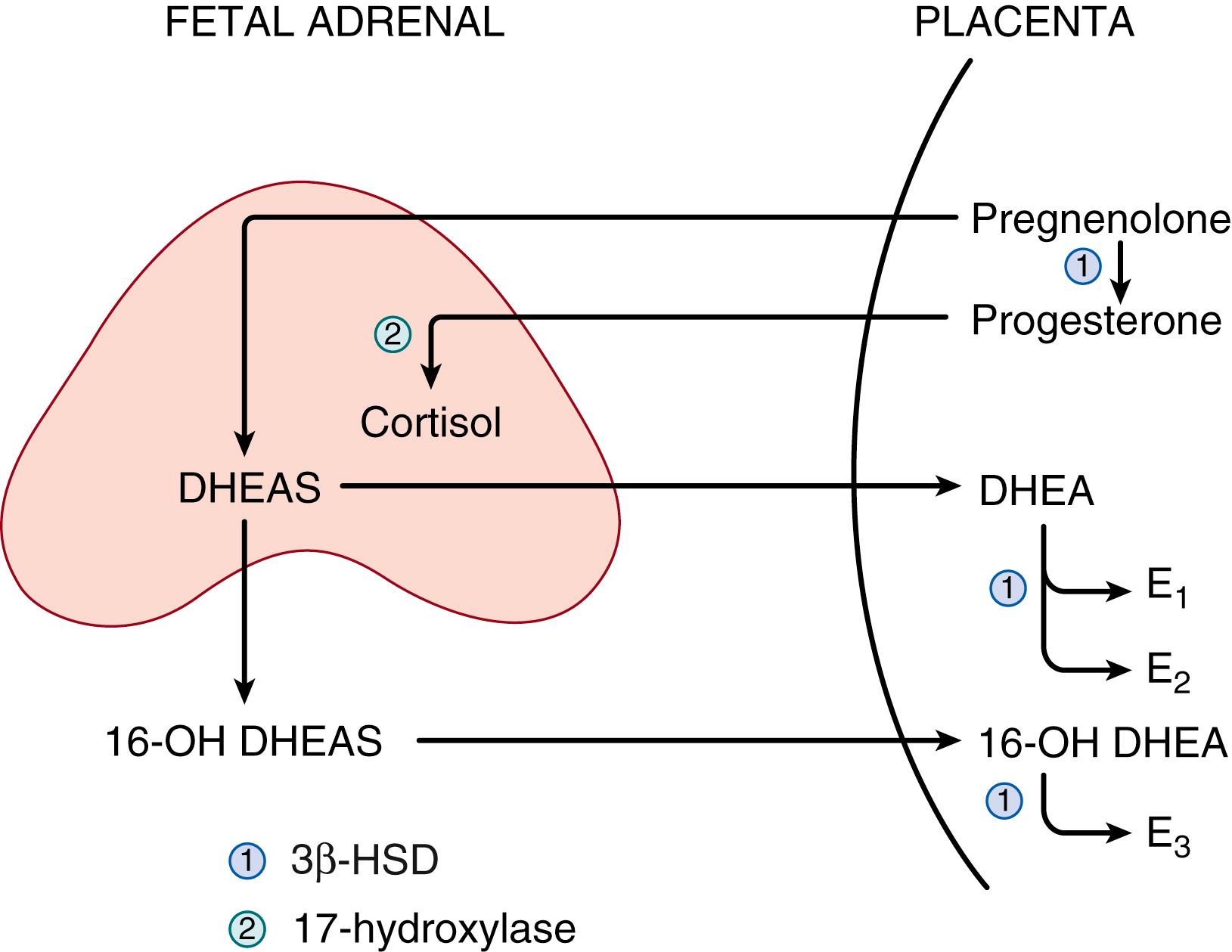
The transitional zone, which appears at 22 to 24 weeks gestation, contains 3βHSD2 and can therefore synthesize cortisol de novo from cholesterol (see Fig. 144.2 ). , Before the appearance of the transitional zone, however, cortisol may be produced by the fetus using placental progesterone as a substrate (see Fig. 144.3 ). The outer, definitive zone of the cortex also begins to express 3βHSD2 toward the end of gestation, but this zone lacks P450C17 and therefore secretes the mineralocorticoid aldosterone (see Fig. 144.2 ). , The zona reticularis, which will not become morphologically apparent until adrenarche, appears to be analogous to the fetal adrenal cortex in that it lacks 3βHSD2 and secretes DHEA and DHEAS.
Expression of these critical branch point enzymes very early in gestation, and their effects on early development have been unclear. 3βHSD2 was generally believed to be absent from the human fetal adrenal cortex in the undisturbed pregnancy until 22 to 24 weeks’ gestation. However, an immunohistochemical study found transient staining for 3βHSD in numerous definitive zone cells and occasional fetal zone cells very early in development, which disappeared by 14 weeks postconception. A subsequent study confirmed this transient expression of 3βHSD2, as well as its regulatory orphan nuclear receptor (nerve growth factor–induced clone B, or NGFIB), in human adrenal cortex during the embryonic period, and additionally documented a brief spike in adrenal cortisol content and secretion during this time ( Fig. 144.4 ). , Biosynthesis was maximal at 8 to 9 weeks postconception, after which 3βHSD immunoreactivity and cortisol content decreased quickly, declining by half at 10 weeks and to undetectable levels by 14 weeks postconception. This early, transitory cortisol production was coincidental with the detection of adrenocorticotropic hormone (ACTH) in the same fetuses. In addition, these adrenocortical cells were responsive to ACTH, strongly suggesting that even at this early stage, adrenal cortical cells are regulated by ACTH secretion.
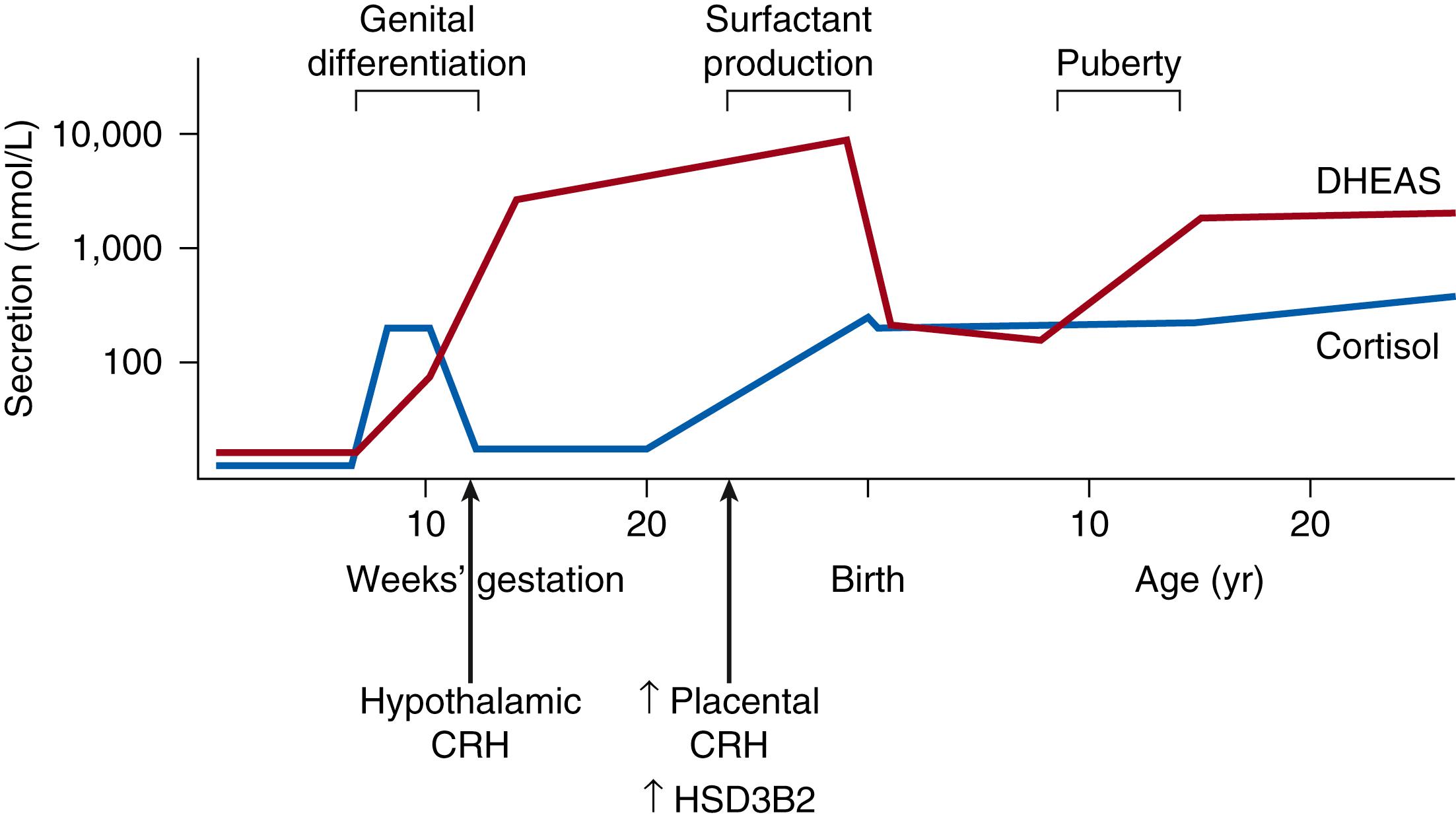
The thought-provoking work just described helps solve the puzzle of how female fetuses affected by P450C21-deficient congenital adrenal hyperplasia become virilized very early in gestation, at a time before the fetus had been thought to produce cortisol. Virilization occurs in congenital adrenal hyperplasia when the cortisol biosynthesis pathway is stimulated by ACTH, and cortisol production is blocked by the absence of P450C21 (see Fig. 144.2 ). This block in cortisol synthesis removes the normal negative feedback of cortisol to the pituitary and results in increased ACTH secretion. In this situation, continuing stimulation of the cortisol synthesis pathway leads to an accumulation of cortisol precursors, which are shunted into increased production of DHEA and DHEAS (see Fig. 144.2 ). Later in gestation, these increased fetal androgens will be converted to estrogens by placental aromatases (see Fig. 144.3 ), thus preventing accumulation in the fetus. , , At this early stage of gestation, however, placental aromatase activity is low, resulting in an accumulation of androgens and virilization of the external female genitalia during differentiation—a process that occurs at 8 to 10 weeks postconception.
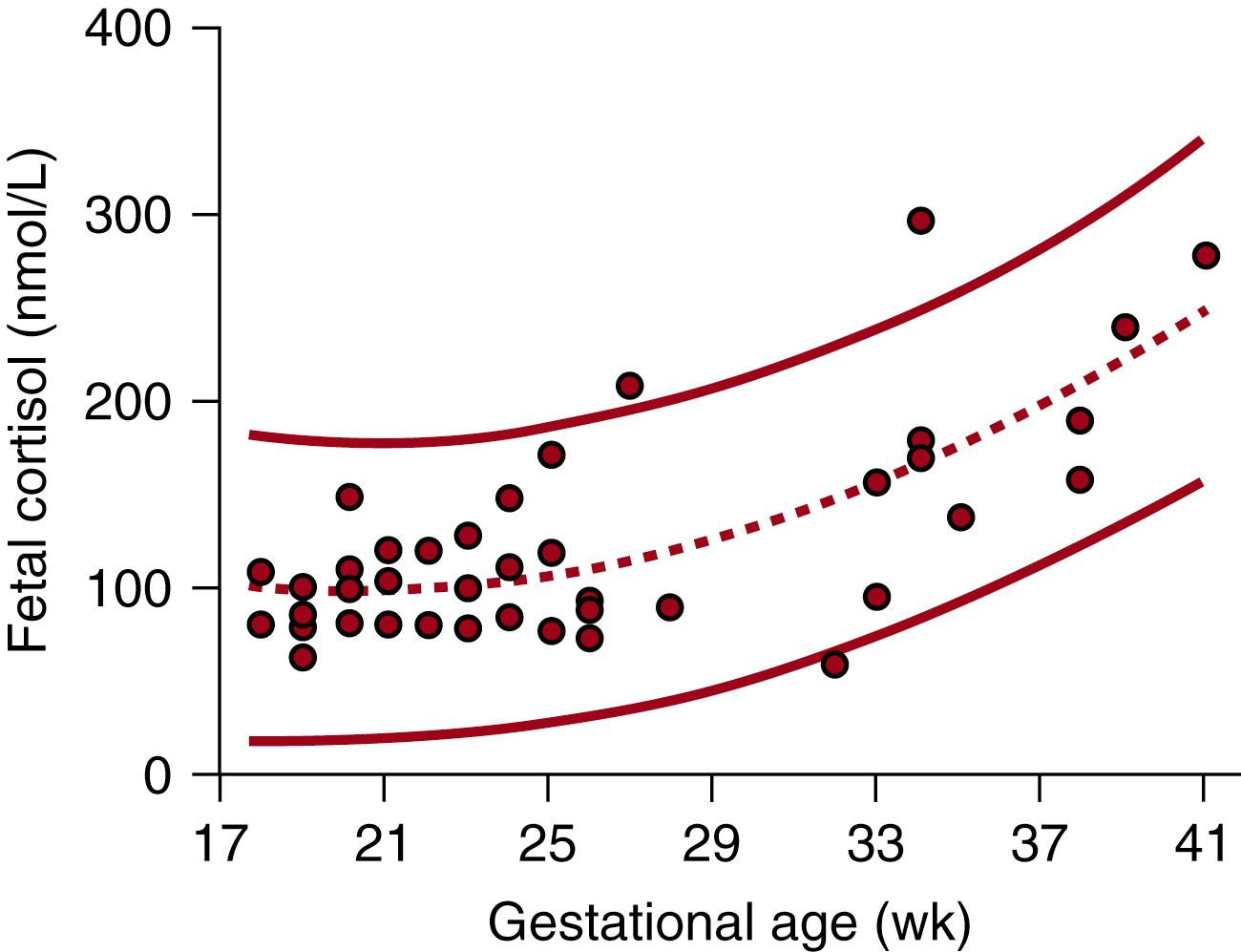
As befits an organ involved in the vital functions of maintaining the pregnancy, initiating parturition, and maturing fetal organs for extrauterine existence, the fetal adrenal cortex is regulated by an intricate web of factors from fetus, placenta, and mother. These factors combine to shield the fetus from premature exposure to excess cortisol early in gestation and then to stimulate the increased cortisol production essential for preparation for extrauterine survival. Normal fetal cortisol concentrations are low until well into the third trimester of pregnancy ( Fig. 144.5 ). The profound effects of premature exposure of the fetus to increased glucocorticoid concentrations have been well documented in animal models, including decreased body, brain, and organ weights. ,
Stimulation of the adrenal cortex by ACTH from the fetal pituitary is necessary for its normal morphologic development and steroidogenic function. The effects of a lack of ACTH in the human are demonstrated in the anencephalic fetus. Stereologic studies of adrenal cortex structure in anencephalic fetuses have shown a striking decrease in both cell number and total volume of the fetal zone and transitional zone (zona fasciculata), with less effect on the zona glomerulosa. , Although ACTH has been detected in placental tissue, outcomes such as these suggest that placental ACTH is insufficient for normal human adrenocortical growth and development.
The specific effects of ACTH on adrenocortical cells have been documented in primate models. Administration of exogenous ACTH or suppression of fetal cortisol synthesis with metyrapone results in hypertrophy of the transitional zone and early appearance of 3βHSD message in both the transitional and definitive zones. , Conversely, administration of betamethasone to suppress fetal ACTH secretion eliminates the transitional zone and decreases mRNA expression of both 3βHSD and the ACTH receptor. , Suppression of ACTH with betamethasone at mid-gestation also resulted in decreased adrenal weight and cell size, cellular disorganization, increased apoptosis, and reduction in expression of mRNA for the P450scc and P450C17 enzymes—effects that were reversed by concomitant treatment with ACTH.
The expression of the ACTH receptor in the adrenal cortex increases through mid-gestation and then decreases substantially by term. In addition, the responsiveness of the fetal and the developing transitional zones to ACTH appear to diverge during the second half of gestation. In the fetal zone, a selective decrease in ACTH receptor expression and a consequently decreased responsiveness to ACTH occurs; in the emerging transitional zone, an increase in ACTH receptor density and 3βHSD expression is seen. , The decline in ACTH responsiveness in the fetal zone may be due to suppression by increasing estrogen concentrations (see later discussion). In contrast to the effects of ACTH on the fetal and transitional zones, the outer definitive zone, the future zona glomerulosa, may be less affected by exogenous ACTH or betamethasone. This is consistent with findings in human anencephalic infants, in whom the growth of the zona glomerulosa is relatively unaffected by the absence of fetal ACTH. ,
ACTH exerts its effects on adrenal cells at least in part through local growth factors, particularly insulin-like growth factor (IGF)-2. A polypeptide growth factor important in the regulation of fetal growth, IGF-2 is highly expressed in the fetal adrenal cortex, and its expression is stimulated by ACTH. In turn, IGF-2 stimulates the proliferation of fetal adrenal cortical cells, increases steroid production, and augments the expression of steroidogenic enzymes. IGF-2 appears to act through the IGF-1 binding protein, possibly in concert with other polypeptide growth factors that have been shown to be mitogenic for human fetal adrenal cells, particularly basic fibroblast growth factor and epidermal growth factor.
Transcription of ACTH’s effect on the fetal adrenal cortex appears to be modulated by members of the nuclear receptor superfamily. Several nuclear receptors essential for human adrenal cortex development have been implicated in the transcription of ACTH’s effect, and it is likely that more will be identified. The orphan nuclear receptor steroidogenic factor (SF)-1 is expressed throughout the adrenal gland and participates in the activation of numerous enzymes in the steroid biosynthetic pathway. Activation of ACTH-dependent signaling cascades has been shown to lead to the cyclic recruitment of SF-1 and multiple rounds of transcription. Human mutations of SF-1 produce adrenal failure, indicating its importance in adrenal development. Another orphan nuclear receptor responsive to ACTH stimulation is NGFIB. NGFIB shows differential expression in the different fetal adrenal zones and may play a crucial role in functional adrenal zonation by upregulating 3βHSD2 gene transcription in the transitional and definitive zones. A third nuclear receptor, DAX-1 (dosage-sensitive sex reversal, adrenal hypoplasia congenita, critical region on X-chromosome, gene 1) appears to be an important negative modulator of ACTH-stimulated glucocorticoid synthesis, repressing SF-1–dependent activation. ACTH has been shown to downregulate DAX-1 as well as stimulate SF-1, a potential mechanism for modulating cortisol production by the adrenal cortex. Deletion or mutation of DAX-1 results in X-linked adrenal hypoplasia congenita, a disorder with profound hormonal deficiencies.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here