Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Early diagnosis is critical to maximize success with femoral head–sparing surgical procedures for osteonecrosis.
One should select the least invasive procedure possible for a given stage of osteonecrosis.
Use of core decompression or percutaneous drilling procedures is appropriate only in precollapse disease.
Success rates with femoral head–sparing procedures are much lower if more than 2 mm of femoral head collapse is present or if the necrotic lesion is large.
Certain nonarthroplasty procedures (e.g., vascularized fibular grafting) are technically complex and should not be attempted without appropriate training.
Many patients will eventually progress to femoral collapse, although nonarthroplasty procedures may delay this by a decade or longer. Nevertheless, it may be preferable to avoid procedures that could complicate future arthroplasty options.
Multiple treatment options are available for osteonecrosis of the femoral head. If the disease has progressed to severe femoral head collapse and/or acetabular damage, only a hip arthroplasty will markedly relieve pain and improve function. However, for lesions that are not as advanced, multiple joint-preserving treatments have been successfully used to improve symptoms and delay or prevent arthroplasty.
Early diagnosis and accurate staging of the disease are important for selecting the optimal treatment. Different treatment modalities are indicated for different stages of the disease, and overall success rates are higher at earlier disease stages. Therefore, it is important to understand how to make the earliest possible diagnosis based on history and physical examination and how to plan treatment based mostly on radiographic indices.
To make an early diagnosis, the clinician should understand that osteonecrosis is frequently associated with one or more risk factors, such as corticosteroid or excessive alcohol use ( Box 57.1 ), which, if present in combination with symptoms, should trigger a low threshold for radiographic evaluation. The most common clinical symptoms of osteonecrosis include deep, throbbing groin pain and limited range of motion, especially internal rotation. Pain also may be localized to the buttock, thigh, or knee. It often occurs with hip movement or weight-bearing activities, although rest pain is noted in more advanced disease. Pain usually has a gradual onset, although acute onset of symptoms may occur. Some patients have minimal pain; others are asymptomatic, irrespective of radiographic appearance.
Traumatic fracture and/or dislocation
Sickle cell disease
Radiation
Chemotherapy
Myeloproliferative disorders
Thalassemia
Caisson disease
Corticosteroid use
Alcohol abuse
Tobacco abuse
Systemic lupus erythematosus
Organ transplant
Gastrointestinal disorder
Pregnancy
Genetic inheritance
Coagulation deficiency
Radiographically, osteonecrosis has a wide range of findings; many different classification systems have been used to characterize this disease and to help stage it for diagnosis, treatment, and prognosis. For example, in one recent report, more than 23 classification systems were cited. Fortunately, most publications have described one of five commonly used systems ( Table 57.1 ). However, the present authors have found it more useful to understand four primary radiographic factors in staging and planning the most appropriate head-sparing procedure for these patients. These radiographic features include (1) precollapse or postcollapse (presence of crescent sign) femoral heads, with precollapse lesions having the best prognosis; (2) size of the lesion, with larger lesions having a worse prognosis than small or medium-sized lesions; (3) amount of femoral head depression, with greater than 2 mm appearing to be the cutoff for not using head-preserving procedures; and (4) acetabular involvement with disease, in which head-preserving procedures should not be used. Some authors have characterized the location of the lesion as important in determining prognosis and a treatment plan, as outlined by the Japanese Orthopaedic Association. For example, lateral lesions have a worse prognosis than medial lesions and central lesions are more intermediate. The present authors have found that location sometimes adds little prognostic information to the size evaluation because most lateral lesions are large and medially located lesions are usually small.
| FICAT AND ARLET | UNIVERSITY OF PENNSYLVANIA a | ASSOCIATION RESEARCH CIRCULATION OSSEOUS | JAPANESE ORTHOPAEDIC ASSOCIATION (OHZONO) b | MARCUS ET AL. | |||||
|---|---|---|---|---|---|---|---|---|---|
| Stage | Description | Stage | Description | Stage | Description | Stage | Description | Stage | Description |
| I | Normal radiographic appearance | 0 | Normal | 0 | None | 1 | Demarcation line | 1 | Mottled areas of increased density on plain radiographs |
| II | Diffuse sclerotic or cystic lesions | I | Positive findings on bone scan or magnetic resonance imaging | 1 | Normal findings on radiography or computed tomography; positive findings with at least one other technique | 2 | Early flattening without demarcation line around necrotic area | 2 | Demarcation zone of increased radiodensity surrounding lesion |
| III | Subchondral fracture | II | Diffuse sclerotic or cystic lesions | 2 | Sclerosis, osteolysis, focal porosis | 3 | Cystic lesions | 3 | Subchondral lucency (crescent sign) |
| IV | Femoral head collapse, osteoarthritis, acetabular changes | III | Stop-off in contour of subchondral bone | 3 | Crescent sign and/or flattening of articular surface | 4 | Flattening of femoral head | ||
| IV | Flattening of femoral head | 4 | Osteoarthritis, acetabular changes | 5 | Flattening and compression of necrotic zone; narrowing of joint space | ||||
| V | Joint narrowing or acetabular changes | 6 | Progressive erosion of femoral head; degenerative arthritis | ||||||
| VI | Advanced degenerative changes | ||||||||
a Further stratified as A, B, or C, depending on severity.
b Stratified by medial to lateral weight-bearing area involvement.
In the following description of various head-preserving procedures, we will discuss where different procedures are used based on these four radiographic features. In addition, if the clinician is reviewing the literature, these features can easily be converted into different classification systems.
To analyze these radiographic features, it is necessary only to get good-quality anteroposterior and frog-leg lateral radiographs, as well as magnetic resonance imaging (MRI) scans. When the disease is obvious (e.g., postcollapse disease with a crescent sign), it may not even be necessary to obtain MRI evaluation. However, MRI examinations are more than 99% sensitive and specific for the disease, and rapid low-cost screening protocols have been developed that take approximately 15 minutes to perform. Other screening tests—such as bone scans, computed tomography (CT) scans, and bone biopsies—although of historical importance, are not necessary for the diagnosis of osteonecrosis unless a patient has a contraindication to undergoing MRI. For example, in one recent study, bone scanning missed 44% of symptomatic osteonecrotic lesions in 48 patients who were otherwise diagnosed with MRI. CT scanning is not recommended because it exposes patients to unnecessary radiation risks.
Once osteonecrosis has been diagnosed and characterized radiographically, a treatment plan can be proposed. In the authors’ opinion, the radiographic evaluation is of paramount importance over other demographic factors in formulating this strategy. However, one may occasionally consider various demographic factors, such as patient age or morbidity, in deciding on a procedure. For example, one would be more likely to try a head-preserving procedure in an 18-year-old patient versus a 62-year-old patient who might be served better with a hip arthroplasty procedure. In addition, patients who have many comorbidities may not want to risk a procedure with a higher risk of failure necessitating reoperation and may opt for a more definitive procedure. With these considerations in mind, the various nonarthroplasty procedures that have been used to treat osteonecrosis of the femoral head will be discussed, including indications and contraindications for each procedure, the surgical technique and relevant variations, postoperative care, results, and complications.
Core decompression is a surgical technique that involves removing a cylindrical core of bone from the proximal femur, creating a tract that extends from the subchondral region of the femoral head to the extraosseous space adjacent to the lateral proximal femur. This technique was originally described by Ficat and Arlet and by Hungerford as a method of diagnosis by histologic examination of removed bone and as a method of pain reduction. This procedure is no longer necessary for the diagnosis of osteonecrosis because MRI is currently the preferred modality. Core decompression continues to be used for pain reduction and to slow the progression of disease, although some controversy continues regarding the degree to which the procedure influences the natural course of this disease. It is believed that bone removal may reduce intramedullary pressure caused by various factors such as increased adipocyte density or venous congestion within the bone, which are associated with osteonecrosis. Multiple animal and human studies have reported neovascularization and improved osseous blood flow following core decompression. This has been described as a simple, low-morbidity method of treating osteonecrosis, although some studies have reported complication rates of 10% or higher.
Core decompression may be used in patients who have precollapse osteonecrosis of the femoral head. Success rates are highest in patients who have Ficat and Arlet stage I disease (changes visible on MRI only) and in those who have smaller lesions encompassing less than 25% of the volume of the femoral head. However, the procedure has been demonstrated to slow or arrest progression in Ficat and Arlet stage II and III disease, albeit less frequently. Because of the minimally invasive nature of the procedure and the low associated morbidity, especially when percutaneous drilling techniques are used, core decompression may be an excellent first choice of surgical treatment for osteonecrosis in many patients with precollapse disease. It should not be used for patients with advanced postcollapse disease or very large stage II and III lesions that have a high risk of progression or have already collapsed.
Little preoperative planning is necessary for this procedure. A recent magnetic resonance study of both hips should be obtained and examined to determine the size and location of the osteonecrotic lesion and to evaluate for lesions in the contralateral hip. Bilateral osteonecrosis of the hips occurs in up to 85% of patients, and most asymptomatic hips progress to symptomatic disease and/or femoral head collapse. Given the low morbidity of core decompression, surgeons may consider decompressing both hips if asymptomatic disease of the contralateral joint is identified on MRI. In addition to the necessary instruments, surgeons should ensure that intraoperative fluoroscopy and a radiolucent table are available for the procedure.
Core decompression has traditionally been performed with trephines or cannulated drill bits that have diameters of between 5 and 10 mm. These instruments are driven through the femoral neck into the head under fluoroscopic guidance. Various materials, such as bone graft and osteogenic proteins, may be placed into the tracts or the channels may be left open. Complications can occur if the drill hole is started too distally relative to the femoral metaphysis, typically distal to the lesser trochanter, which may result in a subtrochanteric fracture. Additionally, the drill hole may be extended too far, damaging the articular cartilage and leading to loose bodies within the joint capsule.
Position the patient supine on a fracture table.
Using fluoroscopy as a guide, make a 2- to 3-cm longitudinal incision in the midlateral thigh at the level of the lesser trochanter and split the fascia lata in the direction of its fibers.
Using a cannulated drill or cortical reamer, create a 10-mm window in the lateral cortex of the femur at the level of the superior margin of the lesser trochanter.
Introduce an 8- to 10-mm trephine through the cortical window over a Kirschner (K) wire, and use image guidance to drive the trephine medially and proximally up the femoral neck toward the center of the lesion, with prior MRI or plain film radiographs as a guide to determine optimal positioning.
Use image intensification to track progress of the trephine in both anteroposterior and frog-leg lateral projections, ensuring that the subchondral bone are not breached, and that the tip does not penetrate the subchondral bone and articular surface of the femoral head ( Fig. 57.1 ).
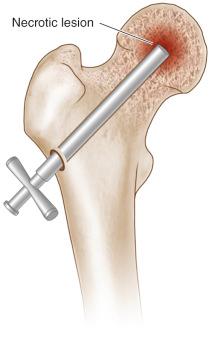
Once the lesion has been reached, the trephine is withdrawn along with the K wire and a core of trabecular bone ( Fig. 57.2 ).
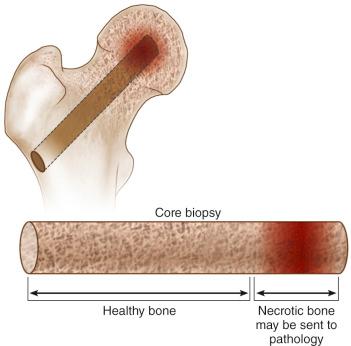
Once the procedure is complete, the wound is closed in layers, with one or two sutures used to close the skin incision.
A newer modified core decompression technique, which is really a percutaneous drilling procedure, has been described by Song and associates and Mont and colleagues. It involves using a smaller-diameter drill bit or a Steinman pin (2 to 3 mm) inserted percutaneously to create the core tracts. Multiple drillings are performed in an attempt to sufficiently decompress the diseased region. In addition, bone matrix or growth factors can be placed into the tracts. Potential advantages of this technique include percutaneous insertion rather than a surgical incision, shorter surgical time, and less tissue damage. In addition, this technique may be associated with a lower risk of fracture or collapse.
Position the patient supine on the fracture table.
Under fluoroscopic guidance, insert a 3.2- to 3.4-mm Steinman pin percutaneously into the lateral thigh at the level of the superior margin of the lesser trochanter.
Keeping the Steinman pin perpendicular to the femoral shaft, penetrate the lateral cortex and enter the intramedullary canal.
Angle the Steinman pin proximally and medially parallel to the neck of the femur. Advance the pin through the medullary canal toward the lesion in the femoral head, checking progress using fluoroscopy in both anteroposterior and lateral views to ensure that the subchondral bone and articular surface are not breached.
Extend the tract to the subchondral bone, taking care to not breach the articular surface.
For small and medium-sized lesions, two passes are made into the diseased area (previously identified using MRI or plain film radiographs), using a single common entry point in the lateral cortex. The pin should not be withdrawn from the wound between passes. The authors make three passes for large lesions.
The Steinman pin is withdrawn, and the entry point into the skin is covered with an adhesive bandage.
Core decompression is a relatively simple and safe procedure that can be used in virtually all patients with the appropriate indications. Use of intraoperative fluoroscopy to guide the path of the intraosseous channel allows accommodation of virtually any of the common anatomic variations of the proximal femur, such as hip dysplasia, slipped capital femoral epiphysis, posttraumatic anatomic changes, or a pistol grip deformity of the proximal femur. Regardless of the femoral anatomy, surgeons should ensure that the femoral subchondral bone and articular surface are not penetrated, and that the decompression tract reaches the lesion in the subchondral space.
Steinberg and coworkers described a variation of the modified Hungerford technique. After the initial core tract is made, two additional passes are made into other areas of the necrotic lesion through the same cortical entry point, using 5- or 6-mm trephines. Then, the large-diameter trephine is reintroduced with the aid of a trocar and is used as a guide to loosely place morselized cancellous bone and/or bone growth–stimulating substances into the decompressed lesion. The rationale for this procedure as described by the authors was to stimulate revascularization and bone growth.
Recommended postoperative care has been similar regardless of the decompression technique used. Patients are typically discharged home on the same day of the procedure or on the following morning. They are mobilized before discharge, using a single crutch or cane in the contralateral hand. In cases of bilateral hip decompressions, two crutches or canes are used. Patients are advised to maintain partial weight bearing until a follow-up visit at approximately 6 weeks after the procedure. Patients are then allowed to progress to full weight bearing as tolerated but are counseled to avoid high-impact activities for 12 months and are encouraged to perform regular home-based abductor-strengthening exercises. One year after the core decompression procedure is performed, patients are evaluated clinically and radiographically for disease progression and/or femoral head collapse. If no evidence of disease progression is found, all restrictions are lifted, although patients are encouraged to return for regular annual follow-up examinations to monitor for radiographic signs of delayed disease progression.
Over the past several decades, considerable variability has been noted in reported results of various decompression techniques for osteonecrosis of the femoral head. The literature includes a paucity of well-designed comparative studies and considerable heterogeneity in study populations in terms of lesion size, location, and radiographic stage. Reporting of results is further complicated by differences in the definition of successful and/or failed treatment, with different authors alternately measuring success as preservation of the native joint, no radiographic progression of disease, or no symptomatic progression.
Overall results of core decompression reported over the last decade as measured by the avoidance of further surgery range from 42% to 86% at mean follow-up times from 24 to 94 months, with most studies reporting survival rates in the range of 70% to 85% over this period ( Table 57.2 ). Success rates as measured by an absence of radiographic progression are more variable and somewhat more modest, ranging from 30% to 86% over a similar range of follow-up times. Several studies have consistently reported that superior survival rates are seen in earlier radiographic stages of the disease (stages I and II) and with smaller, more medially located lesions.
| Author/Year | Technique | No. Hips | Months Follow-Up (Range) | Additional Surgery, n (%) | Radiographic Failure, n (%) |
|---|---|---|---|---|---|
| Chen et al./2000 | Core | 27 | 28 (12–128) | — | 10 (37) |
| Lavernia and Sierra/2000 | Core | 67 | 41 | 16 (24) | — |
| Maniwa et al./2000 | Core | 26 | 94 (53–164) | 8 (31) | — |
| Specchiulli et al./2000 | Core | 20 | 67 | 4 (20) | 4 (20) |
| Piperkovski/2001 | Core | 39 | 48 | 4 (8) | — |
| Yoon et al./2001 | Core | 39 | 61 (24–118) | 19 (49) | — |
| Aigner et al./2002 | Core | 45 | 69 (31–120) | 7 (16) | 12 (27) |
| Hernigou et al./2003 | Core | 189 | 84 (60–132) | 34 (18) | 39 (21) |
| Wirtz et al./2003 | Core | 51 | (36–132) | 18 (35) | — |
| Gangji et al./2004 | Core | 8 | 24 | 2 (25) | 5 (63) |
| Lieberman et al./2004 | Core | 17 | 53 (26–94) | 3 (18) | 3 (18) |
| Bellot et al./2005 | Core | 31 | (1–176) | 19 (61) | 19 (61) |
| Ha et al./2006 | Core | 18 | (50–96) | — | 14 (78) |
| Neumayr et al./2006 | Core | 17 | 36 | 3 (18) | — |
| Marker et al./2008 | Percutaneous | 79 | 24 (20–39) | 27 (34) | 27 (34) |
| Song et al./2007 | Percutaneous | 163 | 87 (60–134) | 50 (31) | — |
| Wang et al./2009 | Percutaneous | 59 | 28 (12–40) | 7 (12) | 14 (24) |
| Yang et al./2010 | Core | 22 | 36 (–) | 10 (45%) | — |
| Mukisi-Makaza et al./2009 | Core | 42 | 132 (–) | 10 (24%) | — |
| Zhao et al./2012 | Core | 44 | — (6–60) | 10 (23%) | — |
| Abrisham et al./2013 | Core | 37 | — | 5 (14%) | |
| Al Omran et al./2013 | Core | 94 | — | 11 (12%) | — |
Some authors raise concerns about the degree, if any, to which core decompression affects the natural course of the disease, noting that many small early-stage lesions do not progress, even without surgical treatment. One well-controlled study did not confirm an effect with core decompression. However, several comparative studies and literature reviews have demonstrated consistently poorer survival rates for hips treated nonoperatively and overall failure rates are approximately double those seen with core decompression.
A comparison of traditional core versus multiple drilling at 3-year follow-up found no outcome differences in precollapse disease; 67% (32 of 48 of traditional), and 72% (18 of 25 of drilling) patients required no further operation. Using multiple drilling, survival in 93 of 120 hips (78%) at 5-year mean follow-up was found. No further surgery was found at a 7.2-year mean follow-up for 88% (52 of 59) of small- to medium-sized lesions. These many recent publications support multiple drilling as an appropriate core decompression alternative.
However, there may be other factors responsible for the results of core decompression. It has been combined with arthroscopy, tantalum rods, calcium bone graft substitutes, platelet-rich plasma, cell-based therapies, and adjunctive therapies (e.g., bisphosphonates, extracorporeal shockwave therapy [ESWT], iloprost). Arthroscopy-assisted decompression may help evaluate articular cartilage, degree of collapse, and labral pathology, plus provide reaming guidance.
In summary, although a number of patients treated with core decompression eventually do progress to further surgical treatment, this procedure is minimally invasive (especially when performed using the percutaneous small-diameter technique), can be performed quickly with minimal technical requirements, has very low morbidity, provides immediate symptomatic relief in the large majority of patients, and may successfully delay further surgical treatment for many years in many patients who otherwise are offered much larger procedures, if not joint arthroplasty. For these reasons, the authors advocate the use of percutaneous core decompression for the treatment of symptomatic precollapse small to medium-sized lesions.
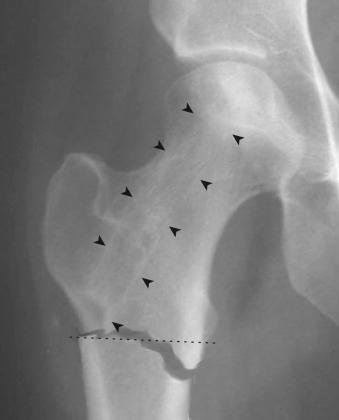
The principal risks of core decompression are related to the anesthesia of the surgery, as the procedure itself has few complications. Authors experienced in core decompression typically report complication rates well below 1%. Patients treated with large-diameter trephine core decompression are at risk for proximal femur fracture should a fall occur in the early postoperative period, when the proximal femur is structurally weakened and the bone has not yet fully healed ( Fig. 57.3 ). Improper placement of the trephine in the subtrochanteric diaphysis of the femur can account for most fractures. However, the rate of these complications is low and can be further minimized by ensuring that an appropriate entry point is used in the metaphysis opposite to the proximal margin of the lesser trochanter and that a period of postoperative protected weight bearing is strictly followed.
Bone grafting is a more invasive procedure than core decompression; thus, it is typically used for patients who have failed core decompression or who would not be appropriate candidates for core decompression owing to large lesions or more advanced stages of disease. The general procedure for bone grafting involves soft tissue dissection to access the femur, removal of a window of cortical bone to access the intramedullary space, debridement of the necrotic bone, placement of the bone graft material, replacement of the bone window, and wound closure. Various types of bone grafting material can be used, including nonvascularized iliac crest, fibula, or tibia autograft or allograft; synthetic bone graft material, such as demineralized bone matrix and corticocancellous bone chips; or vascularized fibular or muscle-pedicle autograft. In addition, biologics such as bone marrow aspirate concentrate and bone morphogenetic proteins 2 and 7 have been added to the graft material in an attempt to stimulate new bone growth. Although a number of procedures have been described, they can be broadly divided into two categories: nonvascularized and vascularized bone grafting. Additionally, a few recent reports have described implantation of nonbiologic porous tantalum metal rods, which may act like a structural bone graft. The rationale for bone grafting is multifold: (1) The technique provides a mechanism to decompress the femoral head, reducing intramedullary pressure; (2) it removes some or all of the necrotic bone, eliminates a source of inflammation, and provides a more suitable environment for the growth of viable bone; (3) it provides structural support with bone graft for the articular surface to prevent further collapse; and (4) it provides a mechanism for the placement of bone growth factors to stimulate healing. An additional rationale for the use of vascularized bone grafting is to introduce healthy viable bone into the lesion as a replacement for necrotic tissue.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here