Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Steatosis, defined as the accumulation of triacylglycerides in hepatocytes, is a frequent finding in most liver biopsies. Based on magnetic resonance measures of lipid signal in European normal-weight healthy adult volunteers, the normal liver contains approximately 3% to 5% lipid, with men having slightly more lipid than women. By convention, histologically evident steatosis that occupies more than 5% of hepatocytes is considered pathological.
Macrovesicular steatosis is an accumulation of lipid droplets of various sizes within hepatocytes (large and small droplet). Cell nuclei may be displaced peripherally when the droplets are large in size. It is common to observe a mixture of small- and large-sized droplets in the same cell and to find isolated droplets of lipid that do not fill the cell in its entirety.
Microvesicular steatosis has a different histological appearance, and clinical significance. The nuclei of affected hepatocytes are typically centrally located within abundant, foamy-appearing cytoplasm. Special stains, such as Oil Red O, may be necessary to confirm lipid accumulation, whereas in macrovesicular steatosis, the large- and small-sized lipid droplets are easily detectable on routine hematoxylin and eosin stain. Diseases associated with microvesicular steatosis are characterized by hepatic failure and lactic acidosis, rather than chronicity and potential fibrosis; the former features are the result of mitochondrial injury. The most common examples of purely microvesicular steatosis are acute fatty liver of pregnancy and Reye’s syndrome. This process has also been reported in the setting of drug toxicity. Drug-related entities are discussed specifically in Chapter 49 .
Alcohol-induced liver disease (ALD) refers to the spectrum of liver disorders directly related to excessive alcohol use. This chapter focuses primarily on the pathological lesions of the ALD subset characterized by accumulation of lipid in the liver parenchyma.
Nonalcoholic fatty liver disease (NAFLD) occurs in patients without significant alcohol use. This clinicopathological entity is characterized by a variety of hepatic parenchymal injury patterns associated with more than 5% of the liver involved with macrovesicular steatosis.
The term nonalcoholic steatohepatitis (NASH) occurs in the same clinical setting as NAFLD, but NASH is a specific pattern of injury in the liver. In addition to revealing a variable amount of macrovesicular steatosis, there is also associated hepatocellular injury (most commonly seen as ballooning degeneration of hepatocytes), inflammation in the hepatic lobules or portal tracts, or both. These lesions and others that may be seen in steatohepatitis, are discussed in detail in this chapter.
The term ALD refers to a spectrum of liver diseases and includes either large-droplet or a mixture of both large- and small-droplet macrovesicular steatosis, either with or without lobular and portal inflammation; steatohepatitis with or without fibrosis; alcoholic hepatitis; alcoholic cirrhosis either with or without steatosis, steatohepatitis, or alcoholic hepatitis; and alcoholic foamy degeneration. Hepatocellular carcinoma (HCC) is a recognized consequence of ALD and cirrhosis.
NAFLD has a more limited pathological spectrum than ALD. NAFLD may manifest as either pure large droplet or a mixed large- and small-droplet macrovesicular steatosis, either with or without lobular or portal inflammation; steatohepatitis with or without fibrosis; and cirrhosis with or without steatosis or steatohepatitis. All of the features of NAFLD may be seen in ALD; therefore the term nonalcoholic fatty liver disease was introduced to distinguish these entities. , HCC may arise as a consequence of NAFLD-induced cirrhosis and is therefore also included within the spectrum of NAFLD lesions. HCC is increasingly recognized in patients with noncirrhotic NAFLD. , However, the true incidence of this neoplastic complication in cirrhotic or noncirrhotic livers is less certain than with ALD.
Although NASH and NAFLD are commonly used terms, neither truly represents the underlying pathophysiological disease process. , Although there are histological similarities with ALD, there are also differences. The relationship between NAFLD and alcohol use may not be as straightforward as originally believed because a few studies indicate that some patients may be at risk for liver disease from both causes simultaneously. Although modest alcohol use may have an effect on insulin resistance, , the presence of any potential benefits remains controversial. ,
For pathologists, microscopically based diagnoses related to fatty liver diseases are descriptive ( steatosis or steatohepatitis ) because in most circumstances, only careful clinical evaluation can help determine the true underlying cause of the patient’s illness. Different clinical situations may result in similar histological findings, and as with chronic hepatitis, knowledge of the patient’s history is essential to establish an accurate etiology. Unlike chronic hepatitis, there are no serological tests available to diagnose NAFLD with complete certainty, although improvements continue to be made in methods of noninvasive evaluation.
The clinical features of ALD vary considerably. Table 50.1 highlights the primary clinical manifestations of the four main clinicopathological subtypes of ALD: fatty liver, alcoholic hepatitis, alcoholic cirrhosis, and alcoholic foamy degeneration.
| Symptoms and Signs | Fatty Liver | Alcoholic Hepatitis | Cirrhosis | Foamy Degeneration |
|---|---|---|---|---|
| Asymptomatic | +++ | + | Portal hypertension | − |
| Right upper quadrant discomfort | +++ | Pain, not mild | + to ++ | + |
| Hepatomegaly | ++ | ++ | + to ++ | + |
| Elevated AST, normal ALT | ++ | + | + (normal if abstinent) | ++ |
| AST/ALT ratio | AST > ALT | AST/ALT >1-3 common | AST/ALT >1 | AST > ALT |
| Marked AST elevation | <5 times normal | As much as 5 times normal | − | ++ |
| Marked alkaline phosphatase elevation | − | +/− | − | ++ |
| Bilirubin elevation | − | >10 mg/dL | − (unless advanced) | ++ |
| Jaundice | − | Cardinal sign | As above | ++ |
| Elevated white blood cell count | − | >10,000/mm 3 | − (may be decreased) | − |
| Fever | − | +++ | + (with infection) | − |
| Ascites | − | +++ | + (advanced) | − |
Patients with ALD and fatty liver may be asymptomatic or may have right upper quadrant discomfort at presentation (see Table 50.1 ). Patients with alcoholic hepatitis usually have abdominal pain and pancreatitis, whereas patients with cirrhosis and alcoholic foamy degeneration often experience only vague abdominal discomfort. In all forms of ALD, the liver is typically enlarged. Fever, an elevated white blood cell count, and jaundice occur in various degrees with the different forms of ALD and may be related to ALD (e.g., alcoholic hepatitis) or to the presence of a secondary infection (e.g., peritonitis caused by alcoholic cirrhosis).
At clinical presentation, patients may have elevated serum levels of aminotransferases (i.e., aspartate aminotransferase [AST] and alanine aminotransferase [ALT]). A ratio of AST to ALT greater than 1 is useful in the evaluation of patients with ALD. AST levels are typically greater than ALT levels in all forms of ALD. Radiographic evaluation plays a role in screening for HCC in patients with cirrhosis and in the clinical care of critically ill patients with pancreatitis and suspected intestinal ileus.
Worldwide, ALD has no social or ethnic barriers, but it is uncommon in very young and very elderly individuals. Both genders may be affected, although women are at higher risk. In the United States, 7.4% of the population meet the criteria for alcohol dependence or abuse. It is the leading cause of end-stage liver disease and liver-related mortality. It follows only heart disease and cancer as the major health issue in the United States. The reported rates of per capita liters of alcohol consumed per year by individuals older than 15 years of age varies throughout the world: 13.9 in the Russian Federation; 12.9 in Germany, France, and the United Kingdom; 8.5 to 9.3 in North America, Japan, and Australia; 5.0 in China, the Philippines, and Vietnam; 1.3 in Iran and Saudi Arabia; and 0.6 in Afghanistan and Pakistan. Alcohol use may combine synergistically with other forms of chronic liver disease to generate progressive disease, malignancy, and morbidity.
Family, twin, and ethnic studies have confirmed genetic susceptibility to ALD. Factors under investigation include gender, genes involved in the metabolism of alcohol, and other enzymes involved in the pathways of oxidative stress, various cytokines (e.g., tumor necrosis factor-α [TNF-α], interleukin-10 [IL-10]), genes involved in the oxidation of hepatic fat, mechanisms of matrix deposition and degradation in fibrosis, and immune reactions to endotoxin and toxic adducts.
Liver disease does not develop in all individuals who abuse alcohol. The risk for ALD, even among heavy drinkers, is less than 10% to 15%. Known risk factors include the amount of alcohol consumed during a 10- to 20-year period (>60 g/day for men; 20 to 30 g/day for women), gender (female > male), alcohol consumption at an early age (<14 years), central obesity, patterns of consumption (nonmealtime > mealtime; daily > weekend only; various types of beverages rather than one type), associated medications (acetaminophen), amount of coffee intake, and genes that regulate expression of proinflammatory cytokines and immune response mechanisms. , A sequence variation in the patatin-like phospholipase 3 (PNPLA3) gene, which encodes adiponutrin, is associated with the development of cirrhosis in patients with European or Native American ancestry. , Genome-wide association studies have also linked two other genes to risk of alcoholic cirrhosis: membrane bound O-acyltransferase domain-containing 7 (MBOAT7) and transmembrane 6 superfamily member 2 (TM6SF2). PNPLA3 and TM6SF2 have also been associated with features of progressive disease in NAFLD patients, suggesting shared pathways of injury.
Alcohol-induced liver damage is a multifactorial process that involves alterations of the gut, gut microbiota, and hepatocellular metabolic pathways as well as activation of the innate and adaptive immune systems. Resultant outcomes are activation of proinflammatory and profibrogenic molecular mechanisms in specific liver cells.
The primary mechanism of alcohol-induced liver injury is hepatocellular oxidative stress. , Ethanol is metabolized to acetate in the liver mainly by two enzyme systems: alcohol and aldehyde dehydrogenases, which produce acetate by sequential oxidation of ethanol and the microsomal ethanol-oxidizing system (MEOS). The direct hepatotoxic effects of alcohol are caused primarily by acetaldehyde. Oxidative stress and free radical production result in mitochondrial damage, depletion of the antioxidants such as reduced glutathione, toxicity by free radicals, and induction of lipid peroxidation. Acetaldehyde-formed toxic adducts bind to proteins and lead to additional hepatocellular injury, serve as neoantigens for initiation of the adaptive immune response, and promote collagen production by hepatic stellate cells. The net effect of acetaldehyde production in hepatocytes is the accumulation of intracellular proteins, lipids, water, and electrolytes with the loss of the hepatocyte structural keratins 8 (K8) and 18 (K18). Histologically, there is cellular enlargement, ballooning, empty-appearing cells, steatosis, and loss of immunoreactivity for K8 and K18.
The key enzyme of the MEOS is the ethanol-inducible CYP2E1, which is located primarily in the endoplasmic reticulum of acinar zone 3 hepatocytes. Activation of this enzyme system results in the production of reactive oxygen species, such as hydrogen peroxide (H 2 O 2 ) and superoxide anions (O 2 − ), which causes further lipid peroxidation of cell membranes. This enzyme system also metabolizes acetaminophen and produces the toxic metabolite N -acetyl- p -benzoquinone imine (NAPQI). Even small amounts of acetaminophen may augment depletion of the antioxidant capabilities of the liver in chronic alcoholism, which may relate to the increased risk of acute liver failure in this situation.
Other sources of ethanol-induced oxidative stress include endotoxin-activated Kupffer cells, functionally impaired mitochondria, and ferric iron accumulation. Oxidative stress promotes hepatocellular injury, apoptosis, and necrosis; proinflammatory cytokine production (e.g., TNF-α, IL-1, IL-6, IL-17); and parenchymal inflammation with polymorphonuclear leukocytes (PMNs) and mononuclear cells and perisinusoidal fibrosis. , ,
Excessive alcohol intake leads to increased gut permeability and increased portal venous exposure to gut-derived endotoxins. This process results in Kupffer cell activation through the CD14/toll-like receptor 4 (TLR4) complex and activation of the innate immune response. , Alcohol consumption also leads to alterations in gut microbiota, reducing populations of beneficial bacteria and inducing dysbiosis. ,
Increased lactate production, another downstream effect of ethanol metabolism, manifests as hyperuricemia and impaired carbohydrate metabolism. Hypoglycemia may occur. Chronic alcohol use impairs protein production and secretion. Multiple aberrations of lipid metabolism result in increased triglyceride production and subsequent accumulation (i.e., steatosis).
The mechanisms of fibrosis are under active scientific investigation. Ultimately, there is an imbalance between collagen deposition and degradation by activated hepatic stellate cells and portal myofibroblasts. Unique to ALD is the additional involvement of hepatocytes and sinusoidal endothelial cells in fibrogenesis through the production of transforming growth factor-β (TGF-β) and fibronectin isoforms. ,
Grossly, steatosis enlarges the liver and imparts a greasy appearance. Livers with advanced fibrosis or cirrhosis may be enlarged and firm, and they contain micronodules (≤3 mm in diameter) scattered throughout the parenchyma. In some cases, fibrotic livers may be small and firm. With time, the nodules may coalesce to form larger ones, at which point determination of the underlying cause may not be possible. HCCs often stand out on a background of cirrhosis as raised, green-tinged or darker nodules, typically larger than 8 mm in diameter.
The histological pattern of tissue injury in patients with alcohol-induced fatty liver disease initially involves acinar zone 3 (roughly equivalent to perivenular) hepatocytes. In patients with cirrhosis, steatosis or steatohepatitis may or may not persist.
Steatosis, the earliest pathological finding in ALD, is not consistently found in all forms of ALD. , Alcoholic steatosis is typically macrovesicular and is characterized by the presence of large intracellular droplets of lipid in hepatocytes (single or multiple) that displace the cell nucleus peripherally. A minor component of lobular chronic inflammation and/or mild portal inflammation may be present, but fibrosis is usually absent.
Steatohepatitis is defined as the presence of both steatosis and hepatocyte injury. Injured hepatocytes may appear ballooned, apoptotic, or lytic. Confluent and bridging necrosis are rarely found in ALD.
Hepatocellular ballooning is considered an essential feature of steatohepatitis by most hepatopathologists. , Ballooned hepatocytes are enlarged cells with rarefied, reticulated cytoplasm ( Fig. 50.1 ) that may or may not contain fat droplets and intracytoplasmic material referred to as Mallory-Denk bodies (MDBs) or Mallory hyaline . Ballooned hepatocytes are located predominantly in zone 3 and are commonly associated with some degree of perisinusoidal fibrosis ( Fig. 50.2 ).
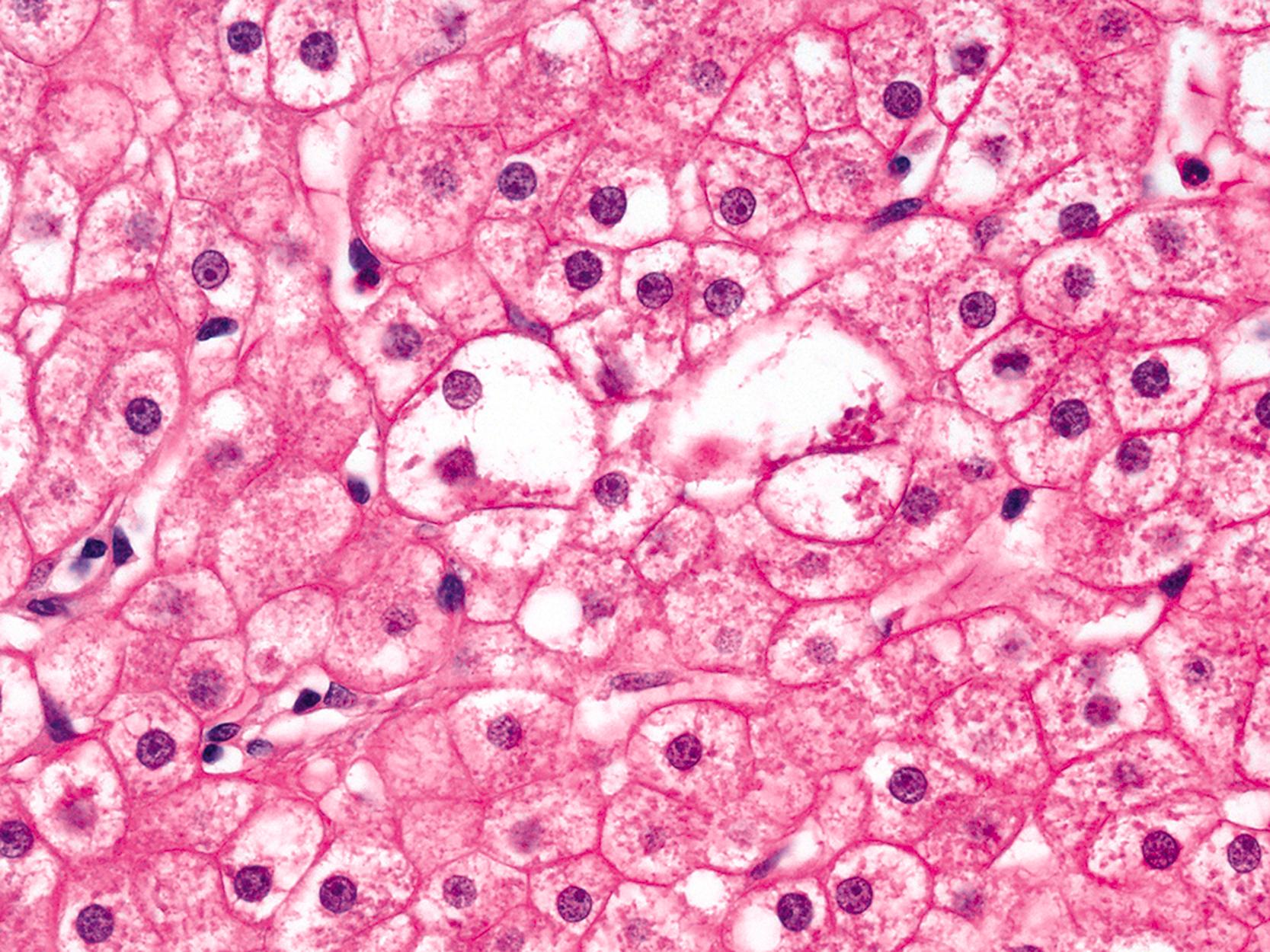
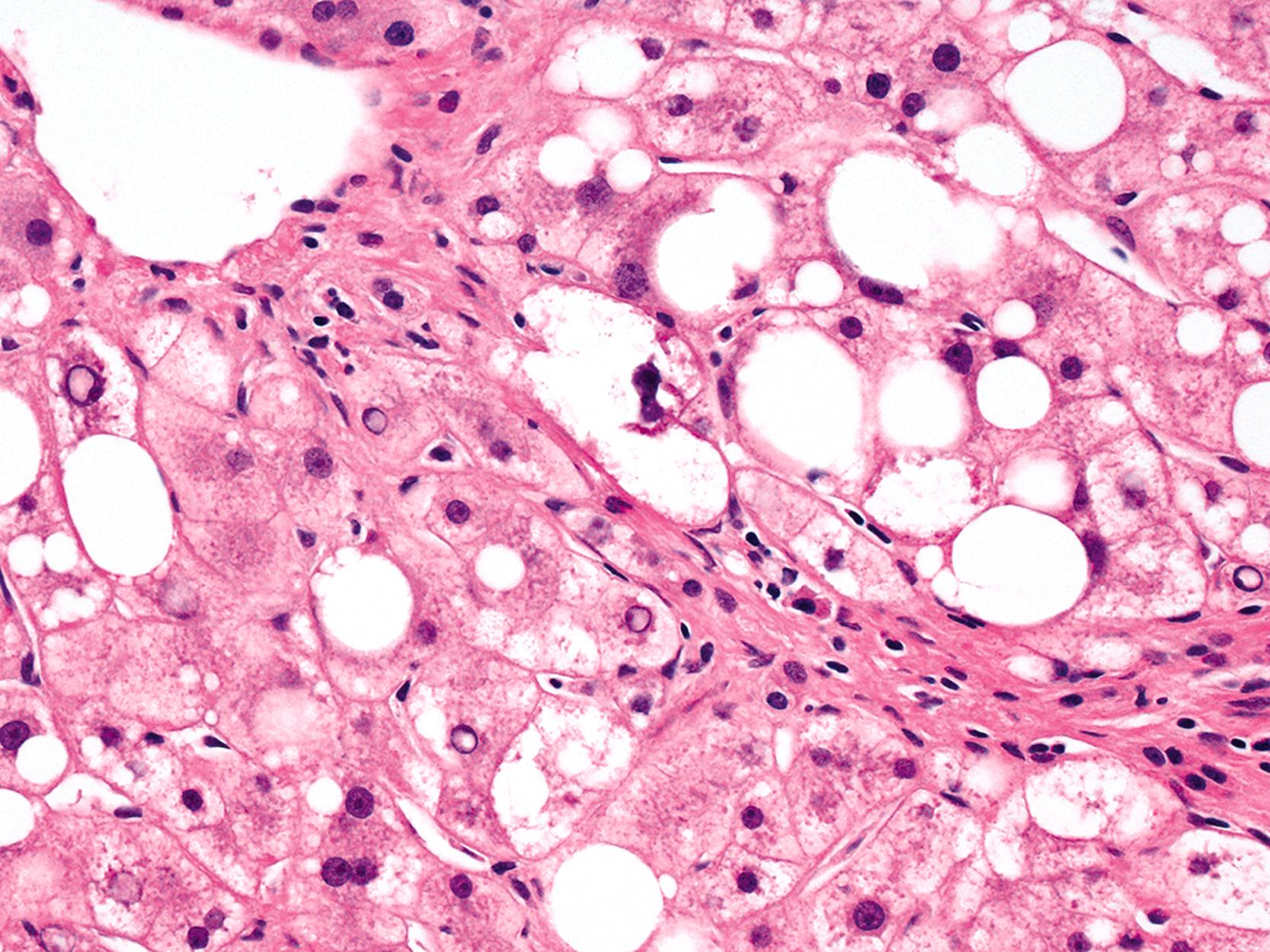
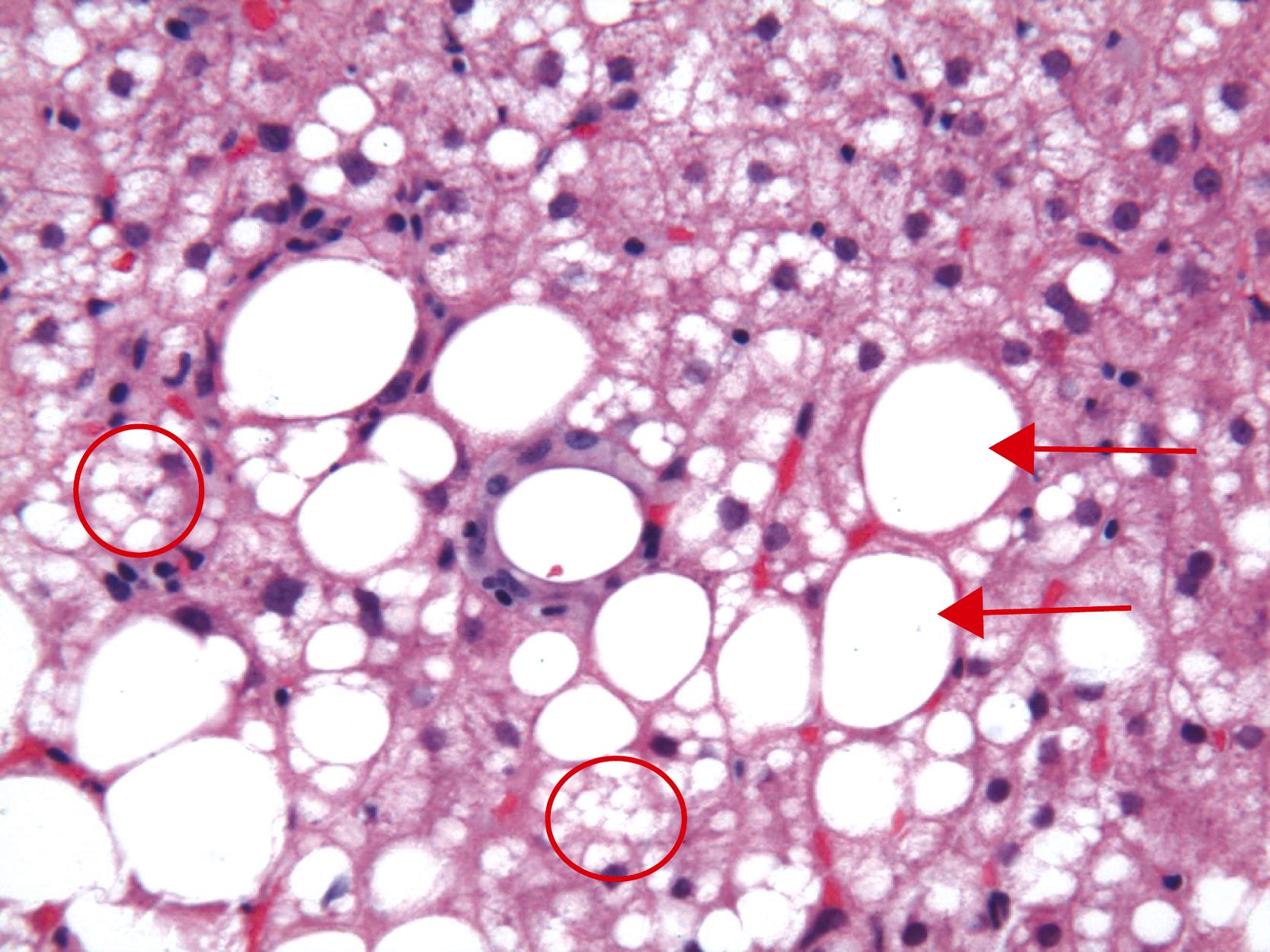
Apoptotic hepatocytes, also called acidophil bodies, are common in patients with alcoholic steatohepatitis. These round, eosinophilic fragments of cytoplasm located in the hepatic sinusoids may or may not retain nuclear pyknotic material. Lobular inflammation in patients with steatohepatitis is usually mild and consists of either mixed acute and chronic or mainly chronic inflammation. Scattered lobular microgranulomas and lipogranulomas are often present. Lobular lipogranulomas may consist of either multiple or single fat droplets surrounded by mononuclear cells and eosinophils ( Fig. 50.3 ). Large portal lipogranulomas are frequently observed in ALD. PMNs may be observed in small clusters adjacent to ballooned hepatocytes or shrunken eosinophilic (apoptotic) hepatocytes that contain MDBs. When PMNs are found next to hepatocytes that contain MDBs, the lesion is often referred to as satellitosis ( Fig. 50.4 ).
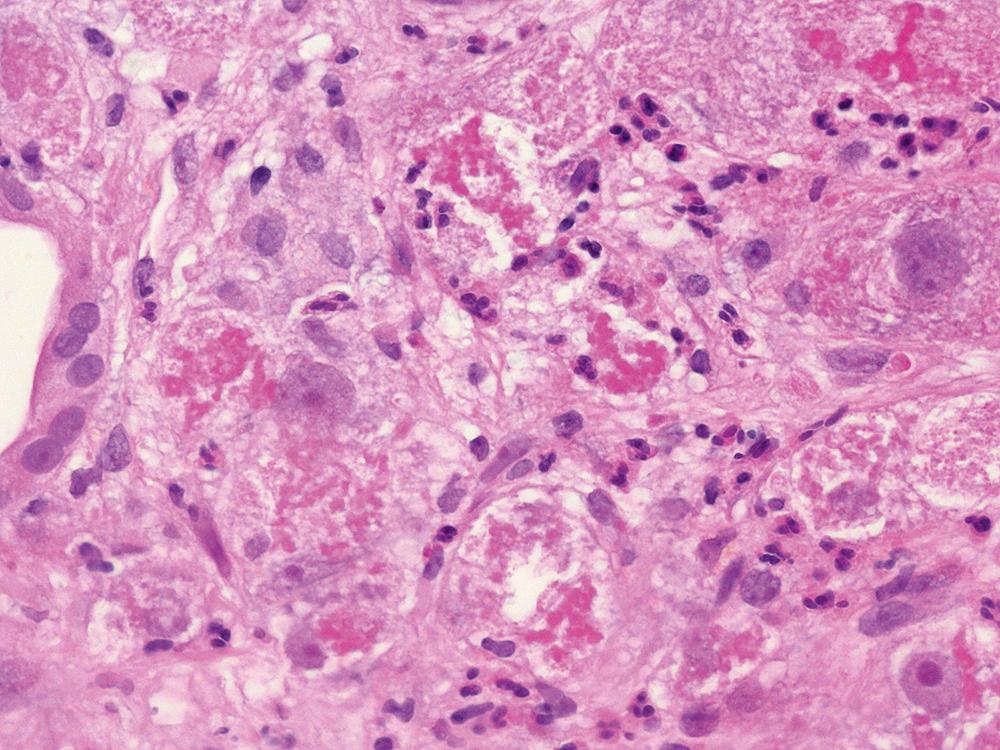
Although common, MDBs and satellitosis are not required for the diagnosis. MDBs are not specific to ALD; these inclusions are seen in NAFLD, chronic cholestatic liver disease, copper toxicity, and amiodarone toxicity. In steatohepatitis of any origin, MDBs are usually found in zone 3 hepatocytes ( Fig. 50.5 ) and are more common in areas of perisinusoidal fibrosis, whereas in chronic cholestasis, copper toxicity, and amiodarone toxicity, MDBs are more common in periportal hepatocytes. , MDBs may also occur in focal nodular hyperplasia, hepatocellular adenoma, and HCC. MDBs can sometimes be visualized by trichrome stains ( Fig. 50.6 ) and confirmed by the use of immunohistochemistry for K8, K18, ubiquitin, or dynactin 4 (DCTN4, formerly designated p62).
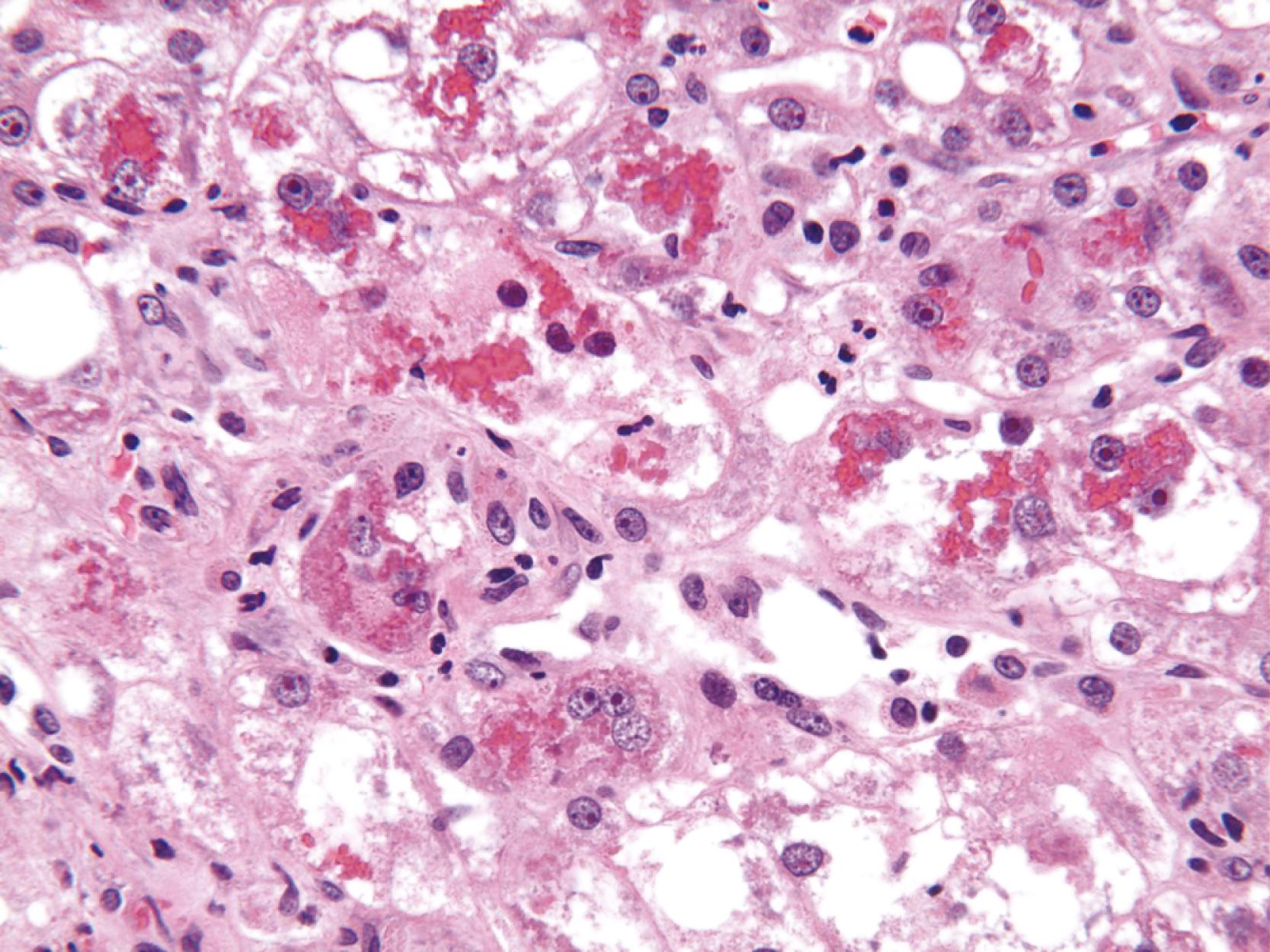
Some cases of ALD may show predominantly portal lymphocytic inflammation in the absence of serological evidence of chronic hepatitis B virus (HBV) or hepatitis C virus (HCV) infection. This feature may correlate with the degree of fibrosis and may reflect an associated autoimmune component to the underlying liver disease. Nevertheless, significant portal and periportal inflammation, particularly when lymphoid aggregates are present, should prompt consideration of concurrent chronic viral or autoimmune hepatitis. Ductular reaction associated with neutrophil infiltration may be related to the proinflammatory and neutrophil chemotactic character of the neo-ductules. Sinusoidal macrophages (Kupffer cells) and possibly portal macrophages are activated by lipopolysaccharide leaking from a gut injured by exposure to ethanol.
Patients with alcoholic hepatitis may not necessarily have steatosis. Histological features of alcoholic hepatitis include hepatocyte ballooning, apoptotic bodies, MDBs with satellitosis, canalicular cholestasis, dense perisinusoidal fibrosis, and a perivenular lesion often referred to as sclerosing hyaline necrosis (i.e., MDBs, satellitosis, and obliterative fibrosis of the outflow vein). Portal hypertension may occur with sclerosing hyaline necrosis. Bridging fibrosis and a ductular reaction may also be present. The absence of steatosis does not rule out alcohol-induced hepatitis.
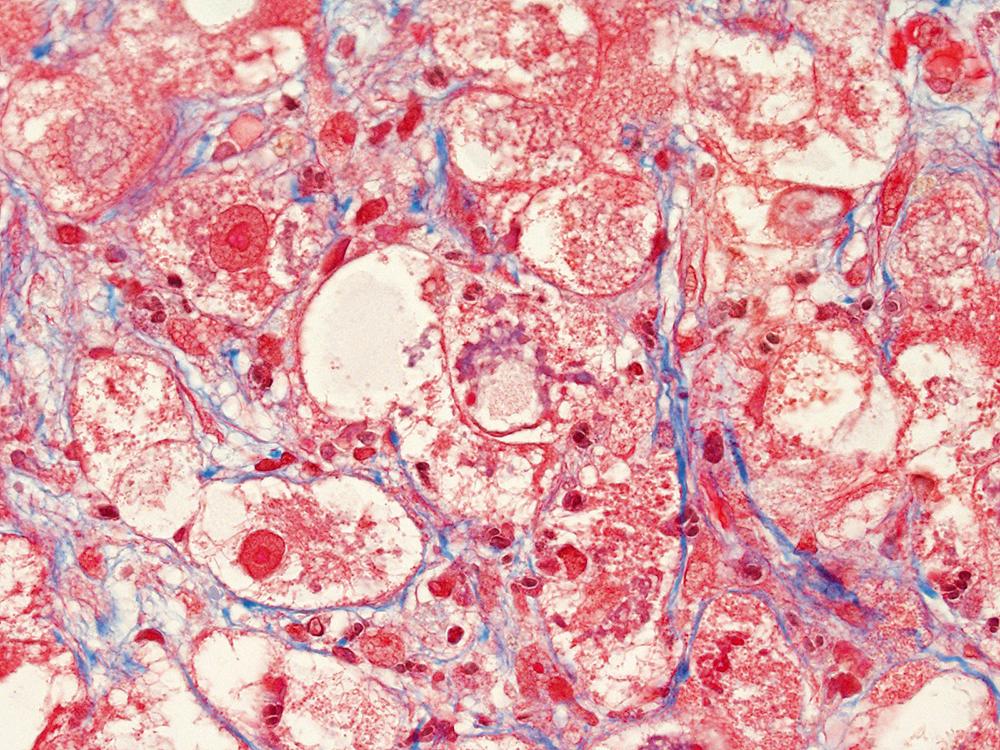
Alcoholic foamy degeneration is an unusual and often serious type of ALD associated with marked elevations of aminotransferases, gammaglutamyltransferase (GGT), alkaline phosphatase, and bilirubin. , Histologically, it is characterized by diffuse, primarily microvesicular steatosis without inflammation or ballooning or by marked fibrosis, and it may be associated with canalicular cholestasis ( Fig. 50.7 ). MDBs are uncommon. Perivenular and perisinusoidal fibrosis may be evident as well. Similar to other disorders that cause microvesicular steatosis, mitochondrial DNA shows evidence of injury. Clinically, the disorder mimics extrahepatic biliary obstruction, but liver biopsy findings can be used to distinguish the two entities. Alcoholic foamy degeneration is reversible with abstinence from alcohol.
The characteristic pattern of early fibrosis in patients with noncirrhotic alcoholic steatohepatitis or alcoholic hepatitis is pericellular or perisinusoidal. This pattern results from deposition of collagen in the space of Disse and is commonly referred to as chicken wire fibrosis ( Fig. 50.8 ).

Fibrosis usually begins in zone 3 of the hepatic acini ( Fig. 50.9 ). This type of fibrosis may be identified with or without steatohepatitis. However, when pericellular or perisinusoidal fibrosis occurs without steatohepatitis, it suggests that the patient had at least one prior episode of steatohepatitis in the past. In ALD, perisinusoidal fibrosis may involve large portions of the lobules and become quite dense in appearance ( Fig. 50.10 ). In these cases, the patient may also have clinical evidence of portal hypertension in the absence of cirrhosis.


Perivenular fibrosis, a thickened sheath of collagen surrounding the outflow veins in the absence of perisinusoidal fibrosis, has been identified in patients with steatosis that subsequently progressed to cirrhosis ( Fig. 50.11 ). This lesion is therefore considered a prognostic indicator of progression if alcohol exposure is continued.

With disease progression, periportal fibrosis may develop ( Fig. 50.12 ), followed in some cases by bridging fibrosis in a central-central, central-portal, or portal-portal pattern. In ALD, regions of parenchymal extinction (i.e., septa) may be quite broad ( Fig. 50.13 ). After cirrhosis is established, areas of perisinusoidal fibrosis may not be evident. Periportal fibrosis and bridging fibrosis are often accompanied by a ductular reaction ( Fig. 50.14 ). In ALD, isolated portal fibrosis in the absence of portal inflammation is uncommon and may be a manifestation of recurrent pancreatitis or biliary obstruction. Itoh et al. observed a pattern of portal fibrosis characteristic of ALD known as holly leaf ( Fig. 50.15 ) . This pattern of fibrosis, which is commonly associated with hemochromatosis, is characterized by portal expansion and nonbridging, small, fibrous extensions or spikes into the surrounding parenchyma that resemble the outline of the holly leaf.




In patients with ALD with bridging fibrosis, a periseptal ductular reaction may be prominent (see Fig 50.14 ). The ductular reaction, characterized by proliferation of K7- and/or K19-positive ovoid cells forming ductular structures in an inflammatory, stromal matrix, is less likely to represent a cholestatic process in end-stage liver disease and is more likely to represent the result of impaired regenerative activity of hepatocytes resulting from the antiregenerative effects of alcohol. ,
ALD-associated cirrhosis may be macronodular, micronodular, or mixed. Micronodular nodules are typically less than 3 mm in diameter ( Fig. 50.16 ). Larger nodules may develop when multiple nodules coalesce to form mixed micronodular and macronodular cirrhosis. Macronodular cirrhosis may be difficult to diagnose in liver biopsy specimens because of the large size of the nodules; clues to abnormal architecture can be sought by use of a reticulin stain ( Fig. 50.17 ). Clusters of oncocytic hepatocytes suggest macronodular cirrhosis (see Chapter 51 ). Phlebosclerosis and occlusive disease of the terminal hepatic venules and sublobular veins occur frequently in patients with ALD-induced cirrhosis ( Fig. 50.18 ).



In ALD-associated cirrhosis, periseptal hepatocellular copper granules ( Fig. 50.19 ) and, uncommonly, α 1 -antitrypsin globules may be found. The role of heterozygosity for genes related to α 1 -antitrypsin in promoting damage in patients with ALD is a subject of ongoing debate, but patients with MZ phenotype are overrepresented in transplant populations and present with more clinically advanced liver disease. Increased iron levels in ALD are discussed later. Pseudotumoral nodules, which correspond to areas of active alcoholic hepatitis in cirrhotic livers, may be recognized on imaging studies and may be confused for HCC. These lesions may result from hypervascularity.

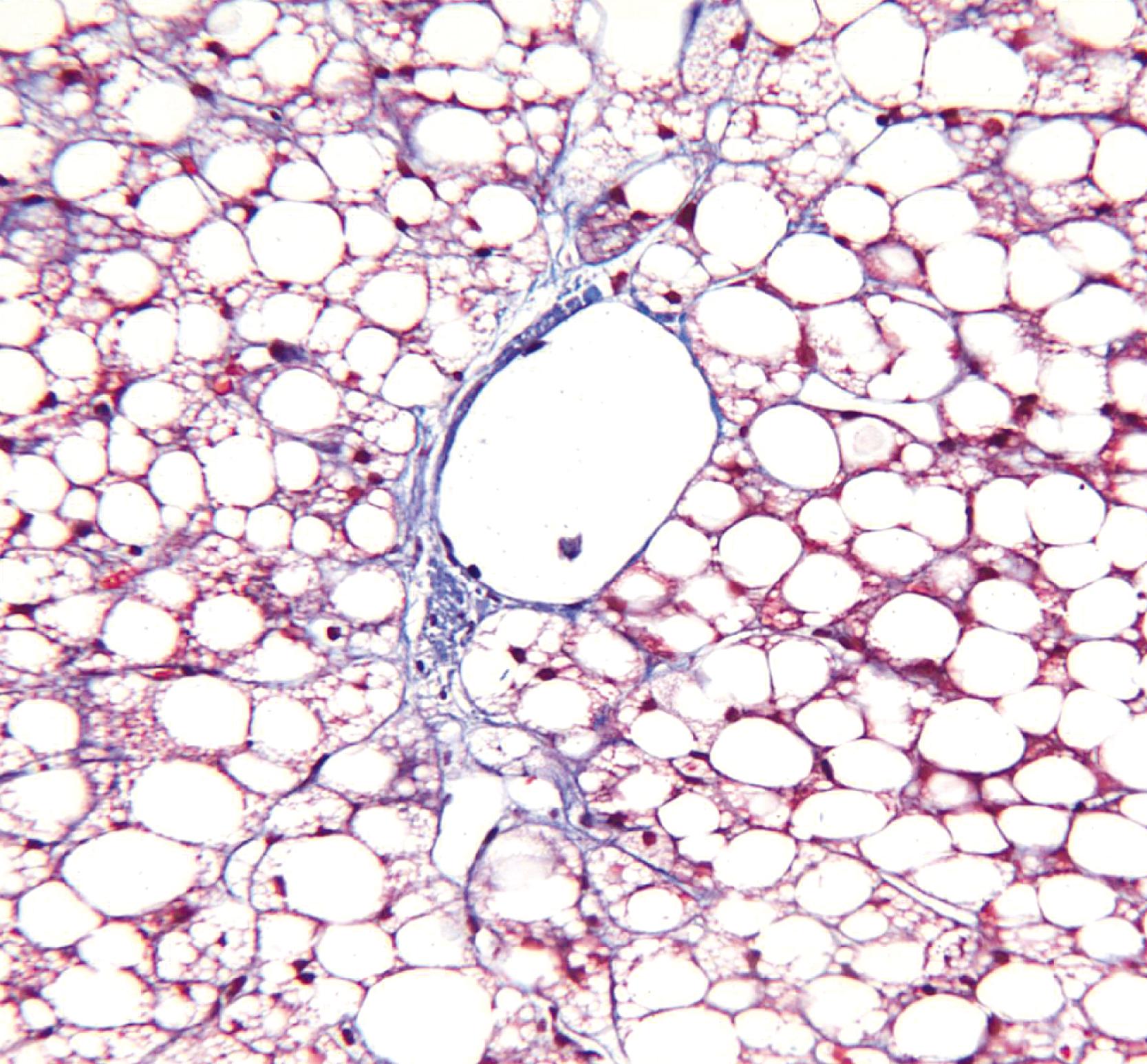
Megamitochondria (i.e., giant mitochondria) are identified by light microscopy as intracytoplasmic, round or cigar-shaped, eosinophilic structures in hepatocytes, and they are most readily seen in hepatocytes that contain microvesicular steatosis ( Fig. 50.20 ). They do not stain with periodic acid–Schiff (PAS) stain, allowing distinction from other round cytoplasmic inclusions such as α 1 -antitrypsin globulin and serum inclusions. Megamitochondria may also occur in NASH, normal pregnancy, acute fatty liver of pregnancy, and Wilson’s disease.

Mildly increased iron deposition is common in most forms of ALD. However, in a minority of cases, it may be moderate or severe. Possible reasons for hemosiderosis in ALD include dysregulation of hepcidin production, intestinal iron absorption, the presence of iron in some types of alcoholic beverages, hemolysis related to spur cell anemia, and upregulation of the transferrin receptor. With a modified Perls stain, iron deposition is usually in hepatocytes when mild, but eventually may accumulate in reticuloendothelial cells as well. In ALD-related cirrhosis, iron deposition may vary in quantity between individual regenerative nodules ( Fig. 50.21 ).

Several pathological processes related to ALD may result in intrahepatic cholestasis: both intrahepatic processes such as alcoholic foamy degeneration, alcoholic hepatitis, and alcoholic cirrhosis as well as extrahepatic processes such as alcoholic pancreatitis, gallstone obstruction, and sepsis. Intrahepatic cholestasis, characterized by canalicular bile thrombi, is observed in 15% to 32% of livers with ALD ( Fig. 50.22 ). Bile stasis in ALD may also be a manifestation of superimposed acute viral hepatitis or drug-induced cholestasis. In patients with established ALD-associated cirrhosis, a copper stain may show mild positivity, which represents chronic retention of bile salts caused by the cirrhotic remodeling.

In advanced cases of ALD, the cytoplasm of some hepatocytes may have a ground-glass appearance because of smooth endoplasmic reticulum proliferation, or it may be deeply eosinophilic, referred to as oxyphil or oncocytic change because of increased numbers of mitochondria. Both changes are considered adaptive in nature. Historically, their presence was considered indicative of ongoing alcohol consumption, but one research group reported that they may also be observed after periods of prolonged abstinence.
Sclerosing hyaline necrosis, which is a marker of severe alcoholic hepatitis, and obliterative vein lesions may be responsible for the development of noncirrhotic portal hypertension in patients with ALD. Sclerosing hyaline necrosis consists of a combination of acinar zone 3 lesions, including dense perivenular and perisinusoidal fibrosis (i.e., sclerosis), occlusion of terminal hepatic venules, MDBs, and liver cell necrosis and loss, resulting in the formation of large perivenular scars (see Figs. 50.11 and 50.18 ). Lesions of the terminal hepatic venules and sublobular veins include perivenular fibrosis, phlebosclerosis, and, less frequently, lymphocytic phlebitis ( Fig. 50.23 ). The number and severity of vascular lesions increases in proportion to the stage of fibrosis.

Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here