Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Various types of ventricular tachycardia (VT) are known to arise from the His-Purkinje network in patients with or without structural heart disease (SHD). This chapter describes the clinical manifestation, mechanism, diagnosis, and therapeutic options in each VT. We first focus on the basic concept of the most common reentrant fascicular VT, which is known as verapamil-sensitive fascicular VT. Recent findings on the VT circuit and characteristics of the rare forms are also addressed. We then describe focal Purkinje VT and ventricular premature contractions (VPCs) arising from the Purkinje system that could trigger ventricular fibrillation (VF). We also describe bundle-branch reentrant VT (BBRVT) and interfascicular VT (IFVT).
Although most VTs are related to SHD, 10% are identified in patients with no apparent structural abnormalities. After right ventricular (RV) outflow tract VTs, left ventricular (LV) fascicular VT is the second most common form, comprising 7% to 12% of such idiopathic VTs. , In 1979, Zipes and associates first described the diagnostic characteristics of this type of VT, which included (1) induction by atrial pacing, (2) VT configuration of right bundle branch block (RBBB) and left axis, and (3) occurrence in patients with no apparent SHD. Belhassen and colleagues reported verapamil sensitivity of this VT in 1981, currently known as the fourth feature. Patients presenting with this VT are typically in young adulthood, and there is a slight male dominance.
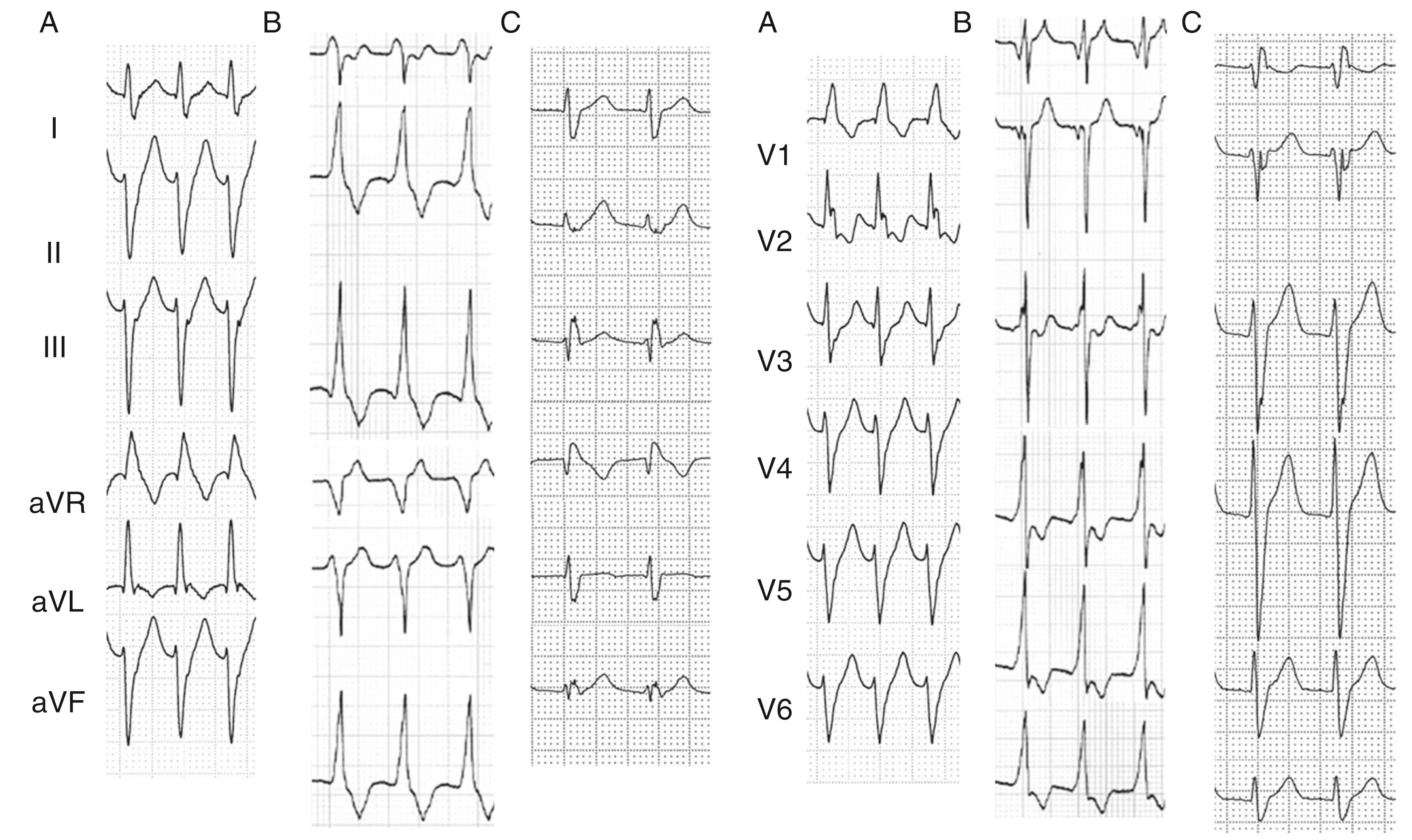
After the initial report by Zipes, two additional types of VT with the same features but different QRS morphologies have been reported. , At present, idiopathic verapamil-sensitive fascicular VT is classified into three forms according to the QRS morphology: (1) RBBB pattern with left axis deviation (common form, left posterior fascicular [LPF] VT, 90%), (2) RBBB pattern with right axis deviation (uncommon form, left anterior fascicular [LAF] VT, 10%), and (3) narrow QRS morphology with normal axis (rare form, upper septal [US] VT, <1%). Fig. 83.1 demonstrates 12-lead electrocardiograms (ECGs) of the three types of VT. More recently, another form of reentrant fascicular VT originating from the LV anterior or posterior papillary muscles was reported.
The mechanism of the verapamil-sensitive fascicular VT is reentry, as ventricular and/or atrial programmed stimulation can induce, entrain, and terminate the tachycardia.
In 2000 Nogami and colleagues first demonstrated macroreentrant circuit of this verapamil-sensitive LPF-VT using a multielectrode catheter placed at the LV midseptal area. During sinus rhythm, Purkinje potentials preceding the QRS complex were recorded along the septal wall from proximal to distal electrodes (basal to apical direction) ( Fig. 83.2A ). During the VT two distinct potentials, middiastolic (P1) and Purkinje, at the presystolic timing (P2) were recorded. Although the P1 potentials propagated in the basal to apical direction, the P2 propagated from apical to basal, as opposed to their activation sequences during sinus rhythm (see Fig. 83.2B ).
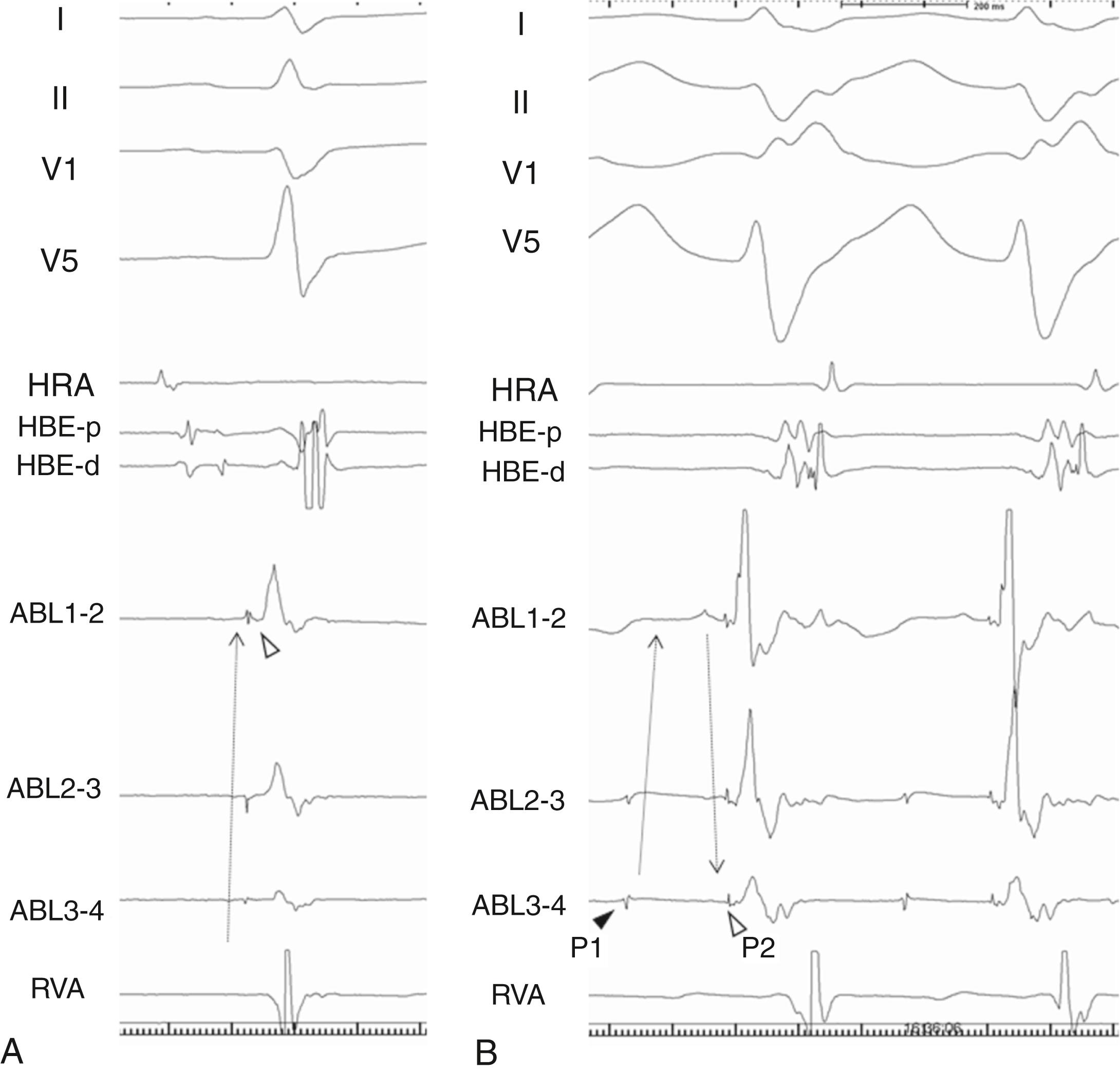
The authors demonstrated that P1 represented a critical component of the antegrade limb of the circuit with decremental properties and verapamil sensitivity, and P2 was the activation of the LPF or Purkinje fiber adjacent to the LPF. Although the authors initially considered P2 as a retrograde limb of the circuit, subsequent analysis demonstrated the LV septum as the retrograde limb and P2 as a bystander. , Although this theory has been a mainstay of the reentrant circuit of the verapamil-sensitive LPF-VT, the P1 potential cannot be recorded in 25%, and the circuit in such cases is unsolved. Another remaining question relates to the observation that the conduction time of the P1 fiber is not long enough to cover the cycle length of the VT, which suggests the existence of a missing segment with slow conduction properties.
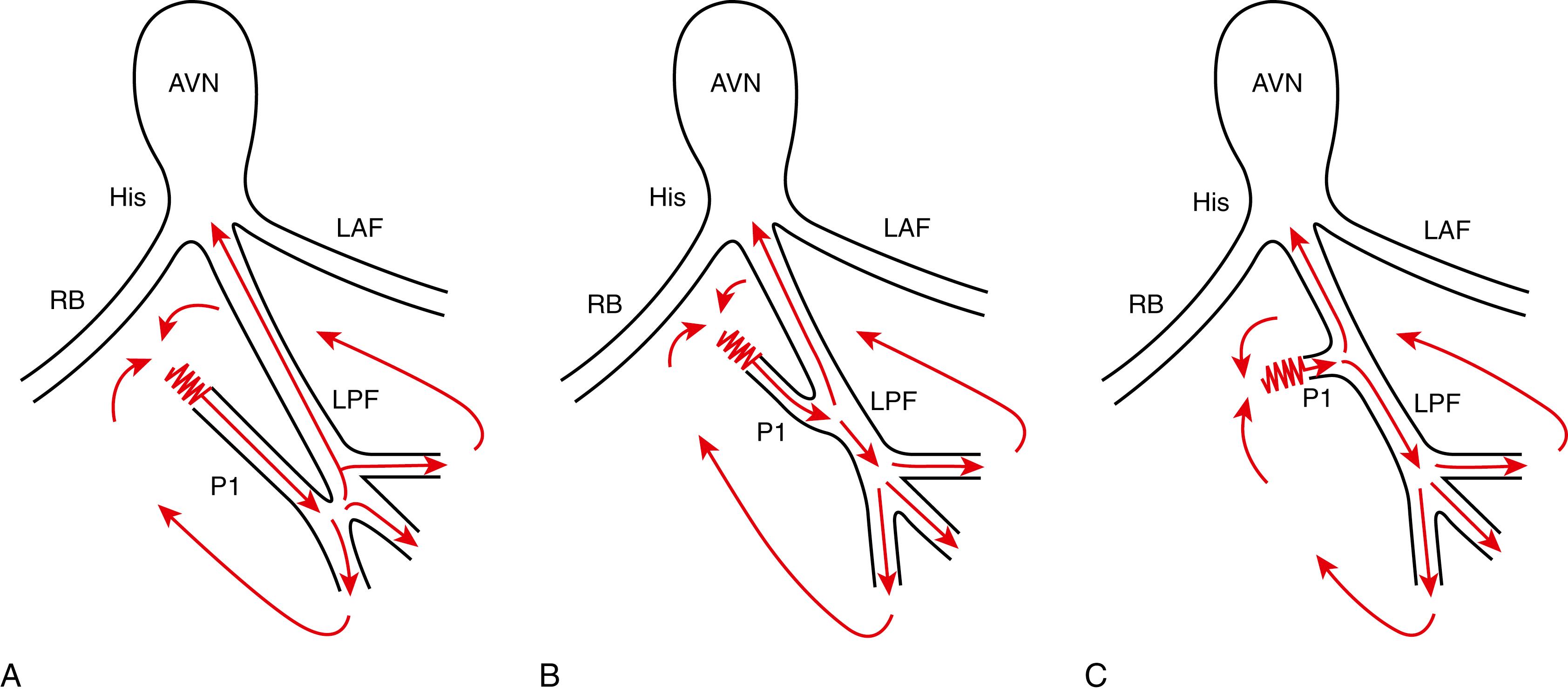
The 2016 article by Liu and associates elegantly demonstrated the components of the reentrant circuit in relation to the HV interval during VT. , They investigated the relationship between the HV interval during VT and the site of the earliest P2 by dividing the LV septum into nine segments from apex to base, showing that a more negative HV interval correlated with a more distal location of the earliest P2. Based on the response to entrainment pacing and single ventricular stimuli during VT, the authors proposed that the reentrant circuit involved the ventricular myocardium, a part of the LPF, a slow conduction zone, and a specially conducting P1 fiber. They hypothesized that the earliest P2 during the VT was the site of connection between the P1 and the LPF, serving as a bridge connection between the distal portion of P1 and ventricular myocardium. In cases without clear P1, P1 fiber was considered to be short or nonparallel to the LPF. At present, it is speculated that the slow conduction zone exists between the ventricular myocardium and the proximal portion of P1. Fig. 83.3 demonstrates the hypothetical schemas of macroreentrant circuit of the LPF-VTs with or without P1 potential recording.
Endocardial mapping during the uncommon type verapamil-sensitive fascicular VT, namely LAF-VT, exhibits P1 in the LV midseptum and P2 near the LAF region. It is considered that the VT activation propagates antegradely over the midseptal P1 fiber similar to the common type LPF-VT and then retrogradely over through the area near the LAF. Although the previously mentioned report by Liu and coworkers did not include LAF-VT, part of the LAF might be involved in the reentrant circuit, bridging the P1 potentials and the myocardium similar to the LPF-VT.
Details of the reentrant circuit of the rare form of verapamil-sensitive fascicular VT (USVT) were unclear until recently. Talib and associates reported that the VT activation antegradely propagated down the LAF or the LPF and then retrogradely through abnormal Purkinje tissue along the LV septum, which is in the opposite direction of their propagation in LPF- and LAF-VTs. USVT often develops after ablation of the other types of verapamil-sensitive fascicular VT. Talib and associates reported half of their USVT cohort had previous LPF region ablation. Conduction delay in the fascicular region created by ablation seems to provide a new substrate for USVT. However, the remaining half of their cohort had no previous ablation, but presented with minor ECG abnormalities, such as small Q waves in the inferior leads and/or S waves in leads I and/or aVF. Regardless of iatrogenic or idiopathic causes, conduction abnormalities in the midseptum are felt to be a substrate for USVT.
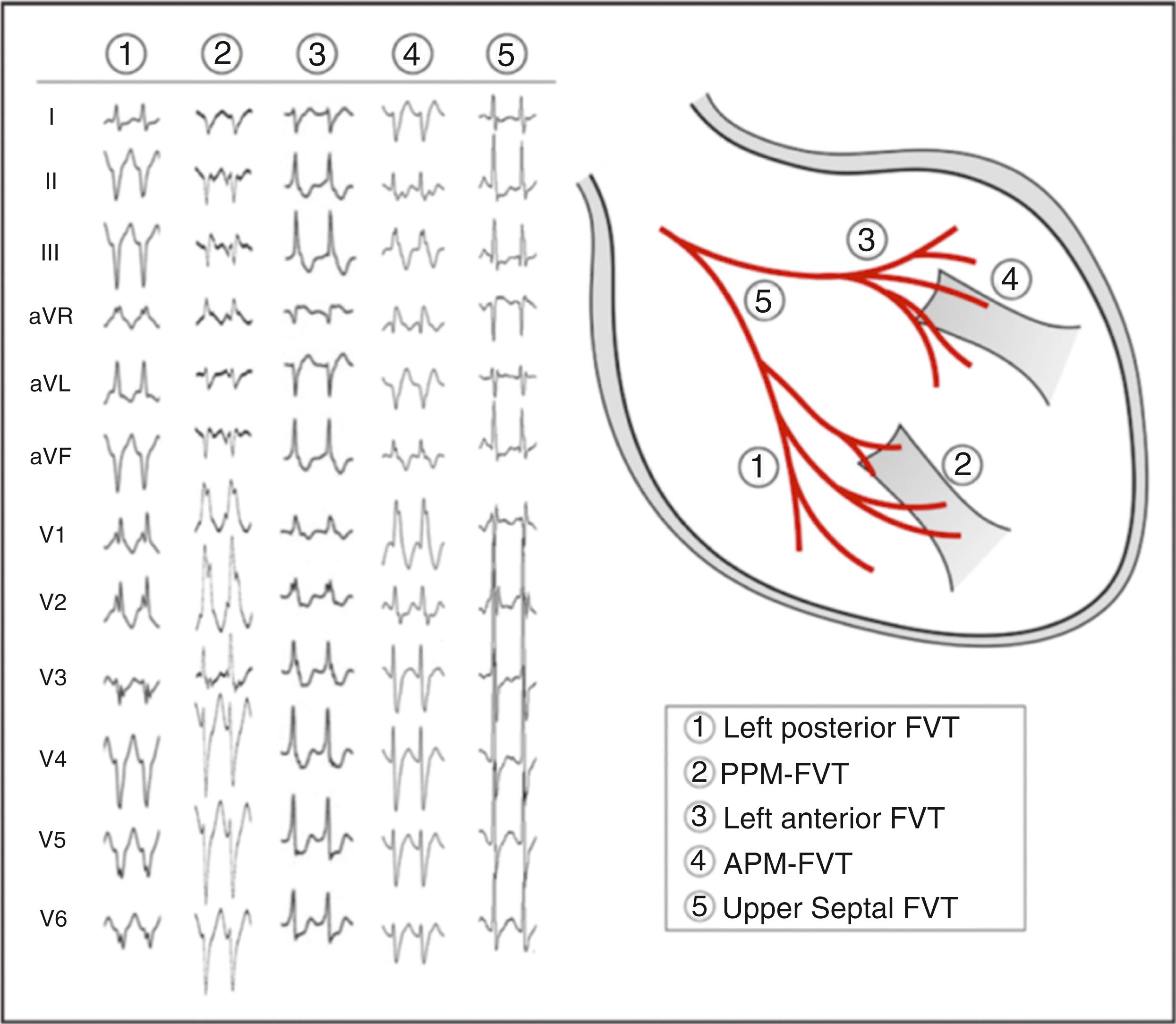
Komatsu and colleagues reported a novel type of reentrant fascicular VT involving the papillary muscles. The P1 potentials were recorded at the anterior or posterior papillary muscles with a basal to apical direction. In contrast, the P2 potentials were recorded with a distal to proximal sequence during the VT. Given that ablation targeting the LPF/LAF region failed and that ablation was successful targeting the P1 at the papillary muscles, the Purkinje network on the papillary muscles are likely to be the critical components for this VT. Fig. 83.4 shows 12-lead ECGs and the origins of five types of fascicular VT.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here