Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
This chapter reviews four of the most common familial neuromuscular diseases that have significant cardiac manifestations: Friedreich ataxia (FA), myotonic dystrophy (DM), Duchenne muscular dystrophy (DMD), and Becker muscle dystrophy (BMD). These syndromes vary significantly in their inheritance patterns, epidemiology, and cardiac manifestations ( Table 76.1 ).
| Familial Cardiomyopathy | Genetics | ECG Findings | Echo Findings | CMRI Findings |
|---|---|---|---|---|
| Friedreich ataxia (FA) | Autosomal recessive Triplet repeat ∼90% have cardiac involvement |
Repolarization abnormalities Inferolateral T-wave inversions Mismatch between increased LV mass and absence of LVH by voltage |
Concentric LVH with no intracavitary gradient Asymmetric septal hypertrophy Globally decreased LV systolic function |
Subendocardial perfusion abnormality in stress CMRI with LGE on LGE CMRI |
| Myotonic dystrophy (DM) | Autosomal dominant Clinical anticipation ∼37%–80% have cardiac involvement |
Atrial fibrillation and flutter Varying degrees of AV block and bundle branch block |
Often normal Rare systolic dysfunction |
Focal fibrosis usually involving midmyocardium to epicardium with endocardial sparing on LGE CMRI |
| Duchenne muscular dystrophy (DMD) | X-linked ∼100% have cardiac involvement |
Sinus tachycardia Right axis Posterior and inferolateral pseudo-infarct pattern with deep inferolateral Q waves, tall R wave in lead V 1 |
Focal hypokinesis basal inferolateral wall MR when involving posterior papillary muscle |
Subepicardial and midmyocardial fibrosis involving inferolateral and anterolateral segments on LGE CMRI |
| Becker muscular dystrophy (BMD) | X-linked ∼60%–70% have cardiac involvement |
Same as with DMD | Same as with DMD | Same as with DMD |
FA is an autosomal recessive disease characterized by spinocerebellar degeneration that leads to progressive ataxia, diabetes mellitus, and cardiac abnormalities. FA is the most prevalent of all spinocerebellar ataxias and occurs in an estimated 1 in 50,000 whites. It was originally described in 1863 by the German neurologist and pathologist Nikolaus Friedreich and is caused by the expansion of glutamic acid (GAA) trinucleotide repeats in the gene encoding frataxin, a protein that plays a central role in mitochondrial iron transport. GAA triplet expansion results in transcriptional gene silencing and loss of frataxin expression. Frataxin deficiency is thought to cause mitochondrial iron accumulation in neurons, cardiomyocytes, and other cell types, which results in cellular and subsequent organ dysfunction, manifesting in the first or second decade of life.
Up to 90% of patients with FA have cardiac involvement, which is characterized microscopically by cardiomyocyte hypertrophy, focal necrosis, and diffuse fibrosis. There are two different cardiac phenotypes. The most common form is hypertrophic cardiomyopathy, which can be further subdivided into asymmetric hypertrophy, which predominantly involves the septum, or concentric left ventricular (LV) hypertrophy. The other observed phenotype is a dilated cardiomyopathy (DCM) with global hypokinesis. There is no apparent relationship between the degree of neurologic and cardiac involvement. Cardiac involvement is not only common in FA but is also a frequent cause of death. In one retrospective study of patients with FA, death from a cardiac cause was the most frequent cause of death (59%), with most mechanisms being congestive heart failure and supraventricular arrhythmias. Compared with noncardiac deaths, cardiac deaths occurred earlier in the disease course (median, 17 years vs 29 years, respectively).
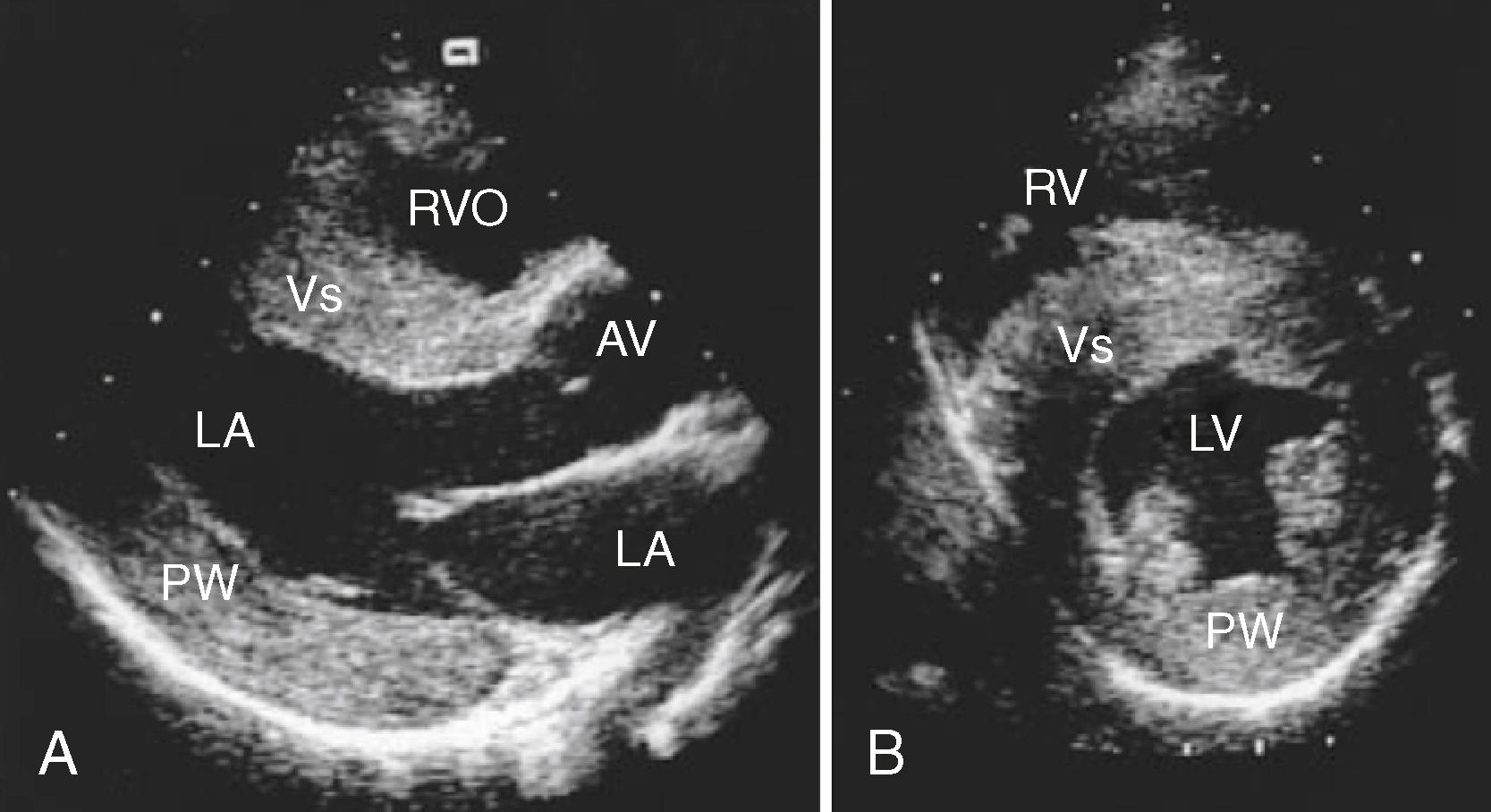
The classic echocardiographic finding in FA is increased LV wall thickness, which most commonly involves the septum ( Fig. 76.1 ). Although the degree of overall cardiac involvement does not correlate with the degree of neuromuscular dysfunction, the degree of septal thickening on echocardiography has been correlated with the number of GAA triplet repeats in some studies. Unlike the asymmetric septal hypertrophy associated with other conditions, such as hypertrophic obstructive cardiomyopathy, intracavitary gradient is not typically seen in FA cardiomyopathy. In addition, in one natural history study, a large majority of FA patients had evidence of diastolic dysfunction, and age did not predict its presence or severity, suggesting that diastolic dysfunction may be an early or intrinsic component of FA cardiomyopathy that correlates more strongly with genetic severity than neurologic disability. Cardiac magnetic resonance imaging (CMRI) in FA similarly reveals the hypertrophy seen on echocardiography. Myocardial perfusion studies using CMRI with an adenosine stress modality have demonstrated a reduced myocardial perfusion reserve index, which appears to parallel development of the metabolic syndrome in these patients ( Fig. 76.2 ). Because impaired perfusion reserve does not appear to correlate with the degree of hypertrophy or fibrosis, this may represent a new therapeutic target in patients with FA.
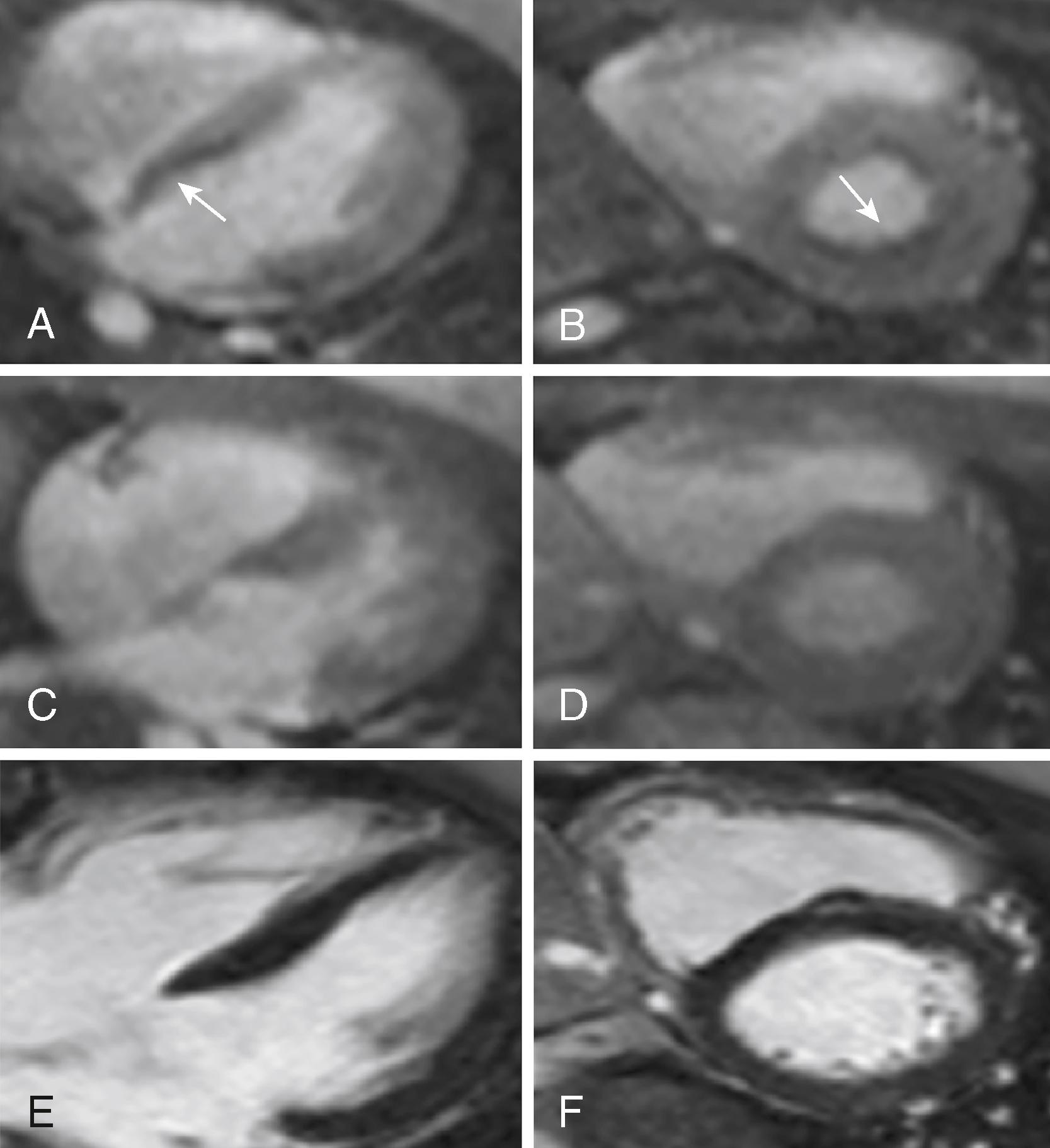
Although guidelines for screening in FA are lacking, a consensus is emerging that patients with this diagnosis benefit from cardiac screening with an annual electrocardiogram (ECG), echocardiogram, and/or CMRI, with follow-up imaging dictated by symptoms and/or changes on the ECG. Treatment of the cardiomyopathy involves early initiation of agents that reduce afterload and that may also reduce fibrosis (e.g., angiotensin-converting enzyme inhibitors and angiotensin receptor blockers). Other treatment modalities, including antioxidants and iron chelation, are under investigation. Preclinical studies have also shown reversal of LV dysfunction in mouse FA models by gene therapy, establishing a proof of concept for the potential of gene therapy in treating FA cardiomyopathy. More recently, cardiomyocytes derived from induced pluripotent stem cells from FA patients were used to demonstrate correction of the frataxin gene and associated cardiomyocyte defects, by zinc-finger nuclease mediated editing of the GAA repeats, thus establishing a target for gene therapy in the future.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here