Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The surgical management of patients with drug-resistant epilepsy is changing rapidly as newer methods of diagnosis and treatment are discovered and enter clinical practice. The International League Against Epilepsy defines drug-resistant epilepsy as seizures that continue despite treatment with at least two properly selected antiepileptic medications. Approximately 30% of patients with ongoing seizures meet these criteria. , For these patients, escalating therapy beyond medications to surgery offers the opportunity to significantly diminish their seizure burden and possibly achieve seizure freedom. Presurgical evaluation is optimally performed by a multidisciplinary team including neurologists specializing in epilepsy, neuroradiologists, neuropsychologists, and neurosurgeons. Together, such a team is best equipped to establish the epilepsy subtype, localize the epileptogenic zone, and identify therapeutic options—including surgery—that offer the best chance of seizure freedom or minimize seizure burden.
The first step in defining these characteristics is noninvasive video electroencephalography to capture electrophysiological changes during the ictal state. Structural imaging of the brain with high-resolution (≥3 Tesla) magnetic resonance imaging (MRI) is also critical to identify structural abnormalities that may be surgical targets. When such lesions are present, there is a greater likelihood of seizure freedom if safe resection is possible. , Other noninvasive imaging tools that are often helpful in delineating the epileptogenic zone include metabolic imaging with interictal positron emission tomography (PET) and peri-ictal single-photon emission computed tomography, both of which may identify metabolic abnormalities corresponding with focal seizure activity. PET can be useful in identifying areas of cerebral hypometabolism, which have shown positive post-surgical outcomes in MRI-negative patients when correlated with EEG. Interictal magnetoencephalography is another tool commonly used to identify the epileptogenic zone. This modality is a non-invasive way to identify the epileptogenic zone by recording interictal spikes that may not otherwise be captured on EEG and has a reported sensitivity of about 70%. Newer modalities that may be helpful in identifying the epileptogenic zone include EEG-functional MRI (fMRI). Detailed functional imaging, including fMRI, is often complementary to conventional MRI and EEG as a surgical planning tool to identify motor, sensory, language, and visuospatial memory architecture that may be anatomically close to or overlap with the epileptogenic zone in patients with extratemporal epilepsy. The Wada test is also useful when mesial temporal lobe structures are involved, as it uniquely evaluates memory function in addition to language lateralization.
For patients with multifocal epilepsy, those with seizure foci in eloquent areas, or those with drug-resistant generalized seizures, resection may not be feasible or safe, and a strategy of neuromodulation may be the best option. Although neuromodulation may not have the same curative potential as resection of a single epileptogenic zone, it is a critical tool in the surgical armamentarium for patients with drug-resistant epilepsy. Neuromodulation methods include vagal nerve stimulation (VNS), responsive neurostimulation (RNS), and deep brain stimulation (DBS).
VNS to treat epilepsy was first pioneered in the late 19th century by James Corning, a neurologist. At that time, it was thought that epilepsy was a result of excessive cerebral blood flow. Corning hypothesized that stimulation of the vagus nerve would reduce sympathetic tone and, along with partial compression of the bilateral carotid arteries, would decrease blood flow and treat epilepsy. Although his experiments were inconclusive, they paved the way for other research on VNS.
In the following half-decade, animal models were used to show that VNS could produce synchronous and desynchronous electroencephalography rhythms, depending on the stimulation voltage used. The first VNS device was placed in a human in 1988, and it was approved for use in the United States by the Food and Drug Administration (FDA) in 1997 after a 1994 randomized, multicenter, double-blind study, EO3. This study enrolled 67 patients with refractory partial seizures and randomized them to “low” or “high” parameters (which included a combination of frequency, pulse width, and duty cycle). After 14 weeks of VNS, 31 patients receiving “high” VNS experienced a mean reduction in seizure frequency of 30.9%, compared with a reduction of 11.3% in the 36 patients receiving “low” VNS. Since the 1990s, VNS has become a standard surgical option in patients with drug-resistant epilepsy.
The method by which VNS exacts its effect on seizure frequency remains unclear, although research is beginning to shed light on potential mechanisms. The vagus nerve is made up of mostly unmyelinated afferent C fibers that project to the nucleus tractus solitarius (NTS) in the brainstem. The NTS fibers extend to various locations in the forebrain and brainstem. The vagus nerve and the NTS network have long been an area of interest. Early studies defined the circuitry through retrograde cell labeling, the use of immunohistochemical stains, and microdialysis of neurotransmitters. Electrophysiological studies then paved the way for new investigational tools to map neuronal pathways in spatial and temporal dimensions. New imaging parameters such as blood oxygen level–dependent fMRI and PET are allowing us to further characterize these pathways. The NTS receives afferent projections from the vagus nerve and then projects to other nuclei, including the locus coeruleus, the dorsal raphe nucleus, the parabrachial nucleus, the paraventricular nucleus of the hypothalamus, the stria terminalis, and the cingulate cortex. Together, these target nuclei appear to be essential in releasing the neurotransmitters necessary to downregulate seizure activity. , ,
In 1997, VNS was approved by the FDA for use in drug-resistant partial epilepsy syndromes in patients older than 12 years of age because of the inclusion criteria of most trials that studied VNS efficacy and safety before its approval. Since then, additional studies have examined VNS efficacy in the younger pediatric population and in the use of various seizure types. In 2013, the American Academy of Neurology released updated guidelines on the use of VNS to treat drug-resistant epilepsy. These guidelines extended the recommended use of VNS to the pediatric population, for patients experiencing either partial or generalized epilepsy syndromes, including patients with tuberous sclerosis, Dravet syndrome, and Lennox-Gastaut syndrome. In this guideline, the authors cited 14 Class III trials where pooled analysis of 481 pediatric patients showed that 55% reached a 50% reduction in seizure frequency with a pooled seizure freedom rate of 7%. They rated the recommendation for use in children as a Level C recommendation.
In 2017, the FDA extended its approval to children as young as 4 years , based on the findings of these studies. Even as the development of more precise methods of seizure localization (e.g., advanced neuroimaging, quantitative EEG, stereotactic EEG) has made the identification of EZs more feasible, VNS therapy remains an option for patients with multifocal epilepsy, generalized epilepsy, or seizure foci in eloquent areas. It should also be noted that up to 20% of patients will have no response to VNS, and while they may meet all the above listed criteria for implantation, there is no presurgical method of identifying those that will gain no benefit. This continues to be an area of frustration for practitioners. , ,
VNS devices are made up of two parts: a pliable, spiral-shaped electrode that wraps around the vagus nerve, and an internal pulse generator (IPG) that is typically implanted below the clavicle. Most VNS implants are placed on the left side because of concern for causing arrhythmias or bradycardia when accessing the right-sided vagus nerve, although there have been reports of successful right-sided VNS placement.
The patient is placed in the supine position with the head turned to the right to access the left anterior cervical triangle and the left chest. A shoulder roll may be placed behind the back to allow for more neck extension. The neck incision should be centered on the medial border of the sternocleidomastoid (SCM) muscle and should measure approximately 2 to 3 cm in length, typically centered around the C5 or C6 vertebral body or thyroid cartilage in an existing neck fold to minimize cosmetic concerns from the scar. The IPG may be implanted subcutaneously in the chest via either a subclavicular incision parallel to and 1 to 2 cm below the clavicle or an anterior axillary incision ( Fig. 104.1 ). An axillary incision also facilitates a subpectoral pocket, which is often preferred in small children.
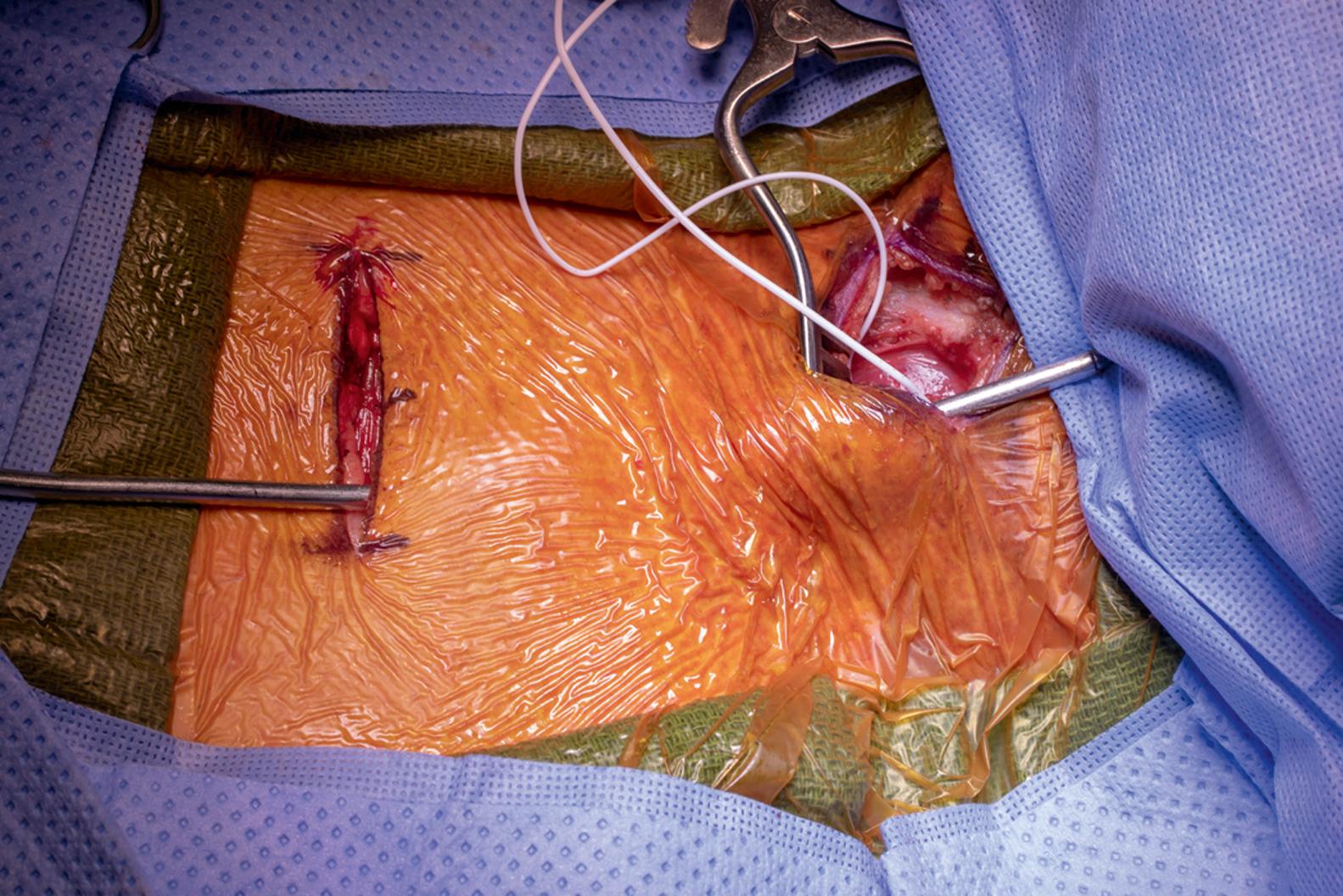
The surgical field is prepped and draped in standard fashion to include the planned incisions, and preoperative antibiotics are administered. The subclavicular incision is opened, and a suitable pocket is made immediately under the pectoralis fascia but above the muscle. In the case of an axillary incision, the pocket should be created in the subfascial space between the pectoralis muscle and the chest wall.
The marked cervical incision for the electrode is then incised, and the platysma is divided. The superficial layer of deep cervical fascia is sharply incised along the medial border of the SCM muscle until the carotid sheath is visible. The carotid sheath houses the internal jugular vein laterally, the internal carotid artery medially, and the vagus nerve posteriorly (typically deep between the two vessels). The carotid sheath is located within the carotid triangle, which consists of the omohyoid as the inferior medial border, the SCM as the lateral border, and the posterior belly of the digastric muscle as the superior border. Localization of the carotid sheath can normally be accomplished by palpating medial to the SCM, feeling for pulsation of the carotid. In some cases, it is necessary to section a portion of the omohyoid to increase visibility of the vagus.
Once identified, the carotid sheath is opened with sharp dissection, taking care to avoid injury to its important vascular structures. The internal jugular vein and carotid artery are then carefully retracted laterally and medially, respectively, by holding onto the adventitia. Once these are retracted, the vagus nerve is identified and dissected from the surrounding areolar tissue with careful blunt and sharp dissection as needed. A vessel loop can then be placed around the nerve to allow for gentle manipulation and to aid in its dissection. This can be done with the operating microscope or loupe magnification. Dissecting at least 3 cm of nerve is necessary for placement of the lead.
Once the dissection is complete and before placing the electrode around the nerve, a tunneling tool is used to create a subcutaneous tract between the cervical and chest incisions, and the lead is tunneled in a cervical-to-chest manner with the helical leads in the neck incision ( Fig. 104.2 ). The VNS lead is made up of three helical coils. From cranial to caudal, the first electrode is the anode, the second is the cathode, and the third coil is an anchor. The electrodes contain two complete coils, and the anchor comprises 2.5 coils. Each electrode is placed in turn, passed underneath the nerve by grasping the connected suture tail. The distal wire is firmly held while the suture tail is brought over the anterior surface of the nerve until the first turn sits flush on the nerve. The second turn of the first electrode is then passed under the nerve is a similar fashion by grasping the suture tail on the opposite end and pulling it over the nerve until it rests flush on the nerve. This process is then repeated for the second electrode and finally the anchor, proceeding from proximal to distal. Once the coils are attached, a redundant electrode is placed distally in the carotid sheath to act as an anchor, and the portion of the lead embedded in silastic is sutured to the deep muscle by using the included pledgets. The final placement of the electrodes is illustrated in Fig. 104.3 .
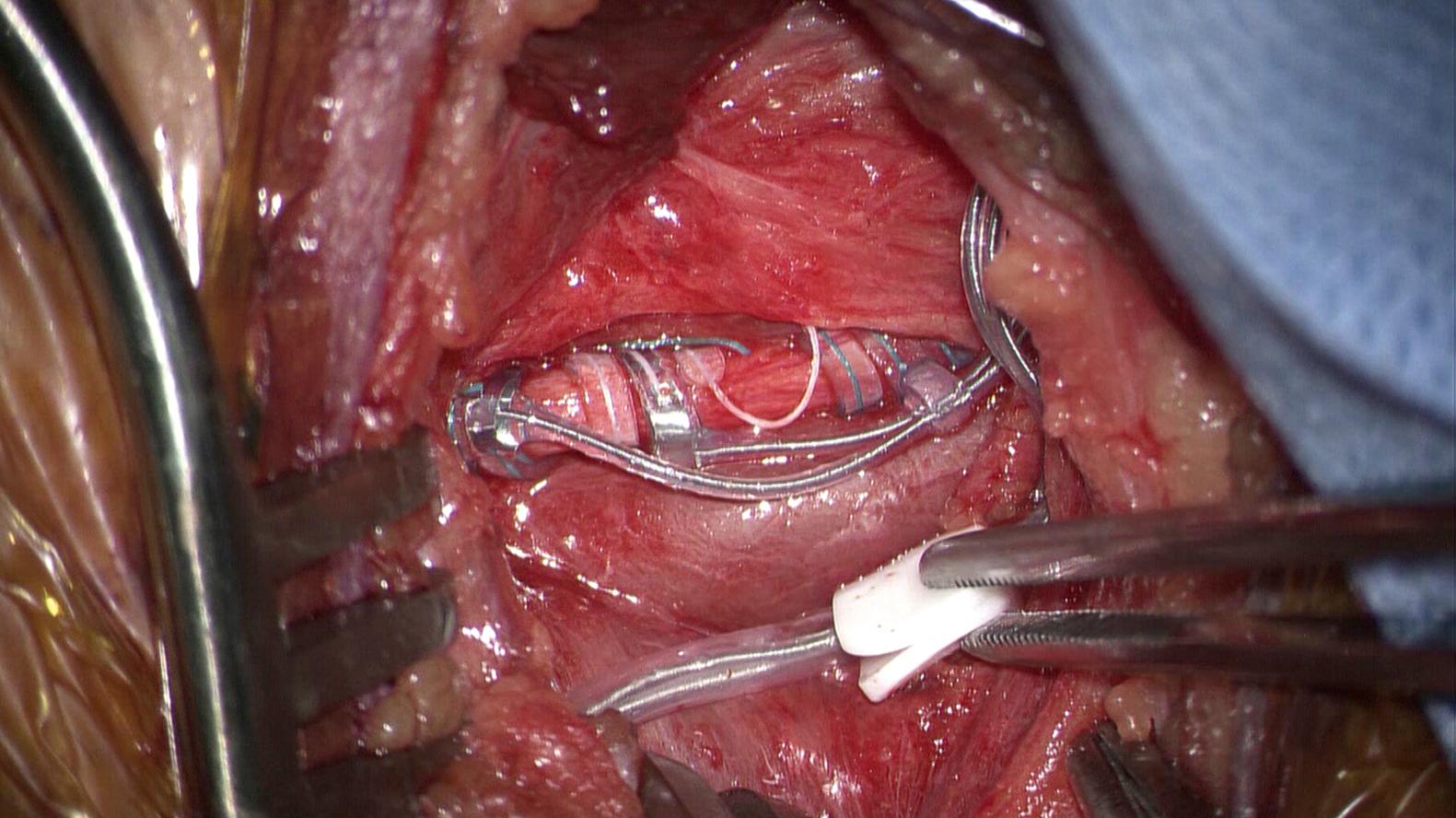
The surgeon’s attention is then turned to the other end of the electrode, where the lead connector pin is inserted into the IPG and secured with a torque-limited screw. After the IPG is secured in the pocket, electrodiagnostic testing is performed. During the electrodiagnostic testing of the lead, there is a risk of severe bradycardia—the anesthesiologist should be warned and the patient should be monitored. If this occurs, the lead should be evaluated because it may be on a cardiac branch instead of the main nerve. The newer models of IPGs including the LivaNova 106 and the LivaNova model 1000 have a cardiac detection mode, which enables the VNS to act as a closed-circuit system. Many seizures are preceded by an elevation in heart rate that activates the IPG when above a certain threshold, thereby stimulating the vagus nerve in an effort to abort the seizure. The wounds are then irrigated with copious amounts of bacitracin-infused saline and closed in a standard multilayer fashion. The IPG is not usually turned on for the first 2 postoperative weeks to allow the patient to recover from surgery before programming.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here