Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Extramammary Paget disease (EMPD) is defined as an intraepithelial adenocarcinoma that primarily involves the epidermis outside the breast.
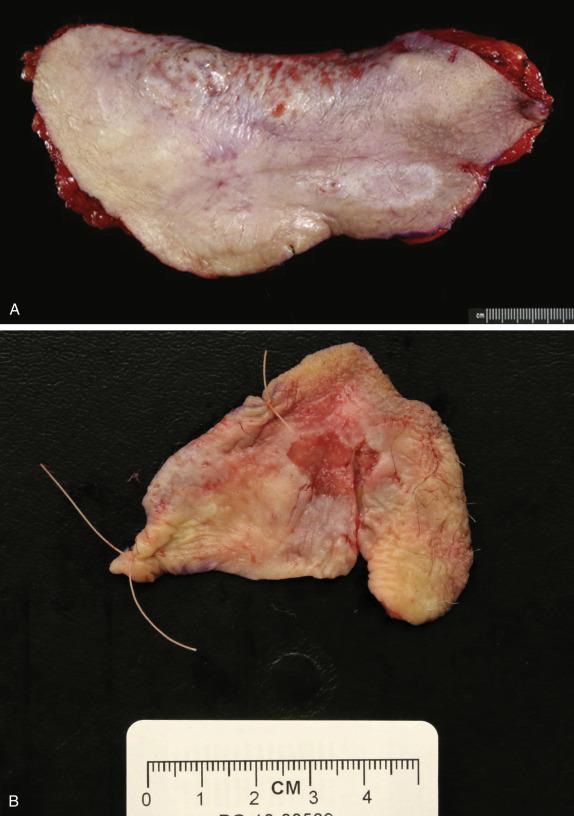
This lesion accounts for only 1% of vulvar cancers. EMPD typically occurs in postmenopausal white women and appears as an eczematous-type plaque, sometimes with excoriation ( Fig. 3.1A ), which can be clinically misdiagnosed as fungal infection or atrophic vaginitis; thus not infrequently there is significant delay in diagnosis. Less commonly it presents as a polypoid, moist and red lesion with ulceration ( Fig. 3.1B ). EPMD more commonly affects the anogenital region, and less commonly the axillae, ears (ceruminal glands), and eyelids (Moll glands). Itching is the most frequent presenting symptom. The labium majus is the most common location (followed by labium minus, clitoris, perineum, and anterior anal area). Bilateral vulvar involvement is frequent.
Unlike Paget disease of the breast, in which an associated mammary ductal carcinoma is almost invariably present, an underlying carcinoma (colorectal, cervical, bladder/urethra, or prostatic) is identified in less than 25% of EMPD. The remainder probably represent in situ malignant transformation of cutaneous sweat ducts.
EMPD appears as an ill-defined, diffuse red plaque that may occasionally become ulcerated; it has a mean size of approximately 6 cm.
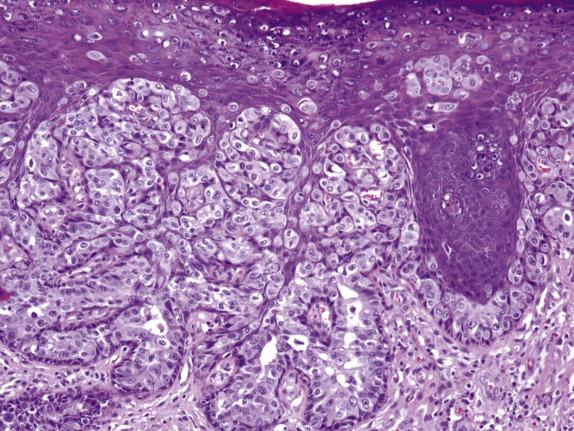
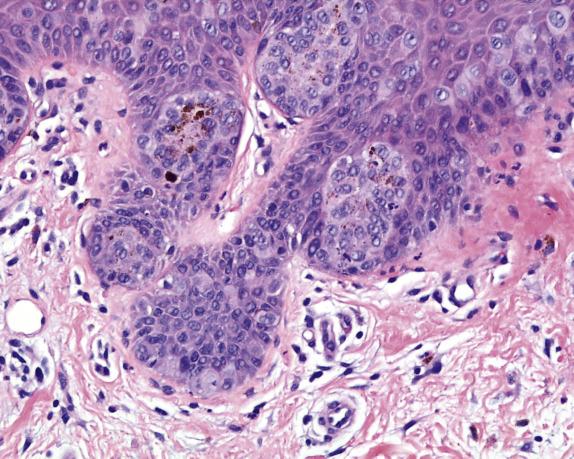
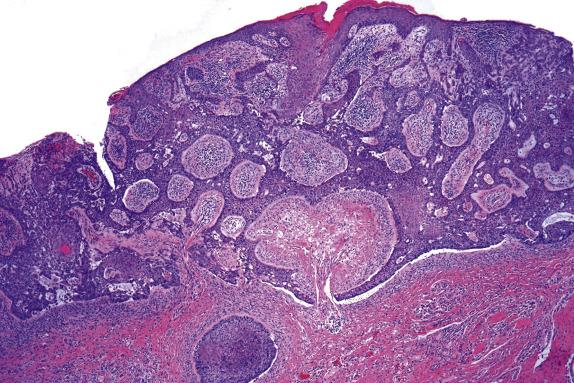
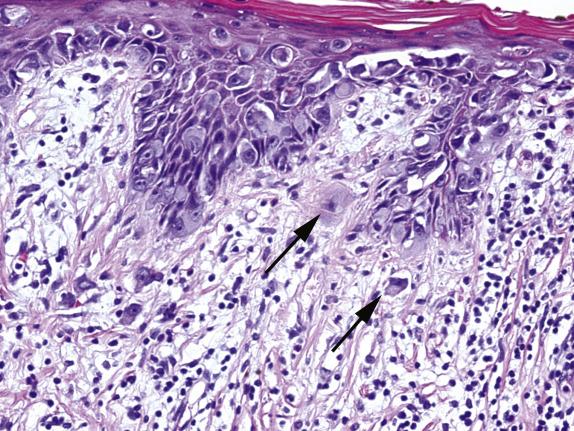
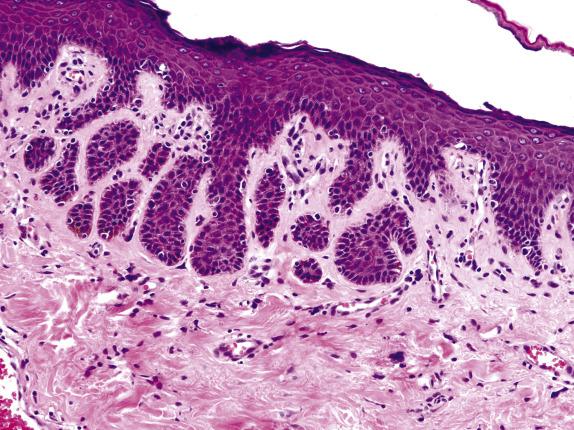
Extramammary Paget disease typically involves the epidermis and epithelium of related adnexal structures as nests and isolated large cells that are sited predominantly in a basal and parabasal location ( Fig. 3.2 ). There is preservation of the normal basal-layer keratinocytes, which frequently appear compressed downward or laterally by the tumor cells ( Fig. 3.3 ). Rarely, the tumor cells form glands within the epidermis. Paget cells have abundant pale cytoplasm with occasional intracytoplasmic vacuoles (signet ring cells), and large vesicular nuclei with prominent nucleoli. They only infrequently may contain melanin pigment due to melanin transfer from melanocytes to the Paget cells ( Fig. 3.4 ).
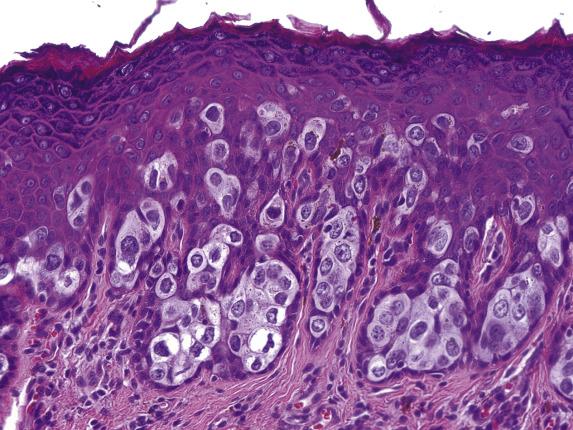
EMPD is associated with proliferative epidermal lesions in one-third of cases, including squamous hyperplasia not otherwise specified, fibroepithelioma-like hyperplasia, and papillomatous hyperplasia ( Fig. 3.5 ). Invasion is seen in less than a third of cases, most commonly as single cells ( Fig. 3.6 ) or in small nests in the upper dermis; overt dermal invasion is uncommon.
Intracytoplasmic mucin (neutral and acidic) is found in the majority of tumor cells, and can be highlighted by special stains (Alcian blue, mucicarmine). Immunoperoxidase studies can be useful in confirming the diagnosis of EMPD, as most express low-molecular-weight cytokeratin, GATA3, monoclonal carcinoembryonic antigen (mCEA) ( Fig. 3.7A ), epithelial membrane antigen (EMA), and gross cystic disease fluid protein 15 (GCDFP-15). S100 protein expression is usually negative. Androgen receptor (but not estrogen or progesterone receptor) expression is detected in approximately 50% of cases.
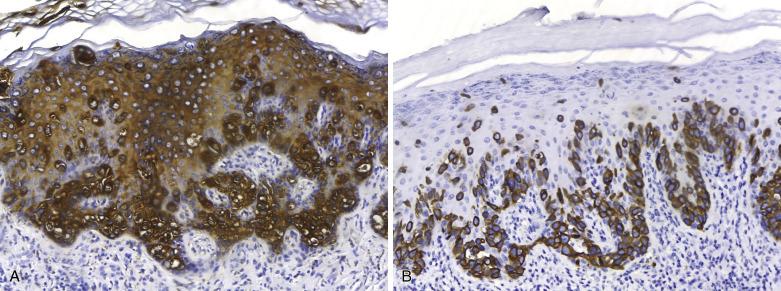
CK7 ( Fig. 3.7B ), CK20, mucin core protein (MUC)2, uroplakin III and GCDFP15 are helpful in the distinction between primary and secondary EMPD. The immunoprofile of primary EMPD is CK7+/CK20−/GCDFP15+/MUC2−. EMPD secondary to urothelial carcinomas is CK7+/CK20+/GATA3+/GCDFP15− ( Fig. 3.8A–C ) and uroplakin III+, whereas EMPD secondary to rectal carcinoma is typically CK7−/CK20+/GCDFP15−/MUC2+. Immunohistochemistry may also help determine the status of surgical margins and possible dermal invasion since it allows detection of isolated Paget cells. Furthermore, immunohistochemistry may aid in cases in which invasion is being considered, as there is differential expression of MUC between primary intraepidermal and primary invasive EMPD: the former coexpresses MUC1 and MUC5, whereas invasive primary EMPD usually lacks MUC5 expression.
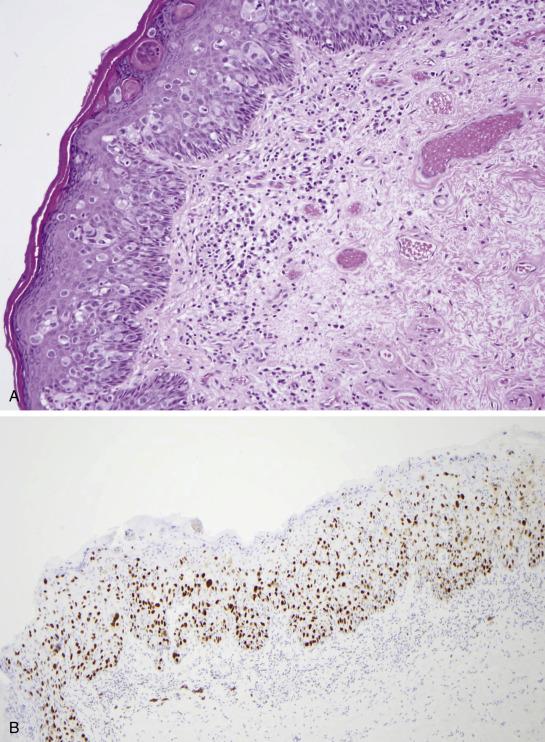
Lastly, the protein expression profile of EMPD suggests a possible cell of origin for cases of primary EMPD, which include Bartholin glands and Toker cells. Bartholin glands are small, apocrine-like structures located in the introitus. Toker cells are mononuclear cells that can be seen in several anatomic areas, including the vulva. These cells are large and arranged as single units at all levels of the epidermis. The fact that the immunophenotype of Toker cells, Bartholin gland cells, and EMPD is very similar (positive for CK7, low-molecular-weight cytokeratin, MUC1, and MUC5AC) would support the possible origin of EMPD from these two sites. It has been hypothesized that EMPD may represent a proliferation of adnexal stem cells residing in the infundibulosebaceous unit of hair follicles and adnexal structures, as the EMPD cells stain for markers typically seen in follicular stem cells located in the hair follicle bulge region, including CK15 and CK19.
Vulvar intraepithelial neoplasia and invasive squamous cell carcinoma may show intraepidermal tumor cells distributed in a pagetoid pattern (so-called pagetoid Bowen disease). However, the atypical cells are usually present at all levels of the epidermis and, unlike EMPD, they also occupy the basal layer and contain keratohyaline granules within tumor cells. A p63 or p40 stain can be useful in this differential diagnosis as they are markers of squamous differentiation. In addition, neoplastic squamous cells are positive for high-molecular-weight-keratins and negative for GCDFP-15- and mCEA. Of note, EPMD may be positive for p16 but usually only weak to moderate positivity. Melanoma should also be considered in the differential diagnosis as both entities may have a pagetoid growth and melanin may be secondarily present in the cytoplasm of Paget cells. Thus, the presence of a melanocytic proliferation along the basal layer of the adjacent epidermis should therefore be sought in every case of putative EMPD. Immunohistochemistry can be useful; however, as EMPD cells may occasionally be S-100-positive, a panel of antibodies is recommended in problematic cases (e.g., cytokeratins, mCEA, HMB-45, MART-1, MiTF, SOX-10). Sebaceous carcinoma often infiltrates the overlying epidermis in a pattern similar to EMPD. However, the cells exhibit multiple cytoplasmic vacuoles that characteristically indent the nuclei and, unlike Paget disease, the neoplastic cells lack mCEA and GCDFP-15 expression. In addition, involvement of the perineum by sebaceous carcinoma is exceptional. Finally, pagetoid dyskeratosis is characterized by the presence of small papules in intertriginous areas. It is considered a reactive process in which a small part of the normal population of keratinocytes is induced to proliferate or develop dyskeratosis. These cells spread in the epidermis in a pagetoid manner and express low-molecular-weight cytokeratin (e.g., CAM5.2). In contrast to EMPD, pagetoid dyskeratosis cells are small, display small pyknotic nuclei, and are negative for mCEA and GATA3.
“Primary” EMPD is associated with an overall good prognosis; however, there is a relatively high recurrence rate even after wide local excision, which can be explained by the presence of frequent microscopic extension beyond the clinically involved area and the presence of multifocal disease; thus, status of resection margins is not predictive of local recurrence and the use of intraoperative frozen section is not helpful in reducing recurrence rate. Location within the perineum may be associated with an increased recurrence rate and shorter time to recurrence. Cases with more than minimally invasive disease (>1 mm) are associated with a worse prognosis, and may result in nodal or distant metastases and secondary death. Sentinel lymph node biopsy may be considered in cases with dermal invasion. Topical imiquimod, photodynamic therapy, chemotherapy, and radiotherapy have been used in nonsurgical cases, with diverse results. The prognosis of EMPD associated with an underlying carcinoma is poor, and related to the prognosis of that primary tumor.
Intraepidermal adenocarcinoma in any anatomic region outside the breast
Rare (<1% of population)
Anogenital region most frequent location (followed by axilla, ear, eyelid)
Pruritus, ulceration, and superinfection
High recurrence rate if incompletely excised
If associated with underlying invasive adenocarcinoma, mortality rate related to primary tumor
Eczematous, itchy plaque
25% associated with internal malignancy (colorectal, cervical, bladder/urethra)
Poor prognosis if more than minimal invasion (>1 mm) or association with internal malignancy
Radical resection treatment of choice
Radiotherapy or chemotherapy in nonsurgical cases (poor response)
Ill-defined, diffuse red plaque, rarely ulcerated
Nests, isolated large cells, or rarely glands mainly located in epidermal basal and parabasal layers “compressing” basal keratinocytes
Atypical cells may be present in upper layers
Cells contain abundant pale cytoplasm with frequent intracytoplasmic vacuoles and large nuclei
Variable mitotic figures
Rarely, intracytoplasmic melanin pigment
CAM5.2 (low-molecular-weight cytokeratin), mCEA, GATA3, EMA, and androgen receptor positive
ER/PR, p63, p40, high-molecular-weight-cytokeratin, melanocytic markers negative
Primary EMPD: CK7+/CK20−/GCDFP-15+/MUC1+/MUC5+
Secondary EMPD (anorectal): CK20+/MUC2+/CK7-/GCDFP15−/GATA3-
Secondary EMPD (urothelial): CK7+/CK20+/uroplakin III+/GCDFP15−
In situ or invasive squamous cell carcinoma with pagetoid growth
Melanoma
Sebaceous carcinoma
Pagetoid dyskeratosis
Mucosal lentigo is a benign lesion defined as a pigmented macule or patch on a mucous membrane. In the vulva, it typically presents as a large and asymmetric area of brown to blue-black hyperpigmentation with irregular borders, and not infrequently can cause clinical concern for melanoma. On rare occasions, it may be associated with Carney complex. Histologically, the epithelium displays hyperpigmentation of the basal keratinocytes predominantly involving the tips of the elongated rete ( Fig. 3.9 ). Other associated findings include mild acanthosis, slightly increased number of melanocytes, and melanophages in the papillary dermis. However, in contrast to melanoma, melanocytes are cytologically benign (small with hyperchromatic nuclei and lacking prominent nucleoli) and are arranged as evenly distributed single cells in the basal epidermis without pagetoid upward migration.
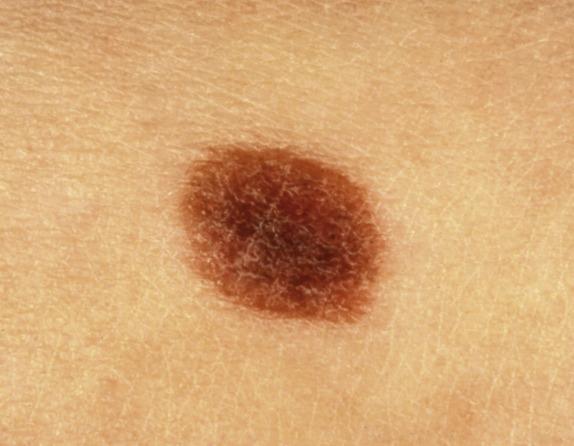
Vulvar melanocytic nevi are uncommon, occurring in approximately 2%–3% of women. In the genital region, nevi often show clinical and histologic features somewhat different from those seen in other anatomic locations. Some authors refer to melanocytic nevi occurring in the genital area, flexural sites (inguinal folds, scrotum, perineal area, and axilla) and acral regions as nevi of “special sites.” Etiologically, it has been suggested that some of the architectural atypical features (single cells, rete ridge bridging) may be related to external trauma to the lesion (rubbing, etc.). It is important to recognize these types of nevi in order to avoid a misdiagnosis of dysplastic nevus or melanoma.
The lesions occur in young women (mean 25 years at diagnosis) and are usually asymptomatic. They are most often discovered during routine gynecologic examination, during pregnancy or at the time of delivery.
Most of the lesions are located in the labium majus or minus, and less commonly in the clitoral area. Most genital-type nevi are macular or papular, symmetrical, small lesions (usually <1 cm) with well-defined margins ( Fig. 3.10 ). They usually exhibit evenly distributed, tan to brown pigment. Around 5% of patients with genital nevi are children (with a majority being affected at birth).
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here