Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
EPO is a member of the type I cytokine family and a multifunctional protein
EPO is synthesized primarily by the kidney as a circulating hormone for erythropoiesis and locally for paracrine/autocrine tissue protection and repair
EPO activates multiple cell types in both its hematopoietic and extrahematopoietic roles
Cross talk between the hormonal and paracrine/autocrine systems is prevented by existence of receptor isoforms having different affinities for EPO, as well as a variable sialic acid content of the oligosaccharide chains (and therefore different circulating half-lives) depending on the site of EPO synthesis
As a response to injury or metabolic stress EPO and its receptor are upregulated locally in a staggered temporal–spatial fashion with receptor before EPO
EPO counteracts inflammation and confers cytoprotection
EPO also activates healing and regenerative processes
EPO analogs have been engineered which are tissue protective but not hematopoietic
Nonhematopoietic derivatives avoid the potential adverse effects of high-dose recombinant EPO administered for tissue protection
Erythropoietin (EPO), long identified as the hormone responsible for regulating hematopoiesis in vertebrates, is a member of the type I cytokine superfamily. Similar to other proteins in this diverse family of intercellular signaling molecules, EPO is multifunctional. From a broad perspective, it appears that EPO evolved to play a central protective role in the adaptive response of organisms to cellular and metabolic stressors, of which the hematopoietic response to systemic hypoxia is only one component. The requirements for EPO to mediate important local and systemic physiological functions have resulted in the evolution of distinct molecular isoforms of EPO and its receptor, which assure that normally no cross talk between different roles occurs. These factors must be taken into consideration to understand the biology of this system, and in particular when attempting to provide therapeutic benefit, e.g., tissue protection, by use of recombinant EPO or its engineered tissue protective derivatives.
This chapter will be divided into two parts: (1) background information concerning the biology of the nonerythropoietic paracrine/autocrine EPO system and (2) nonexhaustive examples of the cytoprotective, antiinflammatory, and repair components of this system in action in representative tissues and organs.
Adaptive responses to stressors have evolved from the complex interaction of organisms with their internal and external environments. The need to detect and respond to hypoxia or diverse molecular signals arising from tissue injury or foreign invaders has resulted in stereotyped responses that are evolutionarily ancient, as shown by the presence of molecules having similar biological functions in a wide variety of organisms ranging from primitive invertebrates to mammals. Historically, the response to tissue injury has been termed the innate immune response (IIR) in which proinflammatory mediators dominate, while the protective responses to hypoxia and cellular metabolic stress have been viewed separately as the hypoxic stress response. However, these physiological processes are interrelated, together forming an integrated stress response system that isolates injury while providing protection to the normal surrounding tissue, as well as actuating the coordinated repair of damage and the restoration of function. Work performed over the last two decades by many individuals has shown that EPO plays key protective and regenerative roles in this complex stress response system (reviewed in Refs. ). Additionally, EPO has recently been documented to play an important role in metabolism and body mass regulation, which can be viewed, in part, as a response to an inflammatory form of cellular stress (reviewed in Ref. ).
The maintenance of adequate tissue O 2 concentration to support cellular metabolism is a crucial physiological function for oxygen-utilizing animals. In vertebrates, delivery of O 2 to tissues occurs via a tightly orchestrated negative feedback control process in which oxygenated hemoglobin within erythrocytes is delivered to tissue beds by the systemic circulation. The key hormone responsible for maintaining adequate circulating hemoglobin concentrations is EPO (see Chapter 11, Chapter 12 ). In the normal adult mammal, for example, hypoxia is detected within the cortex and outer medulla of the kidney by peritubular fibroblast-like type-1 interstitial cells with the resultant synthesis of hypoxia inducible factor (HIF). In turn, HIF activates a wide variety of responses adaptive for hypoxic conditions, including the transcription of the EPO gene. EPO is subsequently released into the circulation to activate the survival of erythrocyte precursors (colony forming units erythroid; CFU-E) residing within the bone marrow. It is important to note that the erythropoietic response depends only in part on adequate circulating EPO concentrations, as the cognate receptor is restricted to a subset of erythroid precursors at a specific stage of differentiation. Therefore, receptor expression is a critical driver of the biological response. This is also a feature of EPO’s nonerythropoietic activities.
EPO-responsive immature red cells express a receptor for EPO that consists of two identical protein monomers (EPOR 2 ) present at relatively low concentrations (∼1000/cell). When inserted into the cell membrane, these subunits migrate to lipid rafts, and if at a sufficient receptor density, spontaneously associate through a leucine zipper mechanism within the cell membrane spanning region. When an EPO molecule bridges across the assembled receptor monomers using distinct recognition binding sites, a conformational change occurs in EPOR 2 that results in autophosphorylation of janus kinase-2 (JAK2). This molecular signal in turn activates multiple secondary cascades that include the (1) signal transducer and activator of transcription (STAT), (2) phosphatidyl inositol-3 kinase (PI-3K), (3) mitogen-activated protein kinase (MAPK), (4) protein kinase C (PK-C) and (5) phospholipase C (PLC) pathways ( Fig. 23.1 ). As a group, the activity of these molecular cascades antagonizes the ongoing programmed cell death of immature erythrocytes, allowing maturation to proceed (see Chapter 11 ). As discussed below, except for PLC, each of these pathways has been documented to transduce, sometimes in a tissue-specific manner, the nonerythropoietic actions of EPO.
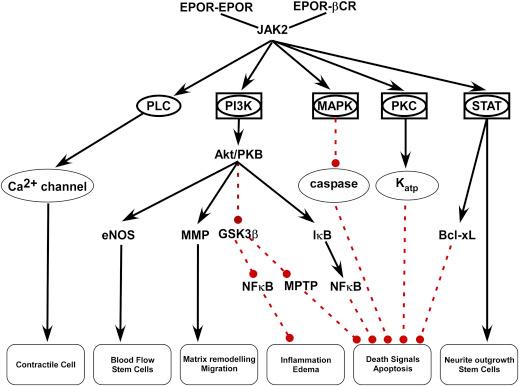
Systemic hypoxia also activates other EPO-dependent biological responses that function to improve or maintain the delivery of O 2 to tissues. Thus, EPO jointly activates adhesion molecules, e.g., endothelial cell selectin expression and vascular cell adhesion molecule, while in synergy with thrombopoietin stimulates the production rate and reactivity of platelets. Together, these actions are strongly prothrombotic, resulting in a state which potentially prevents blood flow into damaged tissues and also reduces the probability of hemorrhagic blood loss. Additionally, EPO improves local tissue oxygen delivery by modulating regional blood flow via stimulating endothelial nitric oxide production. EPO also directly interacts with the neural control of ventilation so as to increase the intake of O 2 during hypoxia. On a longer time scale, EPO improves tissue oxygenation by activating neoangiogenesis via stimulating the mitosis, migration, and differentiation of endothelial cells as well as synergizing with angiogenic factors, e.g., vascular endothelial growth factor (VEGF). Thus, in its hematopoietic role EPO mediates cell growth, survival, differentiation, migration, and function of a variety of cell types in addition to the CFU-E.
Besides the kidney, other tissues and organs synthesize HIF when subjected to hypoxic stress under a wide variety of pathological and physiological conditions. However, mere expression of HIF does result in EPO synthesis, as there is also control provided by tissue-specific repressive factors, e.g., via the GATA box. However, results of study have shown that similar to renal production of EPO, increases in HIF in some nonrenal tissues is accompanied by EPO synthesis. For example, glial cells maintained in vitro produce EPO following exposure to hypoxia, which is abolished by HIF neutralization. Similar to EPO expression, EPO receptor is also upregulated by HIF. In sum, a number of studies have shown that oxygen-deprived cells can produce both EPO and its receptor, thus constituting an autocrine/paracrine system distinct from the hormonal hematopoietic system.
The IIR is an ancient, stereotyped, localized reaction to molecular signals arising from tissue injury and/or pathogen invasion. The key effector molecules are proinflammatory cytokines, e.g., TNFα, which although potentially toxic for foreign invaders, also can damage normal tissues. One important control molecule of inflammation is NFkB which is ordinarily under chronic inhibition by the inhibitor of IkB kinase (IKK). In response to cellular stress, the inhibitor of IKK is itself antagonized and NFkB becomes functional through disinhibition of IKK. Following triggering, proinflammatory cytokines are generated, which if significant concentrations are achieved, kill surrounding normal and abnormal tissues. Once activated, the IIR tends to operate via a positive feedback control system. Too vigorous an IIR is not beneficial, e.g., sustained TNFα production is a common feature of many diseases.
HIF functions as a heterodimer with a hypoxia-sensitive HIF alpha subunit and a constitutively expressed beta subunit (see Chapter 11 ). Under normoxic conditions, HIF is continuously degraded by the O 2 -dependent prolyl hydroxylases (PHD). Tissue hypoxia causes a decrease in PHD activity, which in turn stabilizes HIF heterodimer formation ( Fig. 23.2 ). As a result, a myriad of genes involved in metabolism, angiogenesis, and cytoprotection are activated, including EPO . However, PHD is also a molecular control of the IIR, as IKK is also inhibited by PHD. Specifically, under hypoxic conditions, the resultant PHD inhibition results in activation of IKK, and thus also NFkB, which in turn acts as a transcriptional activator of HIF. In this manner, innate immunity and hypoxic responses are interlinked, a relationship that is evolutionarily ancient, as homologs for HIF and NFkB are expressed by invertebrates and interact in similar ways.
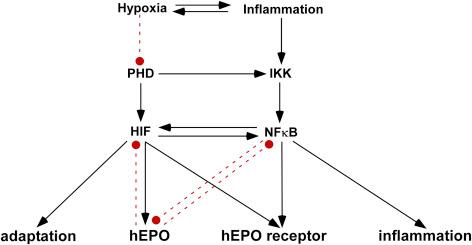
In the complex setting of tissue injury in which both hypoxia and inflammation play important pathophysiologic roles, EPO may be expressed in a distinct temporal pattern by a variety of cells that do not respond to hypoxia alone. As an important example, noninjury-related HIF expression by capillary endothelial cells does not result in EPO expression. In contrast, capillary endothelial cells within the immediate vicinity of an ischemic brain infarct strongly turn on EPO production with 24 h, followed with a delay by microglia (3 days) and reactive astrocytes (7 days). Therefore, capillary endothelial cells within close proximity to a region of injury are a potential source of EPO for any tissue.
As the above discussion has summarized, cellular stress activates a positive feedback system that turns on inflammation. To prevent catastrophic collateral damage there must be control elements capable of containing these processes. Work performed over the last 20 years has shown that EPO is the key negative modulator of these immune responses ( Fig. 23.2 ). EPO synthesis is controlled by the hypoxia response element (HRE) within its structural gene to which HIF binds. However, NFkB also has a binding site adjacent to the HRE that inhibits EPO expression. In addition, products of NFkB activation, e.g., TNFα, can directly inhibit EPO production. At the same time, EPO itself inhibits both HIF and NFkB production. In contrast to the effector molecule EPO, its receptor is strongly upregulated by both HIF and proinflammatory cytokines. Thus, there is a complex interplay between the expression of proinflammatory and protective molecules that are produced in a staggered temporal manner at spatially distinct sites such that the receptor concentration may be high when the ligand concentration is low ( Fig. 23.2 ).
Activity of the paracrine EPO system is critical not only for protection from a wide variety of stressors but also for angiogenesis during development, which is driven in part by hypoxia-induced EPO expression. This is evidenced by the observation that either EPO or EPOR gene knockout results in a dramatic reduction in the number and complexity of vessels in mouse embryos beginning at day 10.5 in development, followed 2 days later by cardiac ventricular hypoplasia, and ultimately death 1 day later. Additionally, local EPO production an important signal in the cyclical angiogenic changes in the adult of the uterus and endometrium as shown by disruption of angiogenesis following administration of a soluble EPOR that complexes and inactivates endogenous EPO. EPO is also required for stem cell differentiation in some tissues. For example, EPO mRNA is detectible and increases as a function of time in the developing human brain and subsides to a much lower level in the adult. Proof of the indispensability of EPO in this setting is demonstrated by gene knockout of EPO which severely disrupts brain development and results in massive losses of neuronal progenitor cells through apoptosis.
In addition to providing a multitude of direct and indirect cytoprotective roles, EPO also participates in all phases of tissue repair and regeneration. Normal healing requires both an inflammatory stage, characterized by programmed cell death, followed by remodeling driven by the growth of new blood vessels. Similar to its function in development, a major function of EPO in repair is to mediate (in conjunction with VEGF) neoangiogenesis resulting in the generation of the microvascular network that underlies wound healing. In this function, EPO activates the homing of endothelial progenitor cells (EPCs) which differentiate to form new vessels and stimulates nitric oxide production which enhances blood flow. EPO also turns on metalloproteinases which reorganize the intercellular matrix, allowing tissue-specific stem cells to migrate within the region of injury and tissue remodeling to occur.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here