Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Extracorporeal membrane oxygenation (ECMO) consists of a specific heart-lung machine that provides circulatory support and/or gas exchange for patients with severe but potentially reversible respiratory or cardiac failure or both. Although the term <ce:italic>extracorporeal life support</ce:italic> (ECLS) might describe the system more accurately, ECMO is actually most often used and universally accepted for describing all its applications.
Different configurations of ECMO (e.g., venovenous [VV], venoarterial [VA], venous to pulmonary artery [V-PA]) can be utilized depending on specific organ failure and severity (e.g., respiratory failure, cardiogenic shock, cardiogenic shock associated with respiratory failure, respiratory failure in association with right ventricular failure). The most rapid way to initiate ECMO urgently (excluding patients undergoing cardiac surgery) is peripheral VA cannulation.
ECMO must be viewed as a “bridging” therapy where the anticipated outcome is either recovery or replacement of the failing heart, lung, or both. Assessment of the likelihood of survival using recently published scoring systems, and of the patient’s potential candidacy for transplant or device (cardiac) should be made prior to initiation. Ideally the decision to proceed with ECMO should be made by a team rather than an individual.
Overall survival of VV ECMO to recovery is now approximately 60% in adults with acute respiratory distress syndrome (ARDS), usually due to viral or bacterial infection, whereas survival of VA ECMO in adults with severe cardiac failure is approximately 40%. Where ECMO is urgently initiated in the setting of <ce:italic>extracorporeal</ce:italic> cardiopulmonary resuscitation (ECPR) survival in adults is 29%.
Vascular access is a critical aspect of ECMO as different sites are associated with different characteristics of flow and gas exchange in combination with the patient’s native heart and lung functions. Vascular complications are common, with arterial complications more frequent and severe than venous complications.
Anticoagulation management is institution specific, varying by the nature of the circuit (how much of the ECMO circuit is heparin bonded), flow (greater anticoagulation needed for lower flows), and which coagulation tests are readily available. Bleeding and clotting complications are common.
The cardiac anesthesiologist plays an important role in the cannulation and decannulation of patients, managing sedation and cardiac support medications for urgent bedside procedures, anesthesia for operative procedures, and in providing echocardiographic assessment of cannula placement and cardiac function. The anesthesiologist-intensivist is an integral part of the management team in the intensive care unit (ICU), providing a continuum of care through ECMO management and potential transition to advanced therapies both in the ICU and the operating room.
The editors and publisher would like to thank Drs. Zaccaria Ricci, Stefano Romagnoli, and Claudio Ronco for contributing a chapter on this topic in the prior edition of this work. It has served as the foundation for the current chapter.
Extracorporeal membrane oxygenation or ECMO refers to a number of configurations of extracorporeal circulatory and/or respiratory support. As the technology evolved, other acronyms such as MCS (mechanical circulatory support) and ECLS (extracorporeal life support) have been used, but in North America ECMO continues to be the most commonly used term to describe a circuit, pump, and oxygenator that can perform the work of the heart and lungs by adding oxygen and removing carbon dioxide from circulating blood for prolonged intervals. Venovenous (VV) ECMO withdraws venous blood and returns oxygenated blood to the right side of the heart supporting only respiration; venoarterial (VA) ECMO withdraws venous blood and returns oxygenated blood to the arterial system, thereby supporting both respiration and circulation. Another configuration is venous-pulmonary artery (VPA) ECMO used to support the right heart and the lungs when there is right heart failure and respiratory failure but the left heart is not supported.
An ECMO circuit comprises cannulae inserted into large vessels to remove and return the blood from the patient, tubing to connect to a nonpulsatile centrifugal pump that generates blood flow, and an oxygenator, where an oxygen-air mixture flows through the blood, referred to as the “sweep.” This is a closed system without a reservoir, with some or all of the circuit components surface bonded with heparin, and is designed for extended use, such as days or weeks. This is in contrast to the extracorporeal circuit used for cardiopulmonary bypass (CPB) in the operating room that uses larger-diameter and longer tubing (larger “prime” volume), and is an open system with a reservoir designed to receive input not only from the venous cannula but also from the operative field. Such CPB circuits may or may not be heparin bonded, and are intended for surgical use of short duration (e.g., hours). There is some evidence in the lung transplant literature that use of ECMO during the surgical procedure may be associated with a smaller systemic inflammatory response than the use of the traditional CPB.
Pumpless extracorporeal lung assist (pECLA), or the Novalung, uses the patient’s arterial pressure rather than a pump to drive blood through an extracorporeal oxygenator. It therefore supports only respiration. This device requires less anticoagulation than traditional ECMO but due to lower flows and the membrane area also has a more limited capacity especially for oxygenation. It has been used to support patients with pulmonary hypertension awaiting lung transplantation, implanted in a pulmonary artery to left atrium configuration. It is available in North America but is less widely used than ECMO and will not be discussed further.
The history of ECMO is inextricably bound to the development of CPB for cardiac surgery. The first successful use of CPB in a human was by Gibbon in 1953, to repair an atrial septal defect in an 18-year-old patient. The following year, Warden and colleagues reported cardiac surgery using extracorporeal circulation, after which there was an ever increasing number of reports from many centers. Among the major limitations in these early reports was the use of “bubble” oxygenators where oxygen is bubbled through a reservoir of blood to achieve gas exchange. These oxygenators are associated with trauma to formed elements of the blood and coagulopathy with prolonged use. The development of oxygenators with membranes separating flow of the respiratory gases from the blood reduced these effects, making the oxygenators suitable for longer-term use. Membrane oxygenators have replaced bubble oxygenators in cardiac surgery and their development led to the first successful reports of prolonged support outside of the operating room, in 1972 by Hill and associates in a 24-year-old trauma patient, and by Bartlett after neonatal cardiac surgery. In 1985 Bartlett and associates reported the successful use of ECMO for neonatal respiratory failure in 11 patients. In the following two decades a number of additional trials showed the benefit of ECMO in neonatal respiratory failure, the most definitive of which was published in 1996 by the UK Collaborative ECMO Trial Group in 185 infants. In the same year Green and colleagues published a trial demonstrating similar benefit in older pediatric patients with respiratory failure. Note the majority of use in the first decades was for respiratory failure, using VV ECMO. During this period the Extracorporeal Life Support Organization or ELSO was founded, first at the University of Michigan in 1989, then a European ELSO group formed in 1991. This organization has played a key role in documenting worldwide ECMO use, and pioneering education, research, and development in all types of ECMO support in all populations. Fig. 85.1 and Table 85.1 illustrate the increase in use of ECMO and the survival data since 1990 ( www.ELSO.org ).
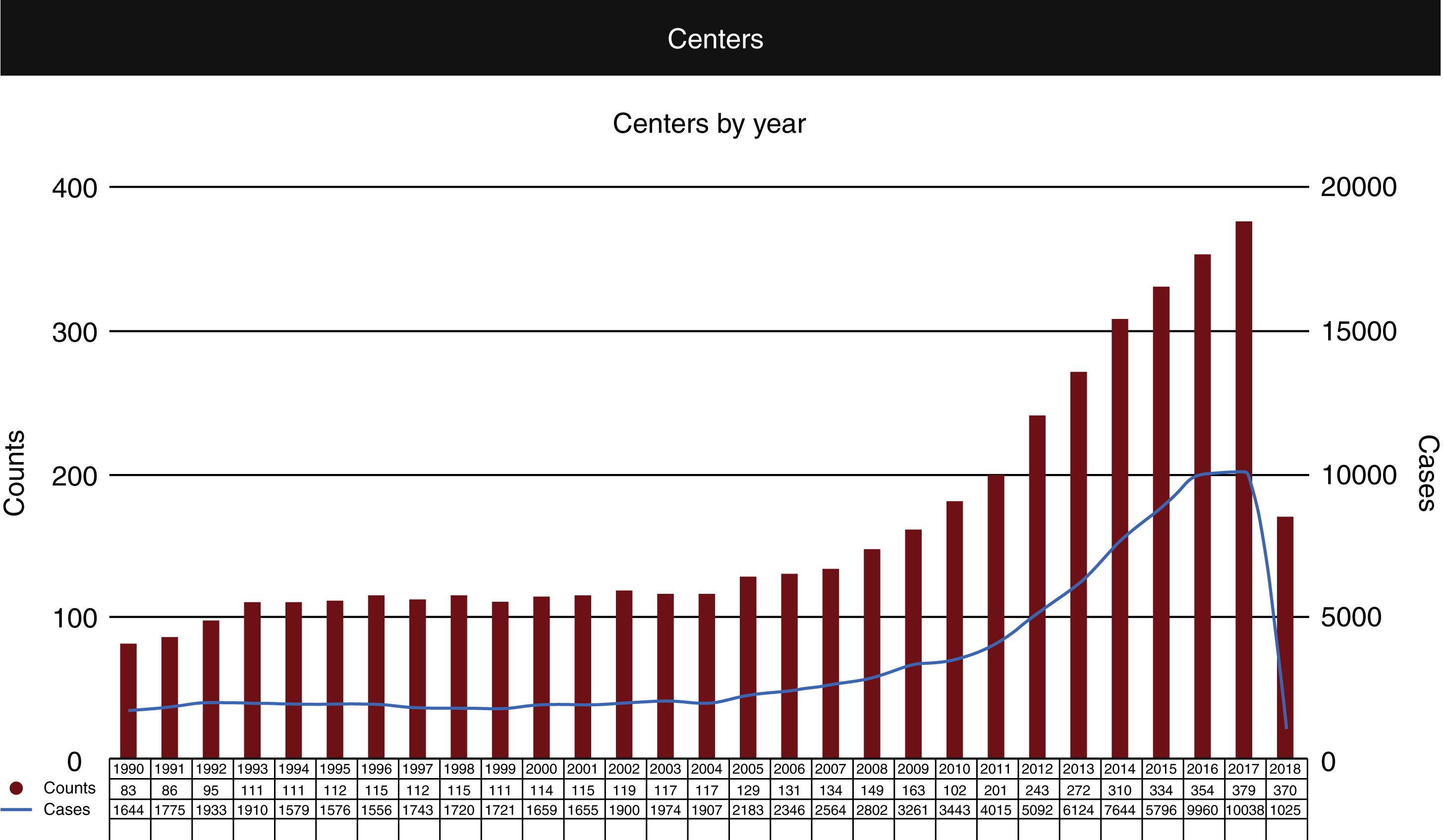
| Overall Outcomes | |||
|---|---|---|---|
| Total Runs | Survived ECLS | Survived to DC or Transfer | |
| Neonatal Pulmonary Cardiac ECPR |
30,934 7,794 1,718 |
25,990 (84%) 5,063 (64%) 1,140 (66%) |
22,662 (73%) 3,281 (42%) 708 (41%) |
| Pediatric Pulmonary Cardiac ECPR |
8,820 10,462 3,946 |
5,953 (67%) 7,177 (68%) 2,262 (57%) |
5,131 (58%) 5,447 (52%) 1,675 (42%) |
| Adult Pulmonary Cardiac ECPR |
16,337 15,942 4,952 |
10,857 (66%) 8,865 (55%) 1896 (38%) |
9,649 (59%) 6,747 (42%) 1,443 (29%) |
| Total | 100,905 | 69,203 (68%) | 56,743 (56%) |
In contrast to the ECMO experience in neonatal and pediatric respiratory failure as previously described, demonstration of benefit in adults took much longer, delayed in part due to the publication of a trial in 1979 by Zapol and associates in 90 adult patients with respiratory failure. This trial had many limitations, including the use of VA rather than VV ECMO, patient selection, anticoagulation technique and bleeding complications, and the use of standard ventilation at the time—relatively high-tidal volume and low positive end-expiratory pressure (PEEP). Poor outcomes deterred adult use of ECMO for more than 20 years. From 2001 to 2006 a large, ambitious British trial, the CESAR trial, was performed to evaluate VV ECMO for respiratory failure in adults. This study was performed during the H1N1 influenza pandemic, and involved transferring patients with severe respiratory failure to a central expert ECMO center, where they were randomly assigned to ECMO or standard therapy. Despite some methodological and statistical limitations, the results supported the use of ECMO performed in a specialized center to improve survival: 63% versus 43% survival with ECMO versus standard treatment. At the same time, another report (case series) of VV ECMO in adults with severe acute respiratory distress syndrome (ARDS) due to H1N1 was reported from Australia and New Zealand (ANZ ECMO). This study found a 79% survival at 30 days in patients who received ECMO. Recently a large multicenter trial of VV ECMO in adults with severe ARDS, the EOLIA trial, was published in 2018, with the authors concluding no difference in mortality between ECMO and conventional therapy at 60 days: 35% versus 46% ( P = .09), with 28% of the control group crossing over to ECMO after randomization and a 57% mortality in this crossover group. Editorial comments on this study have challenged this conclusion, maintaining that it supports the use of early ECMO in adults with severe ARDS. The ELSO database reports survival to discharge with ECMO for adult respiratory failure as 60% with this percentage being relatively stable over 15 years.
Box 85.1 lists common indications for VV ECMO in respiratory failure. As VV ECMO supports only respiratory function, if the patient has right- or left-sided cardiac failure then another configuration of support must be used. The most common indication is ARDS, most commonly due to viral or bacterial infection. As indicated previously, the most studied population is patients with H1N1 viral pneumonia. A commonly used assessment for the severity of ARDS is the Murray score, which is based on four standard criteria: PaO 2 /FiO 2 gradient for oxygen, degree of PEEP, number of quadrants affected as shown on the chest radiograph, and lung compliance. In 2012, the Berlin criteria were published, where the severity of ARDS is rated as mild, moderate, or severe based on the PaO 2 /FiO 2 gradient for oxygen if other criteria are present. In general, patients with severe ARDS (PaO 2 /FiO 2 gradient of < 100 mm Hg with PEEP > 5) are potential candidates for ECMO as the mortality without ECMO is approximately 40%. As described later in the section on the ethics of ECMO, there are studies that evaluate the likelihood of survival at the time ECMO is being considered; this can help guide decision making. It should also be mentioned that from the CESAR trial described earlier, if a patient being considered for VV ECMO is not at an ECMO center or one with expertise in management of ARDS, transfer to such a facility is likely to provide a better outcome even in the absence of ECMO. While not formally studied, many reports indicate that outcomes are better with earlier institution of ECMO, probably at least in part because this permits the use of lung protective ventilation when respiration is supported by ECMO.
Severe ARDS
Murray score of 2.5
Berlin definition
Respiratory failure associated with:
Refractory hypoxemia despite maximum less invasive therapies
e.g., FiO 2 >90%, PEEP >15 cm H 2 O, prone ventilation
Refractory hypercarbia (e.g., PaCO 2 > 80) with acidosis
Injurious ventilating pressures (e.g., plateau pressures >30 mm Hg) with lung-protective tidal volumes
Common clinical conditions
Severe pneumonia (viral or bacterial)
Aspiration pneumonitis
ARDS from any cause
Pulmonary contusion
Status asthmaticus
Severe air leak syndrome
Inhalation injury
Airway obstruction (e.g., mediastinal mass)
Pre and post lung transplant
ECMO for lung transplantation is discussed in the next section.
In keeping with ELSO guidelines there are no absolute contraindications for VV ECMO in adults Box 85.2 . There are, however, conditions known to be associated with a poor outcome, despite ECMO; these should always be considered before initiating ECMO assistance. These conditions include: injurious mechanical ventilation for 7 days or longer, major pharmacologic immunosuppression, and intracranial hemorrhage that is recent or expanding. Specific patient conditions should also be considered. Although no specific age is a contraindication, increased age is considered to increase the risk. A body mass index (BMI) of more than 40 to 45 may be associated with technical difficulties and the risk of not being able to achieve an adequate blood flow. VV ECMO is a bridge to either recovery or lung transplant; if neither of these outcomes appears at all likely then its initiation is not advisable.
Cardiogenic shock
Hypotension/poor tissue perfusion despite maximum medical therapy +/− balloon pump
Combined cardiorespiratory failure
Cardiogenic shock with pulmonary edema and hypoxemia
Urgent ECMO for respiratory failure
As temporizing measure before institution of VV ECMO
Common clinical conditions
Refractory cardiogenic shock (any cause)
Failure to separate from cardiopulmonary bypass
Bridge to durable ventricular assist device or transplant
Intraoperative lung transplant
Unstable arrhythmias
Anaphylaxis
Massive pulmonary embolus
Cardiac arrest without return of spontaneous circulation
VA ECMO, venoarterial extracorporeal membrane oxygenation; VV ECMO, venovenous extracorporeal membrane oxygenation.
The history of ECMO for severe respiratory failure as described previously refers mostly to patients with acute or acute on chronic disease where recovery could be anticipated. Another population is patients with end-stage chronic lung disease awaiting lung transplantation. These patients often have a slow (in years) deterioration in function and an increased need for oxygen support, with the final stage being an acute deterioration where standard therapy with mechanical ventilation ultimately fails. Institution of ECMO to prolong survival until transplant, use of ECMO during transplant surgery, and extension or initiation of ECMO postoperatively for primary graft dysfunction (PGD) or other indications have all become common applications for this advanced therapy. As recently as 2010 there was concern that the use of ECMO resulted in a reduced long-term survival from lung transplantation, but this is no longer the case in 2018. Case reports, single center reports, and surveys have documented that the use of pretransplant ECMO, sometimes for months, was followed by successful transplant and good long-term outcomes. Raleigh and associates compared 10 studies on the use of preoperative ECMO for lung transplant patients and found similar outcomes to the patients who did not need ECMO and Loor and associates described factors that contributed to posttransplant survival in this population. Intraoperative use of ECMO during the procedure has also been shown to reduce the inflammatory response and leads to less PGD when compared to CPB and leads to improved short-term and longer-term outcomes even when compared to no use of either CPB or ECMO intraoperatively. While preoperative ECMO may be VV, VA, or VPA, intraoperatively VA ECMO is usually used due to surgical manipulation of the heart with its attendant hemodynamic compromise as well as the need for one-lung ventilation in patients with end-stage pulmonary disease and elevated pulmonary vascular pressures. Postoperatively, ECMO support may be needed to support the new lungs in the face of PGD, the right heart, the left heart, or any combination of these. Postoperative ECMO support is also associated with excellent outcomes.
VA ECMO can be used to support the heart and lungs temporarily in a patient with poor cardiac function who undergoes an invasive cardiology procedure, to continue postoperative cardiopulmonary support in a cardiac surgery patient who fails to separate from CPB, and in a patient with refractory cardiac failure with or without associated respiratory failure. These conditions may occur due to an acute recoverable illness (e.g., myocarditis) or may be in the setting of acute on chronic heart failure in patients being evaluated for long-term advanced therapies such as a durable ventricular assist device or cardiac transplant. Urgent use of ECMO in the acute setting of in-hospital cardiac arrest (ECPR) is also practiced in some centers. Finally, as VA ECMO can be instituted at the bedside without imaging, it may be the preferred technique for emergent cannulation in any form of respiratory or cardiac failure, with elective conversion to another form (e.g., VV ECMO) once the patient has stabilized.
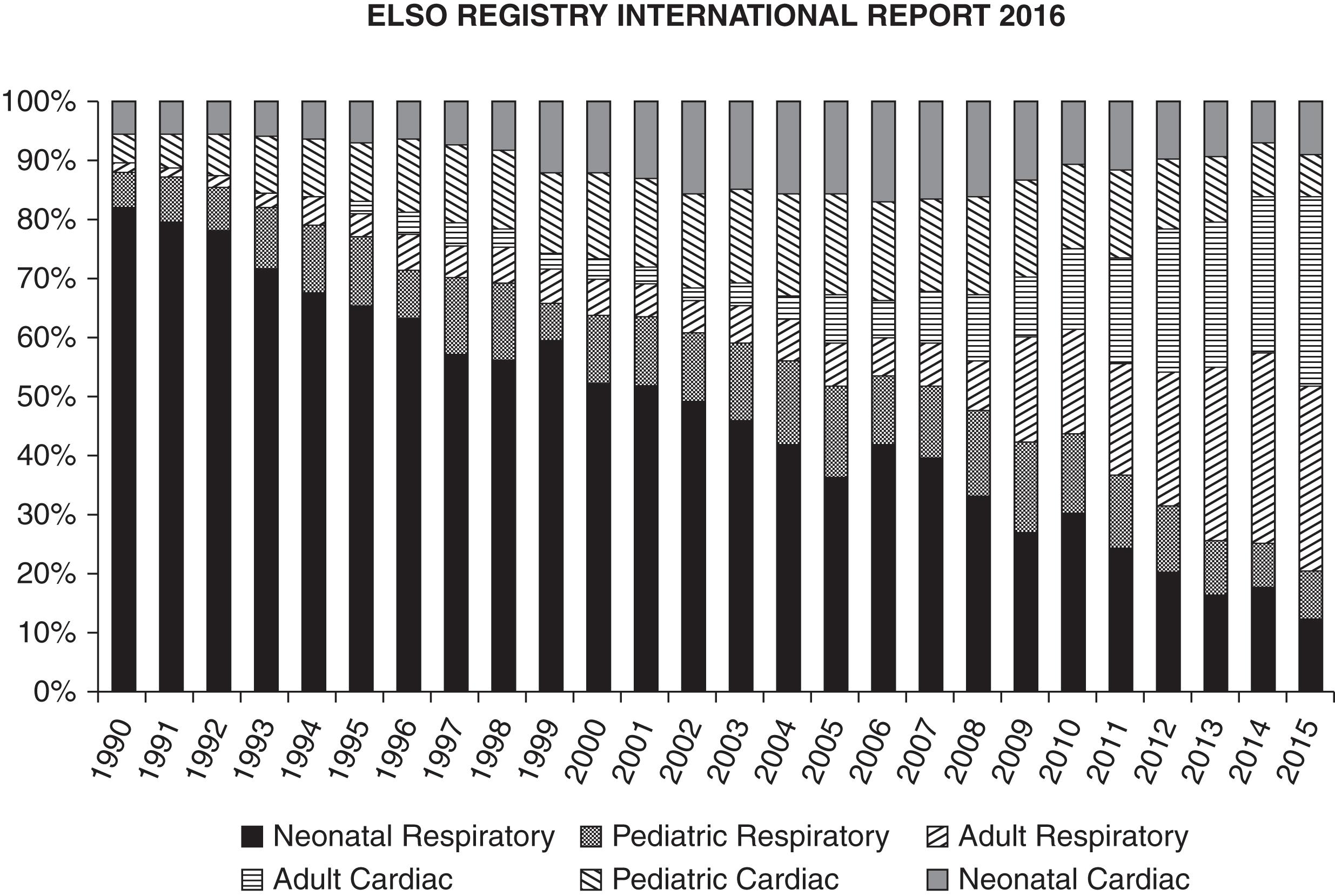
The historical perspective for use of VA ECMO for cardiac or combined cardiorespiratory support in adults is illustrated in the 2016 report from the ELSO registry. Adult cardiac ECMO was in its infancy in 1990 with little increase in use until 2006 when its use began to increase exponentially; there were more than 2000 adult cardiac ECMO runs reported to ELSO in 2015, comprising an ever-increasing proportion of all ECMO runs ( Fig. 85.2 ). This exponential increase was likely fueled by consistent success in the neonatal and pediatric populations; improvements in the ECMO circuits, pumps, and oxygenators; and the success and experience with VV ECMO for adult respiratory failure. Overall survival in adults who receive ECMO for cardiac indications is approximately 40% with a slight increasing trend over the last 10 years.
For periprocedural support of the heart only, short-term devices such as a percutaneous left ventricular assist device (LVAD, e.g., Impella or TandemHeart) are one type of support; the other is short-term ECMO, usually peripheral (femoral) VA ECMO. Recent reviews of temporary circulatory support devices in cardiology compare and summarize risks, benefits, and outcomes with the different approaches in different settings. Use of VA ECMO provides support for both the right and left heart, whereas short-term LVADs support only one ventricle. Surgeons are much more likely to use VA ECMO in the setting of postcardiotomy failure, partly due to familiarity with surgical cannulation (or the presence of central cannulae), but also due to the support this provides for both ventricles and the lungs. Reviews of postcardiotomy ECMO suggest approximately 30% survival to hospital discharge.
For support of the patient with refractory end-stage cardiac failure who may be a potential candidate for durable LVAD or transplant, or who has already been evaluated for such advanced therapy, there are advantages and disadvantages to both the short-term LVAD (Impella or TandemHeart) and ECMO. Such patients always need left ventricular support but if right heart function and pulmonary function are adequate a short-term percutaneous LVAD might be appropriate. As discussed later, a significant advantage of these devices over VA ECMO is decompression of the left ventricle, which is not a feature of VA ECMO. If placed via the axillary/subclavian artery, the patient can be at least somewhat mobile with such a device. If, however the right heart, or lungs, or both, needs support, then VA ECMO is most appropriate. Another issue is urgency or acuity: peripheral VA ECMO can be initiated at the bedside without imaging more rapidly than a temporary assist device. The main problem if this support is needed for days or weeks is the femoral cannulae prevent mobilization. A recent systematic review (publications between 2006 and 2016) of short-term mechanical circulatory support as a bridge to durable LVAD or transplant (or recovery) documented a wide range in the number of days of support (individual study means of up to 47 days) and an overall 45% to 66% of patients surviving to discharge. In this report where the support was via central ECMO (see later), a higher proportion of patients went on to receive durable LVAD or transplant and survived to discharge than those who received peripheral ECMO.
For acute recoverable myocardial illness such as myocarditis, survival is approximately 67% with VA ECMO. This is a better outcome than for other cardiac indications, likely reflective of the younger age of such patients and possibly because in this setting ECMO is usually instituted before cardiogenic shock or arrest.
In the 2016 ELSO report, use of ECMO in the setting of cardiopulmonary resuscitation (CPR) or extracorporeal cardiopulmonary resuscitation (ECPR) in adults comprises approximately 15% of all adult ECMO. Sixty-six percent of all centers reporting to the registry indicate some use in this setting. While referrals for ECMO for respiratory and cardiac failure are often relatively acute and urgent, in the setting of bedside CPR, ECMO needs to be instituted very rapidly; time from starting CPR to ECMO initiation is an important determinant of good outcome. This limits its use to institutions able to support a team that is ready for such rapid activation. Survival to discharge is, not surprisingly, the lowest of all ECMO applications in both adults (29%) and pediatrics (41%). The quality of evidence in published studies comparing ECLS to standard CPR is low, with a great deal of heterogeneity.
Indications for VA ECMO are summarized in Box 85.2 . According to the 2013 ELSO guidelines the most common indication for VA ECMO in adult cardiac failure is the presence of cardiogenic shock with end organ hypoperfusion despite the use of dual inotropes and significant vasopressor requirement. This includes cardiogenic shock with or without myocardial infarction, fulminant myocarditis, peripartum cardiomyopathy, decompensated chronic heart failure, right heart failure, medication or toxic drug overdose, and postcardiotomy shock.
Absolute contraindications to VA ECMO include acute intracranial hemorrhage or massive stroke, active bleeding, and severe aortic insufficiency. Relative contraindications (variable by center) may include contraindication for anticoagulation, advanced age, obesity, active cancer, suicide attempt, chronic hemodialysis, end-stage liver disease, aortic dissection, and lack of social support. As is the case for VV ECMO, if neither recovery nor candidacy for durable therapy (LVAD or transplant) are likely, VA ECMO should not be initiated.
The initiation of any form of ECMO is lifesaving when the heart, or lungs, or both are failing despite maximum medical therapies. It is a very invasive and labor-intensive therapy, with associated severe complications, and may confine a patient to an ICU for days, weeks, or even months. As the practice has evolved over time, many groups have tried to address issues of patient appropriateness, possible exclusion criteria, and prognosis before initiating the therapy. An essential consideration is that ECMO is a “bridge” to something else and cannot be viewed as a long-term solution; Box 85.3 lists the uses of ECMO as a “bridging” therapy to a variety of possible scenarios. The following discussion addresses only the individual patient ethical dilemmas, and not the overriding issue of the use of a limited availability, expensive, and labor-intensive therapy with its cost:benefit implications, and overall implications for health care systems.
| Bridge to Decision | Urgent initiation before the ability to assess likelihood of recovery or candidacy for advanced therapy |
| Bridge to Recovery | Initiation for organ failure that is believed to be potentially recoverable |
| Bridge to Advanced Durable Therapy | Initiation after acceptance for eligibility for device (e.g., VAD) or transplant |
| “Bridge to Nowhere” | Bridge to decision which is likely to be non-recovery and non-eligibility for advanced therapy |
VAD, Ventricular assist device.
When an otherwise relatively young and healthy patient develops an acute severe illness resulting in acute cardiac failure and shock (e.g., viral cardiomyopathy) or acute refractory lung failure (e.g., viral pneumonia), the decision to initiate lifesaving extracorporeal support as a “bridge to recovery” seems relatively straightforward. Similarly, a patient with end-stage disease of any kind who already has a “do not resuscitate” status would likely not be a candidate for ECMO if the heart suddenly failed. Unfortunately, the clinical spectrum of potential candidates runs as a continuum between these two examples. From the patient and family, through all levels of caregivers and decision makers, tools to help assess the likelihood of successful outcome with this advanced therapy are needed.
The ELSO registry database has been used to study the likelihood of survival prior to ECMO initiation, both in respiratory failure ( Table 85.2 ) and cardiac failure ( Table 85.3 ). There are many common elements in these publications, including the duration and degree of respiratory support, age, other organ function, and acidosis. While these publications can be used as a general guide and can be quoted to referring physicians and families, they have to be placed in clinical context; decision making for life-sustaining treatment must be patient-specific. Courtwright and associates make a point of the need to emphasize to the patient’s family the “bridging” nature of ECMO therapy, and that a destination must be formulated at the outset or early on. Because of the need for anticoagulation and the risk for bleeding or thrombosis, patients who are not candidates for anticoagulation (e.g., intracranial hemorrhage) are generally not candidates for ECMO, even though more experience is accumulating with reduced or even no anticoagulation with the use of anticoagulant bonded cannulae, tubing, pump heads, and oxygenators. Patients with underlying severe disease who are not expected to survive more than some predetermined period (i.e., 6 months or a year) independent of the need for ECMO are unlikely to be considered as candidates. Rather than have a single physician or surgeon determine whether ECMO should be initiated in a given patient, especially when a request comes from an outside hospital, many institutions make this a shared decision by a small committee (i.e., 3 individuals) who are all familiar with and participate in ECMO management.
| Parameter | Score |
|---|---|
| Age, Years | |
| 18-49 | 0 |
| 50-59 | −2 |
| ≥60 | −3 |
| Immunocompromised status ∗ | −2 |
| Mechanical Ventilation Prior to Initiation of ECMO | |
| <48 h | 3 |
| 48 h to 7 days | 1 |
| >7 days | 0 |
| Acute Respiratory Diagnosis Group (Select Only One) | |
| Viral pneumonia | 3 |
| Bacterial pneumonia | 3 |
| Asthma | 11 |
| Trauma and burn | 3 |
| Aspiration pneumonitis | 5 |
| Other acute respiratory diagnoses | 1 |
| Nonrespiratory and chronic respiratory diagnoses | 0 |
| Central nervous system dysfunction † | −7 |
| Acute associated (nonpulmonary) infection ‡ | −3 |
| Neuromuscular blockade agents before ECMO | 1 |
| Nitric oxide use before ECMO | −1 |
| Bicarbonate infusion before ECMO | −2 |
| Cardiac arrest before ECMO | −2 |
| PaCO 2 , mm Hg | |
| <75 | 0 |
| ≥75 | −1 |
| Peak Inspiratory Pressure, cm H 2 O | |
| <42 | 0 |
| ≥42 | −1 |
| Total score | −22 to 15 |
| Total RESP Score | Risk Class | Survival |
|---|---|---|
| Hospital Survival by Risk Class | ||
| ≥6 | I | 92% |
| 3-5 | II | 76% |
| −1 to 2 | III | 57% |
| −5 to −2 | IV | 33% |
| ≤−6 | V | 18% |
∗ “Immunocompromised” is defined as hematological malignancies, solid tumor, solid organ transplantation, human immunodeficiency virus, and cirrhosis.
† “Central nervous system dysfunction” diagnosis combined neurotrauma, stroke, encephalopathy, cerebral embolism, and seizure and epileptic syndrome.
‡ “Acute associated (nonpulmonary) infection” is defined as another bacterial, viral, parasitic, or fungal infection that did not involve the lung. ECMO, Extracorporeal membrane oxygenation; RESP, respiratory ECMO survival prediction.
| Parameter | Score | |
|---|---|---|
| Acute Cardiogenic Shock Diagnosis Group (Select One or More) | ||
| Myocarditis | 3 | |
| Refractory VT/VF | 2 | |
| Post heart or lung transplantation | 3 | |
| Congenital heart disease | −3 | |
| Other diagnoses leading to cardiogenic shock requiring VA ECMO | 0 | |
| Age (Years) | ||
| 18-38 | 7 | |
| 39-52 | 4 | |
| 53-62 | 3 | |
| ≥63 | 0 | |
| Weight (kg) | ||
| ≤65 | 1 | |
| 65-89 | 2 | |
| ≥90 | 0 | |
| Acute Pre-ECMO Organ Failures (Select One or More if Required) | ||
| Liver failure ∗ | −3 | |
| Central nervous system dysfunction † | −3 | |
| Renal failure ‡ | −3 | |
| Chronic renal failure § | −6 | |
| Duration of Intubation Prior to Initiation of ECMO (h) | ||
| ≤10 | 0 | |
| 11-29 | −2 | |
| ≥30 | −4 | |
| Peak inspiratory pressure ≤ 20 cm H 2 O | 3 | |
| Pre-ECMO cardiac arrest | −2 | |
| Diastolic blood pressure before ECMO ≥ 40 mm Hg ¶ | 3 | |
| Pulse pressure before ECMO ≤ 20 mm Hg ¶ | −2 | |
| HCO 3 before ECMO ≤ 15 mmol/L ¶ | −3 | |
| Constant value to add to all calculations of SAVE-score | −6 | |
| Total score | −35 to 17 | |
| Total SAVE-Score | Risk Class | Survival (%) |
|---|---|---|
| Hospital Survival by Risk Class | ||
| >5 | I | 75 |
| 1-5 | II | 58 |
| −4 to 0 | III | 42 |
| −9 to −5 | IV | 30 |
| ≤−10 | V | 18 |
∗ Liver failure was defined as bilirubin ≥ 33 μmol/L or elevation of serum aminotransferases (ALT or AST) > 70 UI/L.
† CNS dysfunction combined neurotrauma, stroke, encephalopathy, cerebral embolism, as well as seizure and epileptic syndromes.
‡ Renal dysfunction is defined as acute renal insufficiency (e.g., creatinine > 1.5 mg/dL) with or without RRT.
§ Chronic kidney disease is defined as either kidney damage or glomerular filtration rate < 60 mL/min/1.73 m 2 for ≥ 3 months.
¶ Worse value within 6 hours prior to ECMO cannulation. VA ECMO, Venoarterial extracorporeal membrane oxygenation; VF, ventricular fibrillation; VT, ventricular tachycardia.
| Drug | Pro | Absolute/Relative Contraindications |
|---|---|---|
| No anticoagulation | Avoids anticoagulation in high-risk patients (hemorrhagic CVA, postoperative bleeding, etc.) | High risk for thrombus/embolism/short circuit life span |
| Unfractionated heparin | Most frequently used | HITT |
| Low-molecular-weight heparin | Infrequently used | Very dependent on renal function and patient weight, HITT |
| Argatroban | No concern for HITT | Hepatically cleared |
| Bivalirudin | No concern for HITT, short half-life | Renally cleared |
| Heparin bonded circuits | Decreases fibrin coating of circuits | HITT |
Overall survival for adult respiratory and cardiac ECMO is approximately 60% and 40%, respectively. Although this is certainly a major advance for diseases that were previously not survivable, the other side of this coin is that mortality remains at 40% and 60%. It makes good sense to engage palliative care and/or an ethics committee and other counselling services, where available and appropriate, at the outset for this therapy. When ECMO is being considered but there is uncertainty about the likelihood of recovery or candidacy for advanced durable therapy, discussions with family and caregivers should be similar to those made regarding “do not resuscitate,” assessing the values and goals of the patient. Counselling for ICU caregivers as well as family, including postmortem “debriefings,” can be very valuable to help staff deal with end-of-life issues. There are few settings where withdrawal of therapy can so immediately result in death, where patients have intact neurologic function but are on the “bridge to nowhere”; it can be extremely troubling to all involved when ECMO is stopped.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here