Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
This chapter describes the novel concept of expanded carrier screening (ECS), whereby individuals are simultaneously screened for up to 200 genetic conditions.
Different laboratory techniques are used in ECS and include targeted genotyping and next-generation sequencing approaches.
The choice of conditions to be included in ECS varies among laboratories, and no consensus presently exists.
Various screening strategies exist, including premarital carrier screening, cascade screening, stepwise screening and couple screening.
An important aspect in offering ECS is patient education.
In 1968, the World Health Organization (WHO) proposed guidelines for establishment of screening programs ( Box 26.1 ). These initial guidelines provided general recommendations regarding the types of disorders appropriate for screening. These recommendations predated the advent of genetic testing. Thus, in 1998, the WHO proposed modified guidelines that would specifically apply to genetic screening ( Box 26.2 ).
Is the disease an important health problem?
Is there a recognisable latent or early symptomatic stage?
Do we know the natural history of disease?
Is there an effective treatment for patients with recognised disease?
Is there a suitable test that will identify the disease in its early stages?
Is the test acceptable to the population?
Do we agree on who treats the disease?
Are facilities for diagnosis and treatment available?
Case finding should be ongoing.
The cost of case finding (including diagnosis and treatment) should be economically balanced in relation to possible expenditures on medical care as a whole.
Genetic screening should be voluntary, not mandatory.
Genetic screening should be preceded by adequate information about the purpose and possible outcomes of the screen or test and potential choices to be made.
Anonymous screening for epidemiologic purposes may be conducted after notification of the population to be screened.
Results should not be disclosed to employers, insurers, schools or others without the individual’s consent to avoid possible discrimination.
In rare cases in which disclosure may be in the best interests of the individual or of public safety, the health provider may work with the individual towards a decision by him or her.
Test results should be followed by genetic counselling, particularly when they are unfavourable.
If treatment or prevention exists or is available, this should be offered with a minimum of delay.
Newborn screening should be mandatory and free of charge if early diagnosis and treatment will benefit the newborn.
In 2006, the WHO stated that genetic prevention services are needed to effectively reduce the burden of congenital and genetic disorders. Yet in many countries, the level of genetic services is currently inadequate and insufficient. Frequently, these services are available only to the wealthy and well-educated. Indeed, the provision of genetic services must be weighed responsibly and fairly against the competing health requirements in each country. However, epidemiologic data regarding the prevalence of congenital and genetic disorders and cost-effectiveness analyses of screening programs indicate that many countries would actually benefit from incorporating preventive genetic approaches into their health services. Unfortunately, despite the growing number of genetic technologies that hold great promise, these are underused in clinical practice and fail to reduce the burden of inherited diseases. It is at this juncture that the introduction of expanded carrier screening (ECS) may provide a feasible universal solution to these shortcomings.
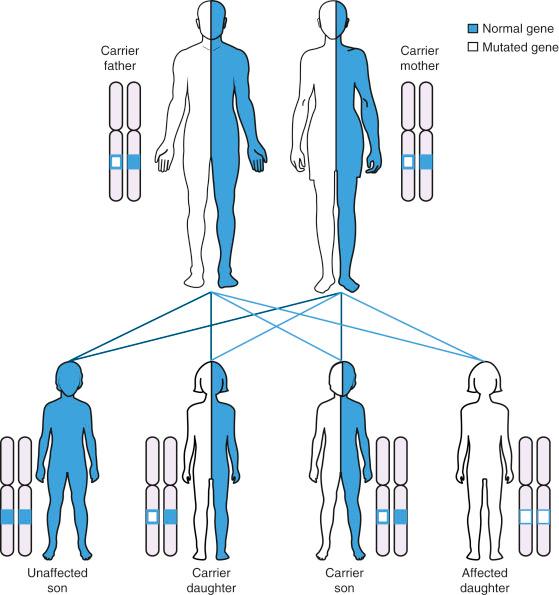
One of the first successful programs has been the community-wide screening for Tay-Sachs disease (TSD) carriers, established in the 1970s among Eastern European (Ashkenazi) Jews. TSD is caused by deficiency of β-hexosaminidase A activity, which is inherited in an autosomal recessive (AR) manner ( Fig. 26.1 ). Unaffected heterozygote carriers may be ascertained by detection of reduced enzymatic activity in serum and blood lymphocytes. Wide-scale voluntary screening programs for TSD were initiated among Ashkenazi Jews first in the United States, Canada and Israel but were eventually adopted in many countries worldwide. In the first 30 years after the implementation of these programs, more than 1.4 million individuals were screened (mostly from North America and Israel), and more than 1400 couples have been identified to be at risk for offspring affected with TSD. These programs resulted in a significant reduction in the incidence of TSD among Ashkenazi Jews, with the majority of affected individuals being from other unscreened ethnicities.
β-Thalassemia is a relatively common condition among individuals of Middle Eastern and Mediterranean origin. It is characterised by deficient synthesis of β-haemoglobin chains, resulting in severe anaemia. Heterozygote carriers have mild anaemia and reduced red blood cell indices. Screening is based on the results of complete blood count (CBC) showing a low mean corpuscular volume (MCV) and low mean corpuscular haemoglobin (MCH). Haemoglobin electrophoresis is used to differentiate carriers from those with iron deficiency, with high sensitivity and relatively good correlation with phenotype. Because the CBC is relatively inexpensive, easy and widely available, screening programs for β-thalassemia are widely available. For a detailed description, see Chapter 27 .
Screening methods for both TSD and β-thalassemia were initially based on nonmolecular testing (enzymatic testing and CBC, respectively). Currently, however, most carrier screening programs are based on molecular methodologies. Screening for cystic fibrosis (CF) has become the prototype model, following the discovery of the causative role of the CFTR gene in 1989. CF is an AR disorder characterised by progressive lung disease, pancreatic dysfunction and male infertility. It is one of the most common severe genetic diseases in caucasians, with a carrier frequency of about 1 in 25. Although more than 2000 mutations have been described in the CFTR gene, each ethnic group may have a unique set of mutations, with the delF508 mutation being the most prevalent and accounting for about 70% of CF alleles. In 2001 the American College of Obstetricians and Gynecologists (ACOG) and the American College of Medical Genetics (ACMG) set guidelines for prenatal and preconception CF carrier screening. Initial guidelines recommended limiting screening to caucassian individuals or those with a family history of CF. However, in 2011, the guidelines were updated, stating that it has become increasingly difficult to classify individuals with CF into distinct ethnic categories. Thus the Committee agreed that offering CF screening to all couples planning a pregnancy is reasonable. For couples in whom both partners are carriers of mutations in the same AR gene, there is a reproductive risk of 25% for affected offspring. Knowing this before delivery provides an opportunity for genetic counselling during which reproductive options such as prenatal diagnosis by chorionic villus sampling or amniocentesis may be discussed. Knowing the carrier status before pregnancy also provides the option of preimplantation genetic diagnosis of embryos attained by in vitro fertilisation.
Although many genetic disorders have an ethnic predilection, only a few are universally frequent in diverse populations. One such example is spinal muscular atrophy (SMA), a severe AR neuromuscular disease caused by degeneration of motor neurons in the spinal cord, resulting in progressive muscle weakness and paralysis. It is for this reason that the ACMG recommends that carrier screening be offered to all couples, regardless of race or ethnicity. The same is also true for some CF mutations (e.g. delF508 mutation), which account for approximately 70% of CF alleles worldwide. This mutation is thought to have occurred more than 52,000 years ago, in a population genetically distinct from any present European group. Panethnic screening for CF is currently recommended by ACOG and ACMG. Similarly, fragile X syndrome (FXS), a condition associated with intellectual disability (ID) and autistic behaviour, is prevalent in most populations. Indeed, it is the most common cause of inherited mental retardation. The prevalence of the fragile X syndrome is estimated to be about 1 in 4000 males and about 1 in 7000 females. The clinical phenotype in males includes varying degrees of ID with mild dysmorphic features such as relative macrocephaly, large ears, a prominent jaw and macro-orchidism after puberty. Affected females usually present with a less severe phenotype and may exhibit only subtle cognitive impairment. The disorder is caused by a CGG triplet repeat expansion mutation in the FMR1 gene on chromosome Xq27.3. Unaffected individuals usually have less than 55 CGG repeats. Individuals with the fragile X syndrome have more than 200 CGG repeats, a full mutation. Individuals with 55 to 200 CGG repeats are considered to have a premutation . Female premutation carriers are at risk for having offspring with further CGG expansion, which may ultimately result in a full mutation (>200 repeats). There is a strong correlation between the number of CGG repeats in the carrier mother and the risk for expansion to full mutation in the offspring. Population-based carrier screening for fragile X has been widely used in Israel since the mid-1990s. It is now included in most basic screening panels for all ethnicities.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here