Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Skeletal muscle fibers are innervated by large myelinated nerve fibers that originate from large motoneurons in the anterior horns of the spinal cord. As discussed in Chapter 6 , each nerve fiber, after entering the muscle belly, normally branches and stimulates from three to several hundred skeletal muscle fibers. Each nerve ending makes a junction, called the neuromuscular junction , with the muscle fiber near its midpoint. The action potential initiated in the muscle fiber by the nerve signal travels in both directions toward the muscle fiber ends. With the exception of about 2% of the muscle fibers, there is only one such junction per muscle fiber.
Figure 7-1 A and B shows the neuromuscular junction from a large myelinated nerve fiber to a skeletal muscle fiber. The nerve fiber forms a complex of branching nerve terminals that invaginate into the surface of the muscle fiber but lie outside the muscle fiber plasma membrane. The entire structure is called the motor end plate. It is covered by one or more Schwann cells that insulate it from the surrounding fluids.
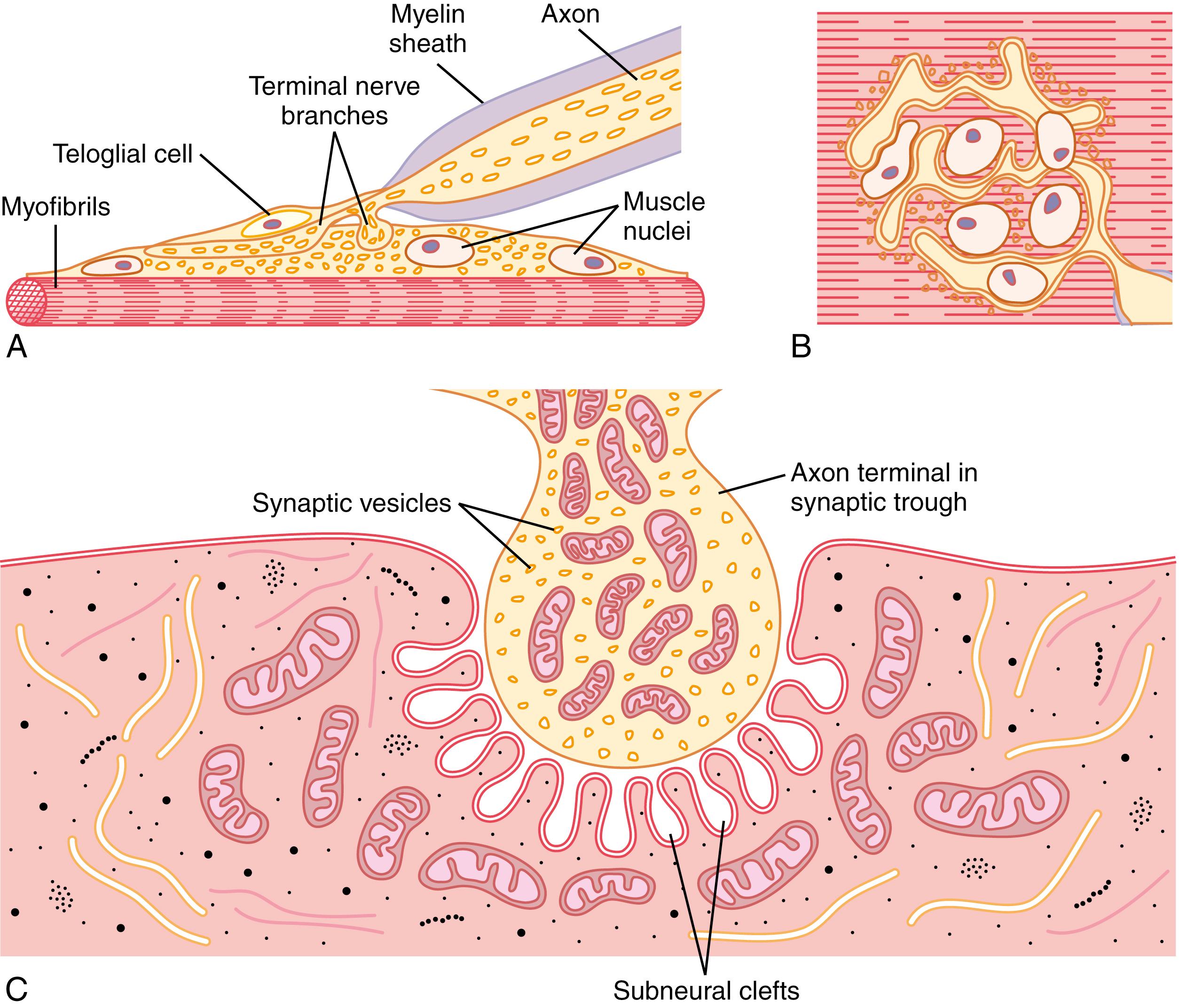
Figure 7-1 C shows the junction between a single axon terminal and the muscle fiber membrane. The invaginated membrane is called the synaptic gutter or synaptic trough , and the space between the terminal and the fiber membrane is called the synaptic space or synaptic cleft, which is 20 to 30 nanometers wide. At the bottom of the gutter are numerous smaller folds of the muscle membrane called subneural clefts , which greatly increase the surface area at which the synaptic transmitter can act.
In the axon terminal are many mitochondria that supply adenosine triphosphate (ATP), the energy source used for synthesis of a transmitter, acetylcholine, which excites the muscle fiber membrane. Acetylcholine is synthesized in the cytoplasm of the terminal but is absorbed rapidly into many small synaptic vesicles , about 300,000 of which are normally in the terminals of a single end plate. In the synaptic space are large quantities of the enzyme acetylcholinesterase , which destroys acetylcholine a few milliseconds after it has been released from the synaptic vesicles.
When a nerve impulse reaches the neuromuscular junction, about 125 vesicles of acetylcholine are released from the terminals into the synaptic space. Some of the details of this mechanism can be seen in Figure 7-2 , which shows an expanded view of a synaptic space with the neural membrane above and the muscle membrane and its subneural clefts below.
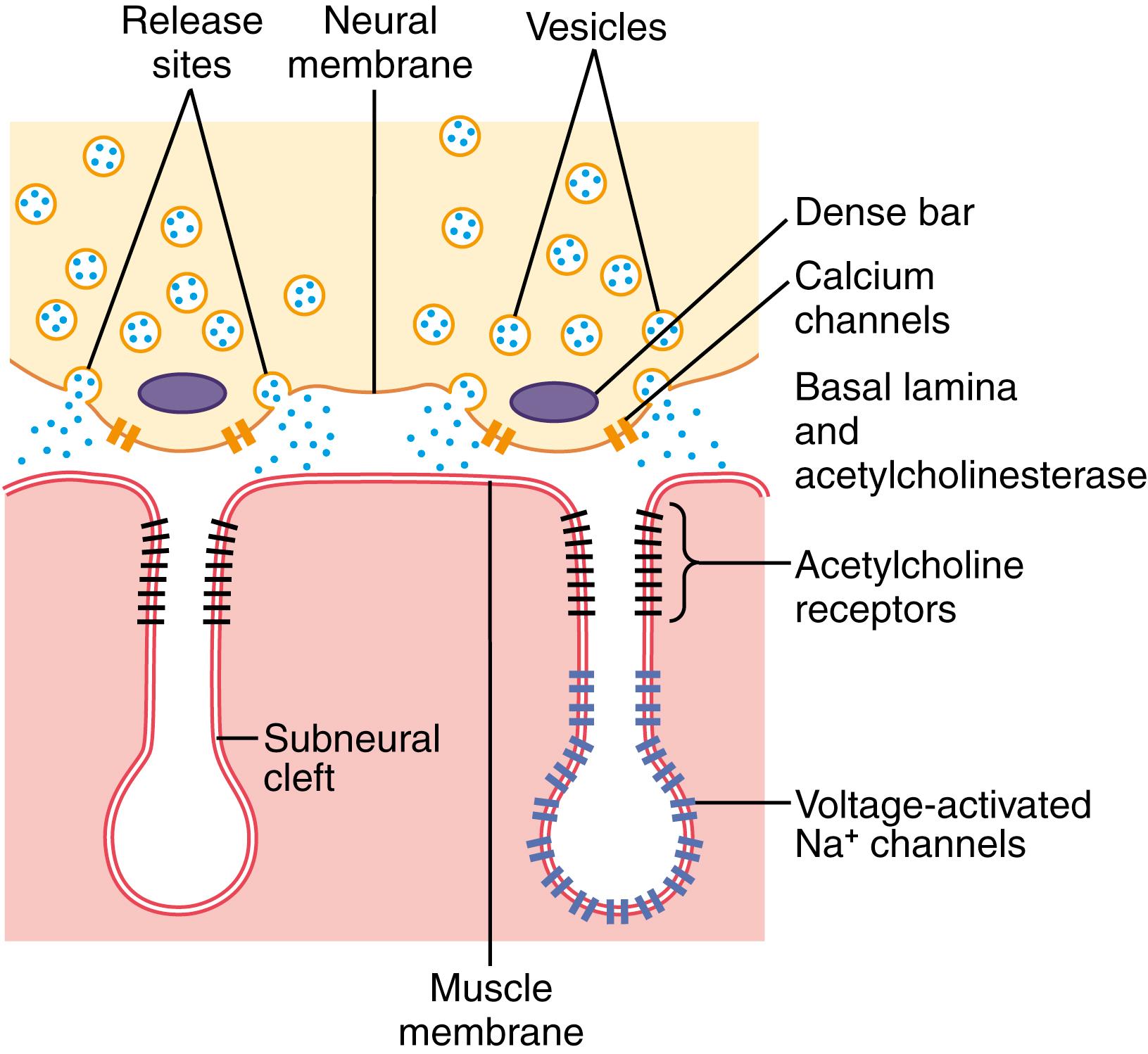
On the inside surface of the neural membrane are linear dense bars , shown in cross section in Figure 7-2 . To each side of each dense bar are protein particles that penetrate the neural membrane; these are voltage-gated calcium channels . When an action potential spreads over the terminal, these channels open and allow calcium ions to diffuse from the synaptic space to the interior of the nerve terminal. The calcium ions, in turn, are believed to activate Ca 2+ -calmodulin–dependent protein kinase , which, in turn, phosphorylates synapsin proteins that anchor the acetylcholine vesicles to the cytoskeleton of the presynaptic terminal. This process frees the acetylcholine vesicles from the cytoskeleton and allows them to move to the active zone of the presynaptic neural membrane adjacent to the dense bars. The vesicles then dock at the release sites, fuse with the neural membrane, and empty their acetylcholine into the synaptic space by the process of exocytosis .
Although some of the aforementioned details are speculative, it is known that the effective stimulus for causing acetylcholine release from the vesicles is entry of calcium ions and that acetylcholine from the vesicles is then emptied through the neural membrane adjacent to the dense bars.
Figure 7-2 also shows many small acetylcholine receptors and voltage-gated sodium channels in the muscle fiber membrane. The acetylcholine-gated ion channels are located almost entirely near the mouths of the subneural clefts lying immediately below the dense bar areas, where the acetylcholine is emptied into the synaptic space. The voltage-gated sodium channels also line the subneural clefts.
Each acetylcholine receptor is a protein complex that has a total molecular weight of approximately 275,000. The fetal acetylcholine receptor complex is composed of five subunit proteins, two alpha proteins and one each of beta, delta , and gamma proteins. In the adult, an epsilon protein substitutes for the gamma protein in this receptor complex. These protein molecules penetrate all the way through the membrane, lying side by side in a circle to form a tubular channel, illustrated in Figure 7-3 . The channel remains constricted, as shown in part A of the figure, until two acetylcholine molecules attach respectively to the two alpha subunit proteins. This attachment causes a conformational change that opens the channel, as shown in part B of the figure.
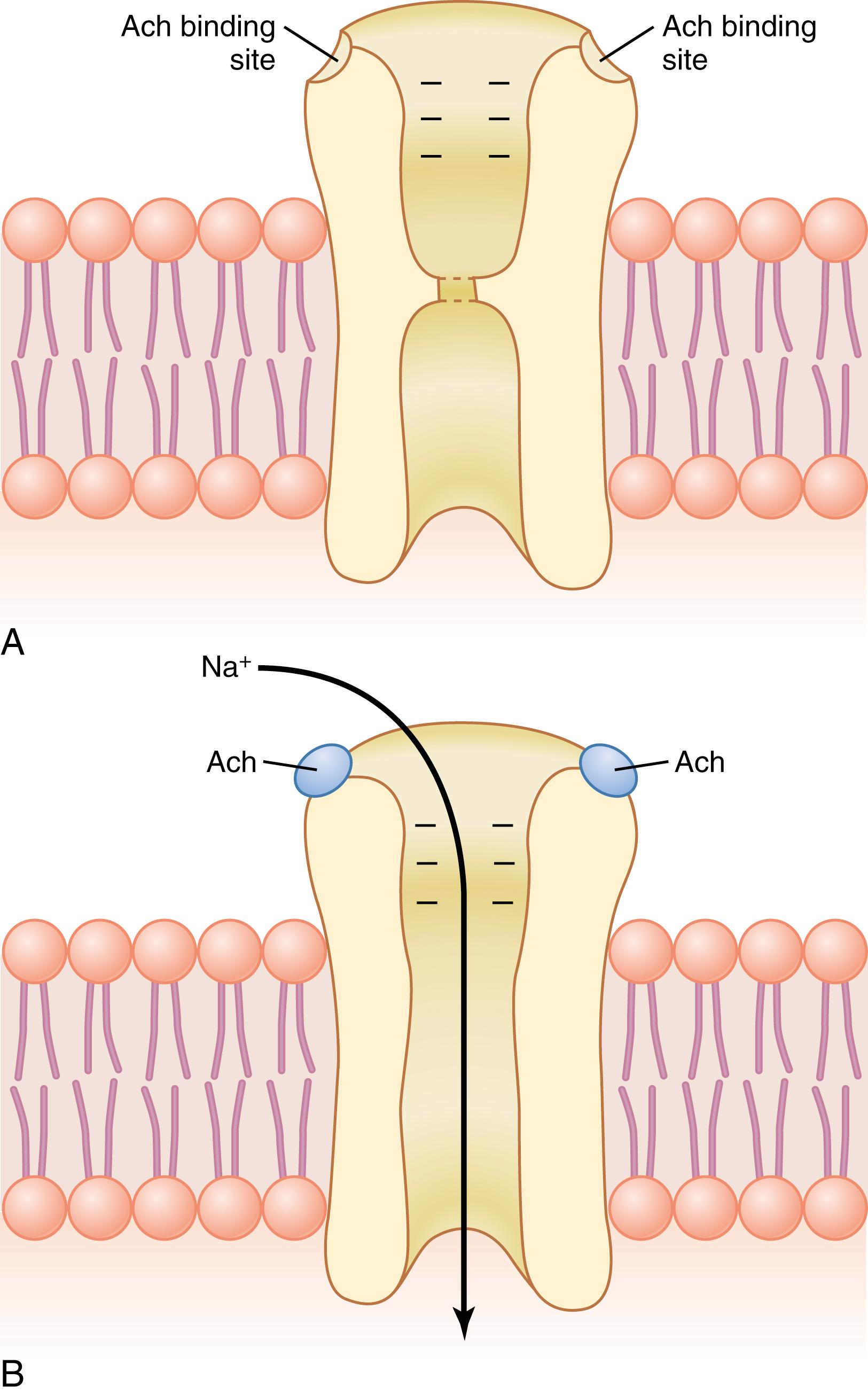
The acetylcholine-gated channel has a diameter of about 0.65 nanometer, which is large enough to allow the important positive ions—sodium (Na + ), potassium (K + ), and calcium (Ca 2+ )—to move easily through the opening. Patch clamp studies have shown that one of these channels, when opened by acetylcholine, can transmit 15,000 to 30,000 sodium ions in 1 millisecond. Conversely, negative ions, such as chloride ions, do not pass through because of strong negative charges in the mouth of the channel that repel these negative ions.
In practice, far more sodium ions flow through the acetylcholine-gated channels than any other ions for two reasons. First, there are only two positive ions present in large concentrations—sodium ions in the extracellular fluid and potassium ions in the intracellular fluid. Second, the negative potential on the inside of the muscle membrane, −80 to −90 millivolts, pulls the positively charged sodium ions to the inside of the fiber while simultaneously preventing efflux of the positively charged potassium ions when they attempt to pass outward.
As shown in Figure 7-3 B , the principal effect of opening the acetylcholine-gated channels is to allow sodium ions to flow to the inside of the fiber, carrying positive charges with them. This action creates a local positive potential change inside the muscle fiber membrane, called the end plate potential. This end plate potential normally causes sufficient depolarization to open neighboring voltage-gated sodium channels, allowing even greater sodium ion inflow and initiating an action potential that spreads along the muscle membrane and causes muscle contraction.
The acetylcholine, once released into the synaptic space, continues to activate acetylcholine receptors as long as the acetylcholine persists in the space. However, it is rapidly destroyed by the enzyme acetylcholinesterase , which is attached mainly to the spongy layer of fine connective tissue that fills the synaptic space between the presynaptic nerve terminal and the postsynaptic muscle membrane. A small amount of acetylcholine diffuses out of the synaptic space and is then no longer available to act on the muscle fiber membrane.
The short time that the acetylcholine remains in the synaptic space—a few milliseconds at most—normally is sufficient to excite the muscle fiber. Then the rapid removal of the acetylcholine prevents continued muscle re-excitation after the muscle fiber has recovered from its initial action potential.
The sudden insurgence of sodium ions into the muscle fiber when the acetylcholine-gated channels open causes the electrical potential inside the fiber at the local area of the end plate to increase in the positive direction as much as 50 to 75 millivolts, creating a local potential called the end plate potential . Recall from Chapter 5 that a sudden increase in nerve membrane potential of more than 20 to 30 millivolts is normally sufficient to initiate more and more sodium channel opening, thus initiating an action potential at the muscle fiber membrane.
Figure 7-4 illustrates an end plate potential initiating the action potential. This figure shows three separate end plate potentials. End plate potentials A and C are too weak to elicit an action potential, but they do produce weak local end plate voltage changes, as recorded in the figure. By contrast, end plate potential B is much stronger and causes enough sodium channels to open so that the self-regenerative effect of more and more sodium ions flowing to the interior of the fiber initiates an action potential. The weakness of the end plate potential at point A was caused by poisoning of the muscle fiber with curare , a drug that blocks the gating action of acetylcholine on the acetylcholine channels by competing for the acetylcholine receptor sites. The weakness of the end plate potential at point C resulted from the effect of botulinum toxin , a bacterial poison that decreases the quantity of acetylcholine release by the nerve terminals.
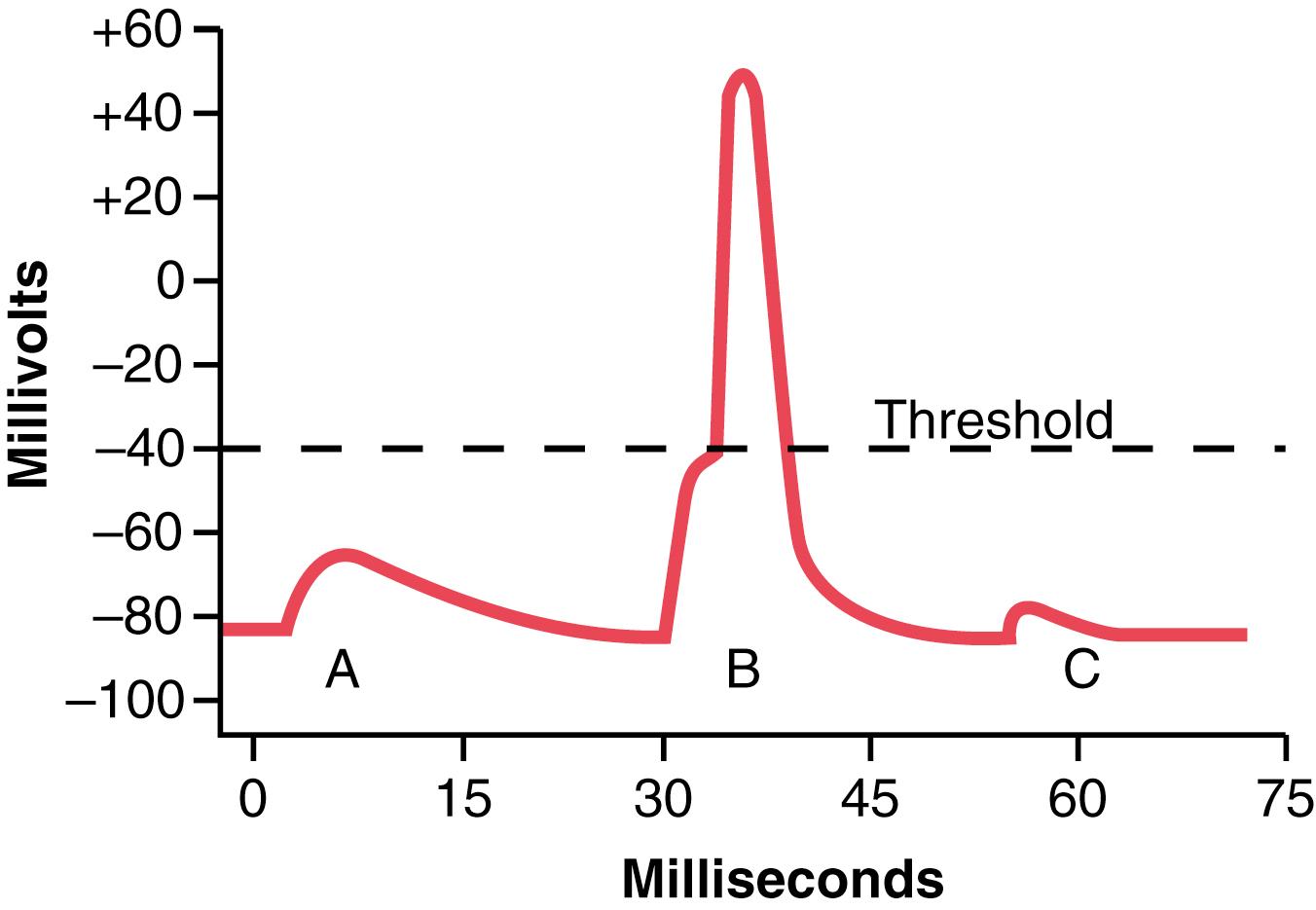
Ordinarily, each impulse that arrives at the neuromuscular junction causes about three times as much end plate potential as that required to stimulate the muscle fiber. Therefore, the normal neuromuscular junction is said to have a high safety factor . However, stimulation of the nerve fiber at rates greater than 100 times per second for several minutes may diminish the number of acetylcholine vesicles so much that impulses fail to pass into the muscle fiber. This situation is called fatigue of the neuromuscular junction, and it is the same effect that causes fatigue of synapses in the central nervous system when the synapses are overexcited. Under normal functioning conditions, measurable fatigue of the neuromuscular junction occurs rarely and, even then, only at the most exhausting levels of muscle activity.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here