Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Diagnosis of hydrops fetalis requires two or more of the following sonographic abnormalities: scalp and body wall edema (defined as skin thickness >5 mm), ascites, pleural effusion, pericardial effusion, polyhydramnios, and placental thickening (≥4 cm in the second trimester and ≥6 cm in the third trimester).
Immune hydrops refers to cases in which the underlying condition is due to fetal anemia developing from maternal red blood cell alloimmunization. Nonimmune hydrops refers to cases resulting from all other conditions.
Almost 90% of cases of hydrops are secondary to a nonimmune mechanism.
For women with a prior history of a fetus or child with alloimmunization, defined as requiring intrauterine transfusion or exchange transfusions after birth, the use of serial maternal titers to identify the fetus at risk is not indicated because these measures are no longer predictive in these circumstances.
Measurements of the peak systolic velocity (PSV) in the middle cerebral artery (MCA) are highly predictive of fetal anemia and are the preferred method to manage pregnancies at risk for immune hydrops.
If MCA PSV measurements reach a value greater than 1.5 MoM (multiples of the median), fetal blood sampling via cordocentesis should be performed.
During fetal transfusions, the blood should be transfused at a rate of 5 to 10 mL/minute, and if feasible, an attempt should be made to reach a final hematocrit of 50% to 65%.
The most common causes of nonimmune hydrops are cardiovascular conditions, which include arrhythmias, structural abnormalities, and diverse cardiomyopathies.
A fetal echocardiogram should be part of the initial evaluation of nonimmune hydrops.
Hydrops fetalis has been defined as the pathologic accumulation of excessive fluid within two or more fetal compartments. These features can be detected sonographically and include scalp and body wall edema (defined as skin thickness greater than 5 mm), ascites, pleural effusion, pericardial effusion, presence of polyhydramnios (amniotic fluid index greater than 25 cm or maximum verticle pocket greater than 8 cm), and placental thickening (≥4 cm in the second trimester and ≥6 cm in the third trimester) ( Figs. 17-1 through 17-8 ).
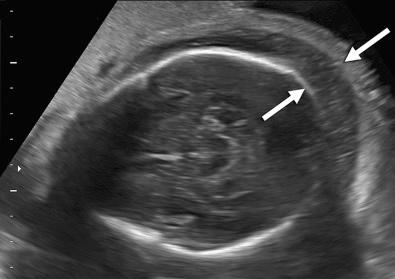
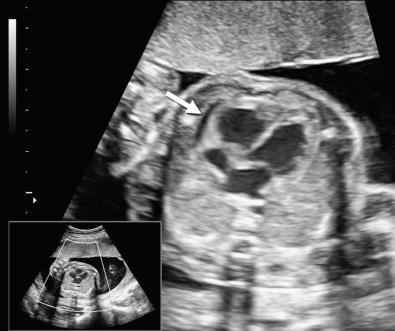
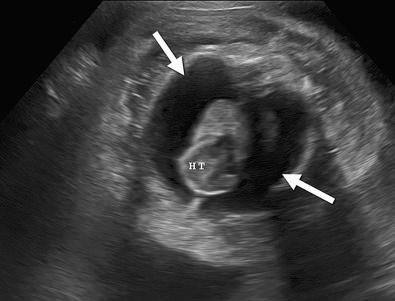
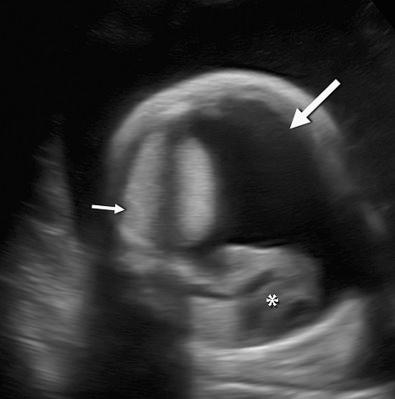
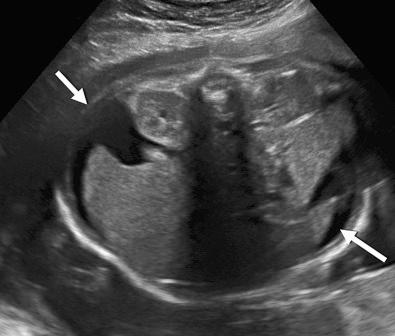
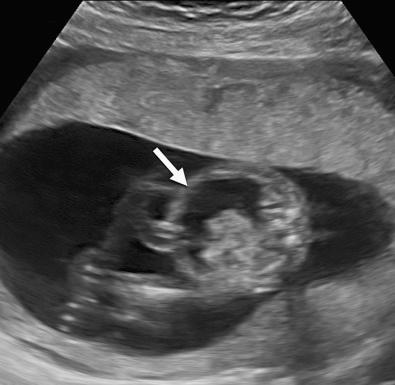
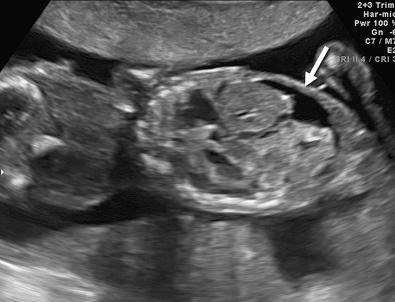
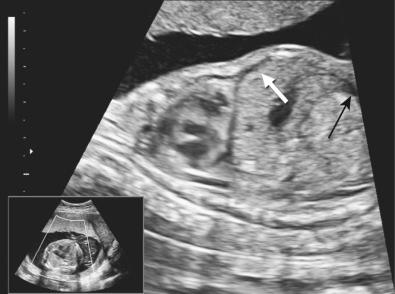
The use of this term should be limited to cases that meet the criteria outlined here because the diagnosis is associated with a poor prognosis and the workup for the cause may be extensive. The presence of one but not two of these criteria may be secondary to different causes and may also be associated with a better outcome than hydrops fetalis. In certain situations, the finding of a single abnormal fluid accumulation may be a sign of progressive and evolving hydrops fetalis and may warrant more frequent imaging and surveillance.
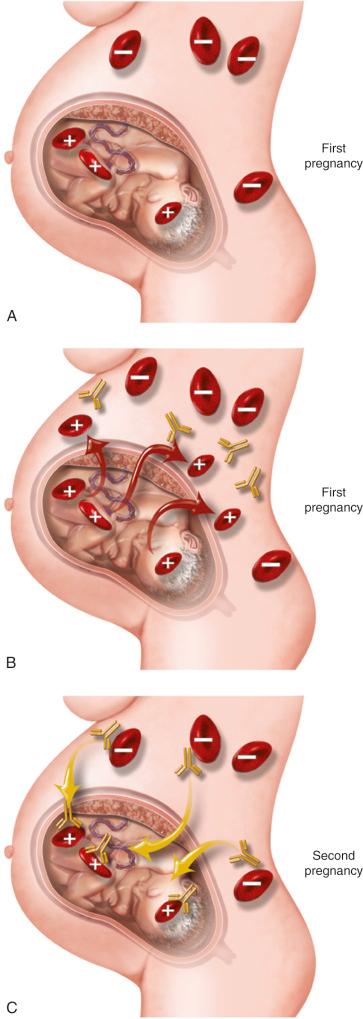
Based on the cause, the condition should be classified either as immune or nonimmune hydrops. Immune hydrops refers to all cases in which the underlying condition is due to fetal anemia developing from red blood cell (RBC) alloimmunization. The term nonimmune hydrops was reserved for the cases that resulted from all other conditions. Immune hydrops was probably first described in 1609 when a French midwife delivered a set of twins: the first one was hydropic and stillborn, whereas the second twin was noted to be severely jaundiced and subsequently died, most likely from kernicterus. This association was then again reported in 1932 when Diamond and colleagues described a disease characterized by anemia and jaundice that was associated with erythroblastosis and RBC hemolysis. Although the clinical features were described, the cause and pathogenesis were largely unknown. In 1938 Darrow described the concept of an antigen-antibody reaction leading to icterus gravis neonatorum. In 1940, the work by Landsteiner and Weiner led to the discovery of the rhesus (Rh) antigen that was expressed on the surface of RBCs. Later, in 1941 Levine and associates described the pathophysiology of the disease in a case series involving five patients. They observed that Rh-negative women form antibodies to Rh-positive RBCs, and transplacental passage of these antibodies to the fetus can lead to erythroblastosis fetalis.
Prevention of immune hydrops would have to wait decades. William Pollack, working in collaboration with Vincent J. Freda and John G. Gorman, developed a gamma globulin from anti-D serum. This gamma globulin was administered to RhD-negative inmates who then failed to develop anti-D antibodies when exposed to RhD-positive RBCs. At the same time, Finn and colleagues were also investigating the potential prevention of Rh hemolytic disease. They injected radioactive-labeled Rh-positive RBCs into Rh-negative men; they were able to demonstrate that administration of anti-D gamma globulin not only led to Rh-positive cells being coated by antibodies but also to a much more rapid destruction of these cells. Later, Hamilton treated RhD-negative women following their first delivery and showed that this strategy prevented formation of anti-D antibodies in the second pregnancy and led to improved perinatal outcomes. A subsequent multicenter clinical trial directed by Pollack led to the widespread use of anti-D immune globulin in clinical practice. The work by these pioneers helped the development of the current practice guidelines presently used throughout most of the world.
The true incidence of hydrops fetalis is difficult to quantify. Many studies differ on the definition of cases included in their series as well as the study population. The reported incidence ranges from 1 : 424 to 1 : 2000 live births admitted to a neonatal intensive care unit (NICU). The widespread use of anti-D immune globulin has led to a decrease in the overall incidence of immune hydrops over the past few decades. Macafee and coworkers reported that in 1970, 82% of the hydrops cases were secondary to immune hydrops. The incidence of nonimmune hydrops has been reported to range from 1 : 1500 to 1 : 3800 births. More recent publications have reported a shift in incidence; currently almost 90% of cases of hydrops are secondary to a nonimmune mechanism.
The pathophysiology of immune hydrops is exemplified by RhD isoimmunization. However, several other RBC antigens have been linked to the development of immune hydrops ( Table 17-1 ). The RhD antigen is one of the most immunogenic antigens expressed on the surface of RBCs ; other antigens differ in their ability to cause the disease. Maternal alloimmunization results from the exposure to foreign RhD-positive cells that eventually lead to an immunologic response. The most common cause of this phenomenon is a transplacental passage of fetal cells into maternal circulation, such as during a miscarriage, amniocentesis, trauma, or delivery ( Fig. 17-9 ). Using Kleihauer-Betke stain, as many as 75% of women have some evidence of the presence of fetal RBCs in maternal circulation during pregnancy or delivery.
| Antigen System | Specific Antigen | Antigen System | Specific Antigen | Antigen System | Specific Antigen |
|---|---|---|---|---|---|
| Frequently Associated With Severe Disease | |||||
| Kell | -K (K1) | ||||
| Rhesus | -D -c |
||||
| Infrequently Associated With Severe Disease | |||||
| Colton | -Co a -Co3 |
MNS | -Mt a -MUT -Mur -Mv -s -s D -S -U -V w |
Rhesus | -HOFM -LOCR -Riv -Rh29 -Rh32 -Rh42 -Rh46 -STEM -Tar |
| Diego | -ELO -Di a -Di b -Wr a -Wr b |
||||
| Duffy | -Fy a | ||||
| Kell | -Js a -Js b -k (K2) -Kp a -Kp b -K11 -K22 -Ku -Ul a |
||||
| Rhesus | -Be a -C -Ce -C w -C x -ce -D w -E -E w -Evans -e -G -Go a 7 -Hr -Hro -JAL |
Other antigens | -HJK -JFV -JONES -Kg -MAM -REIT -Rd |
||
| Kidd | -Jk a | ||||
| MNS | -En a -Far -Hil -Hut -M -Mi a -Mit |
||||
| Associated With Mild Disease | |||||
| Dombrock | -Do a -Gy a -Hy -Jo a |
Gerbich | -Ge2 -Ge3 -Ge4 -Ls a |
Scianna | -Sc2 |
| Other antigens | -Vel -Lan -At a -Jr a |
||||
| Duffy | -Fy b -Fy3 |
Kidd | -Jk b -Jk3 |
||
The risk of fetal-maternal hemorrhage also increases with gestational age: 0.01 mL of fetal blood may be detected in 3% of pregnancies the first trimester and 46% during the third trimester. The probability of alloimmunization is directly proportional to the amount of fetal blood that crosses the placental barrier. It has been estimated that 0.25 mL of fetal blood is the minimum amount required to lead to alloimmunization. For these reasons, delivery poses the greatest risk for Rh sensitization. The proper administration of RhD immune globulin, however, can effectively prevent the formation of maternal antibodies. Transfusion of incompatible blood can also lead to maternal sensitization; this is the most common mechanism explaining alloimmunization from other RBC antigens.
The genetics of the Rh system are complex. The Rh factor is among the largest of the blood groups and contains well over 50 different recognized antigens. Rearrangements in the Rh locus can result in hybrid proteins that can lead to additional antigen expression. Only a small number of these antigens are actually clinically relevant. During pregnancy there are three antigens that can lead to the formation of antibodies and alloimmunization, Rh (D) and Rh (CcEe). These antigens are encoded by two genes located on the short arm of chromosome 1, the RhD and RhCE genes. Each gene contains 10 exons. It has been speculated that through a mechanism involving alternative splicing these two genes could direct the synthesis of different proteins corresponding to different variations in Rh antigens. Fischer and Race have proposed a concept to explain the inheritance of the alleles in the Rh genes. In the majority of patients, three pairs of Rh antigens are inherited from each parent—Cc, Dd, and Ee. An individual can be heterozygous or homozygous for each allele. The Rh-positive or Rh-negative status of a patient is determined by the presence or absence (or possible nonexpression) of the D antigen site. The Rh (CcEe) has fewer alleles and a limited number of mutations reported. The Cc and the Ee alleles are codominant and expressed in a heterozygous fashion. It should also be noted that the symbol “d” simply indicates the absence of D antigen; there is no “d” allele.
The D antigen is a transmembrane protein located on the surface of the erythrocyte that contains several extracellular loops. More than 150 different alleles for the Rh (D) gene have been reported, most likely a byproduct of numerous mutations within the gene. The complete absence of the RhD antigen is called a negative allele. The gene frequency varies according to race and ethnicity. Rh-negative individuals are reported in approximately 1% of the Chinese and Japanese populations, whereas the Basque tribe in northern Spain has a reported incidence of approximately 30%. In the patient population of the Americas, the difference among races and various ethnic backgrounds is also noticeable. Caucasians of European descent have an Rh-negative incidence of 15%, whereas Hispanics and African Americans have an incidence closer to 8%. Among several African populations, a variant of Rh (D) gene referred to as the RhD pseudogene (Rh ψ) has also been described. This gene contains all the 10 exons normally found on the Rh (D) gene but has a difference in gene expression due to the presence of a stop codon in the intron between exons 3 and 4. Because of this change, the Rh (D) protein is not synthesized, and the patient is phenotypically Rh (D) negative. This gene is detected in more than 60% of Rh-negative black Africans.
After the first exposure to the D antigen, a weak immunologic response is mounted over 6 weeks to a year. The predominant immunoglobulin produced in this first interaction is immunoglobulin (Ig) M. This large protein does not cross the placenta, and therefore the first pregnancy is usually not affected. However, a second exposure to the antigen can cause B lymphocytes to differentiate into plasma cells and proliferate. This amnestic response in the immune system is characterized by the production of IgG almost entirely; these IgG antibodies can cross the placental barrier. Two IgG subclasses have been shown to be produced during this stage, IgG1 and IgG3. Disease severity appears to be lower in cases in which IgG1 is almost exclusively present.
Fetal erythropoiesis begins in the yolk sac by day 21. The Rh antigen can be expressed as early as day 30 of gestation. After the second exposure, there is an increase in the production of IgG antibodies leading to an increase in maternal titers. These antibodies recognize and attach to the foreign antigen on the fetal RBC surface. The IgG antibody lacks the ability to bind complement, and therefore, the antibody-coated fetal RBCs are sequestered by macrophages and are destroyed by the reticuloendothelial system extravascularly. This chain of events leads to the development of fetal anemia.
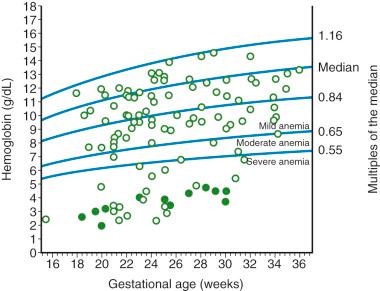
As the life span of the fetal RBC is reduced, a greater burden is placed on the hematopoietic system. It has been proposed that medullary hematopoiesis is stimulated in the early stages of mild anemia and that recruitment of extramedullary sites occurs only after anemia has progressed and become severe. This is evidenced by the fact that in severe disease, both the liver and the spleen become enlarged because of congestion and increased extramedullary hematopoiesis, causing the release of nucleated RBC precursors (erythroblasts). In a study evaluating umbilical cord samples from 127 pregnancies, Nicolaides and associates showed that significant erythroblastosis was present only when the hemoglobin deficit was greater than 7 g/dL ( Fig. 17-10 ). Severe anemia can lead to decreased oxygen delivery and hypoxia in certain end organs, causing cardiac output to increase as a compensatory mechanism. However, these mechanisms may be inadequate to maintain adequate perfusion. The loss of the buffer function of fetal RBCs also contributes to worsening acid-base disturbances. Soothill and colleagues reported an increase in the lactate concentration in the umbilical artery when hemoglobin levels were below 8 g/dL and an increase in umbilical vein concentration when this level dropped to 4 g/dL. This finding implies that disease progression leads to a shift in cellular metabolism to a predominantly anaerobic state.
The exact mechanism leading to fetal hydrops is incompletely understood. It is well known that anemia must be severe before hydrops develops and that fetal hemoglobin must fall below six standard deviations from the mean for that gestational age. Initial studies indicated that low fetal albumin levels leading to decreased colloid pressure and increased extravasation could be a contributing factor leading to hydrops. However, more recent studies have shown that hypoalbuminemia is found in only a small percentage of fetuses with hydrops and that drops in albumin levels appear to be a component of the very late stages of the disease. Other factors may also lead to increased vascular permeability facilitating the development of hydrops. Berger and coworkers demonstrated that concentration of ferritin and lipid-peroxidation products were increased, leading to the suggestion that iron overload and free radical damage may contribute to the pathogenesis. Cardiac dysfunction is also common among fetuses affected by severe anemia and could possibly play a role in the disease process. Affected fetuses develop an increase in both biventricular cardiac diameter and umbilical venous pressure. The increase in venous pressure may potentially lead to increased fluid extravasation, favoring the development of hydrops. The increase in venous pressure could also lead to an obstruction of lymphatic flow. Interestingly enough, it has also been shown that umbilical venous pressure decreases as early as 24 hours following therapeutic fetal intravascular transfusion.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here