Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Recognition of different types of pancreatic fluid collections is crucial for appropriate management.
EUS-guided drainage is the treatment of choice in the management of pancreatic pseudocysts, with clinical outcomes comparable to surgical cystogastrostomy.
The management of walled-off necrosis (WON) is more complex. If the percutaneous/surgical and the endoscopic approach are both feasible and available, the endoscopic approach is preferred. Management is multidisciplinary and is tailored to the size and location of the fluid collection. An algorithmic step-up approach including percutaneous drain insertion and endoscopic necrosectomy may be required to achieve successful clinical outcomes.
Major outcome of EUS-guided WON drainage, including number of interventions needed and complications, does not seem to differ between LAMS and plastic double pigtail stents.
Identification of disconnected pancreatic duct syndrome is crucial in patients with WON, as they require plastic transmural stents to remain in situ indefinitely to minimize risk of recurrence.
EUS facilitates transmural drainage of postoperative abdominal and pelvic fluid collections adjacent to the stomach, duodenum, rectum, or colonic lumen and within the reach of an echoendoscope. Both procedures are safe, with a treatment success rate greater than 90%. Adverse events are mild and can be managed conservatively in most patients.
Pancreatic fluid collections (PFC) can be broadly divided into four main types: (1) acute peripancreatic fluid collection, (2) pancreatic pseudocyst, (3) acute necrotic collection, and (4) walled-off necrosis (WON), with classification depending on the presence of a mature wall and the degree of solid, necrotic debris.
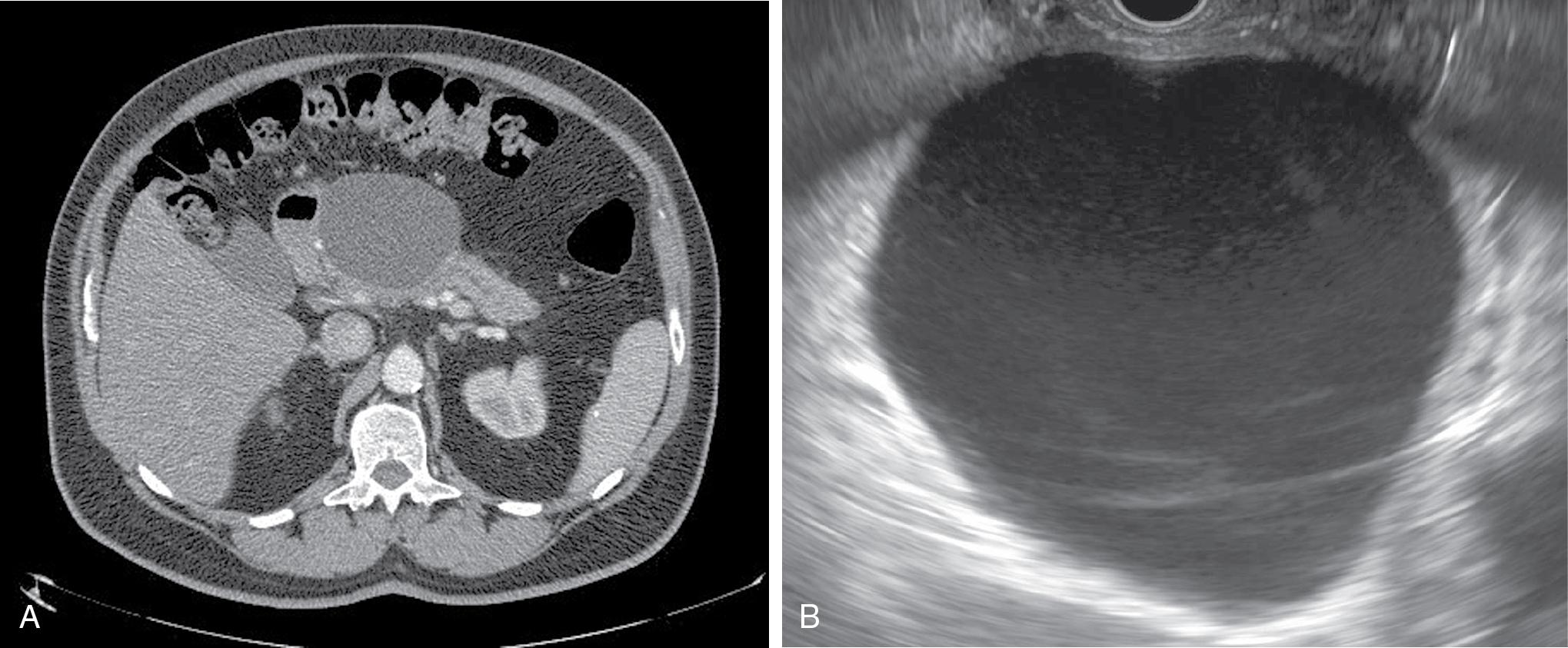
Acute peripancreatic fluid collections and pancreatic pseudocysts both result from acute interstitial edematous pancreatitis and hence contain no necrotic material. Acute pancreatic fluid collections usually develop less than 4 weeks from the onset of acute pancreatitis and can be differentiated from pancreatic pseudocysts by the absence of a mature outer wall. On the other hand, pancreatic pseudocysts are characterized by the presence of a mature wall, which usually requires 3 to 4 weeks to develop from the inciting event ( Fig. 21.1 A and B).
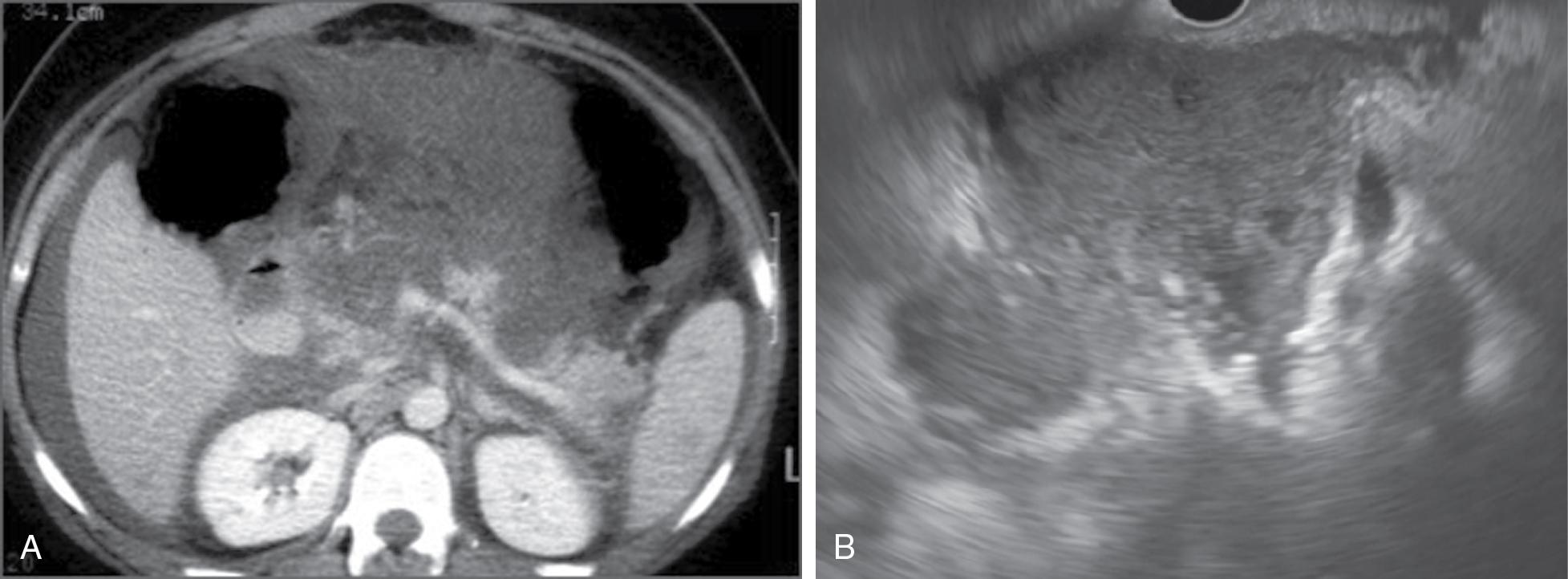
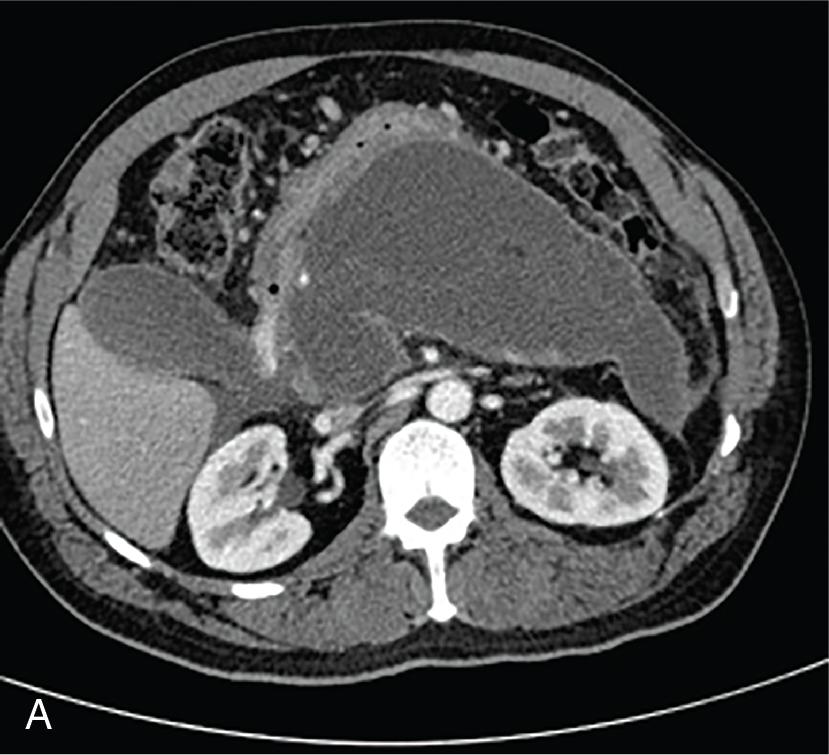
Acute necrotic fluid collections and WON occur as a result of acute necrotizing pancreatitis and hence are characterized by the presence of solid, necrotic debris of varying quantity. Similar to the difference between acute peripancreatic fluid collections and pancreatic pseudocysts, acute necrotizing fluid collections are present early on in the course of acute necrotizing pancreatitis ( Fig. 21.2 A and B), whereas WON develops after sufficient time has passed for a mature wall to form around the necrotic collection (usually >4 weeks after the onset of acute pancreatitis) ( Fig. 21.3 A and B) and ( Table 21.1 ).
| PFC Type | Etiology | Time for Development (Weeks) | Presence of a Mature Wall | Necrotic Debris |
|---|---|---|---|---|
| Acute peripancreatic fluid collection | Interstitial pancreatitis | <4 | No | No |
| Pseudocyst | Interstitial pancreatitis | ≥4 | Yes | No |
| Acute necrotic collection | Necrotizing pancreatitis | <4 | No | Yes |
| Walled-off necrosis | Necrotizing pancreatitis | ≥4 | Yes | Yes |
Computed tomography (CT) with contrast enhancement is the most commonly performed imaging modality to diagnose PFCs; however, studies have shown superiority of magnetic resonance imaging (MRI) over CT for quantification of necrotic debris. , The differentiation between necrotic and nonnecrotic PFCs is in turn critical owing to the difference in clinical outcomes, with significantly higher treatment success rates, lower adverse events rates, fewer reinterventions, and shorter duration of hospitalization observed following endoscopic drainage of pseudocysts compared to WON. Therefore, pseudocysts and WON must be recognized as separate disease entities warranting different management strategies. Furthermore, PFCs can arise in peripancreatic regions with extension into the retroperitoneal space, such as the paracolic gutters, which can pose additional management challenges. In this chapter, we outline the various EUS-guided treatment strategies that can be adopted to maximize treatment success.
Proven or suspected infection is a clear indication for drainage of WON as well as pseudocysts. In case of infected necrosis, the general consensus is that interventions should be avoided in acute peripancreatic or necrotic collections and should ideally be delayed until a mature wall has developed around the fluid collection. , This usually occurs about 4 weeks after the onset of necrotizing pancreatitis. It has been hypothesized that early drainage of infected necrosis would lead to better outcome. However, a recent randomized trial showed similar outcome but with less interventions if the procedure is postponed until collections became walled off.
Patients with asymptomatic sterile WON or pseudocysts do not require drainage, regardless of the PFC size. In patients with persisting symptoms despite conservative management, such as intractable abdominal pain, failure to thrive, gastric/intestinal outlet obstruction, and biliary obstruction, drainage should be considered. When considering intervention for sterile WON, it is important to note that over 70% of patients develop clinically relevant postprocedural iatrogenic infection that may warrant additional procedures to achieve optimal outcomes.
Surgical interventions in pancreatic pseudocysts primarily consist of either open or laparoscopic cystogastrostomy or cystoenterostomy. However, as pseudocysts are primarily fluid-filled and lack necrotic debris (unlike WON), the larger transmural conduit that can be created via surgical cystogastrostomy is not required. In a randomized trial comparing surgical cystogastrostomy with EUS-guided cystogastrostomy for drainage of pancreatic pseudocysts, high treatment success was achieved with both modalities (95% in endoscopy vs 100% in surgery group); however, endoscopic drainage was associated with significantly shorter hospital stay, better physical and mental health and lower costs. Pseudocysts, especially in case of chronic pancreatitis, are often caused by a (permanent) disrupted pancreatic duct. Besides avoidance of an external drain, EUS-guided transluminal drainage also obviates possible development of a pancreatocutanenous fistula due to an underlying disrupted pancreatic duct. Therefore, EUS-guided drainage is the modality of choice in the management of uncomplicated pseudocysts.
There has been a paradigm shift in the surgical management of WON in the last decade from invasive to minimally invasive techniques. Open surgical necrosectomy is now no longer recommended due to the high morbidity (40% to 55%) and mortality (15% to 20%) associated with this technique. , Minimally invasive surgical drainage options currently include cystogastrostomy with laparoscopic transabdominal necrosectomy and percutaneous drainage followed by videoscopic-assisted retroperitoneal debridement (VARD). In a randomized trial, the step-up minimally invasive surgical approach (consisting of percutaneous drain placement followed by minimally invasive surgical drainage) to open necrosectomy, the latter technique was associated with a significantly higher rate of major adverse events. Since then, the drainage first, followed by necrosectomy only in case of lack of clinical improvement, approach became the standard of care. Percutaneous or transmural drainage obviates the need for necrosectomy in 45% to 67% of patients. , , In addition, two recent randomized trials that compared step-up endoscopic approach (transmural drainage followed by endoscopic necrosectomy) to the step-up minimally invasive surgical approach (percutaneous drain insertion followed by minimally invasive surgical necrosectomy or laparoscopic cystogastrostomy with debridement) demonstrated comparable clinical outcomes pertaining to major complications and mortality. , However, endoscopic therapy was associated with less pancreatic fistula formation, shorter duration of hospitalization, lower costs, and better quality of life. Long-term (at least 5-year follow-up) results have confirmed durability of these outcomes. Therefore, when the requisite expertise is available and the collection can be drained via the gastrointestinal tract, endoscopic drainage (with or without necrosectomy) should be undertaken as first-line therapy in the management of WON.
In case the collection cannot be drained endoscopically, percutaneous drainage, followed by VARD/minimally invasive necrosectomy if warranted, should be the first-line treatment option.
Percutaneous drain placement can be performed as adjunctive therapy in patients with large collections extending to the lower abdomen or pelvis. It is preferable to undertake percutaneous drainage in conjunction with EUS-guided transmural drainage as a part of “dual-modality” approach (see section on Dual Modality ). However, no clear guidelines exist regarding the size of drain required or optimal duration of insertion. Also, adverse event rates associated with percutaneous drainage can range from 9% to 50%, which include bleeding, colonic perforation and pancreaticocutaneous or pancreaticoenteric fistula formation.
Since the first description of the single-step technique in 1998, EUS-guided drainage is now the standard of care technique for endoscopic drainage of PFCs. , The treatment success following EUS-guided drainage of pancreatic pseudocysts is as high at 73% to 100%, in contrast to the suboptimal treatment success rates of 60% to 70% observed with WON. , In the following section, various EUS-guided drainage techniques will be described, with the aim of optimizing clinical outcomes and minimizing adverse events.
Prior to EUS-guided drainage of PFCs, the following steps should be undertaken:
History and physical examination, with review of vital signs and laboratory values to determine indication for drainage, presence of systemic inflammatory response syndrome (SIRS), sepsis, and organ failure.
Review of the most recent cross-sectional imaging to assess location, size, presence of solid debris and a mature wall.
Optimization of coagulation parameters (INR ≤1.5, platelet count >50,000/mm 3 ).
Optimization of nutritional status with enteral feeding via nasojejunal or percutaneous gastrojejunal tube if unable to tolerate adequate oral diet.
Administration of broad-spectrum intravenous antibiotics prior to instrumentation. Antibiotics should be continued postdrainage in case of WON containing significant necrosis.
Multidisciplinary care in consultation with pancreatic surgeons and interventional radiologists especially in case of (infected) WON.
Prior to drainage of any PFC, a careful EUS examination should be performed, as EUS has been shown to alter the management in up to 37.5% of patients referred for endoscopic drainage, due to the several reasons. First, the suitability for endoscopic drainage as determined by the presence of a mature wall around the PFC that is adherent to the gastric or duodenal wall can be confirmed on EUS, especially if less than 3 to 4 weeks have passed since the inciting event. If the wall is still immature without adequate adherence, endoscopic drainage should, if clinically possible, be postponed ( ![]() ). Second, the presence of malignancy should be excluded as etiology of the PFC on EUS, which has been shown to occur in 1.25% of patients referred for endoscopic drainage (
). Second, the presence of malignancy should be excluded as etiology of the PFC on EUS, which has been shown to occur in 1.25% of patients referred for endoscopic drainage ( ![]() ). Finally, in absence of a definitive episode of clinical pancreatitis, other types of cysts that can mimic PFCs such as duplication or mucinous cysts should be excluded. In one study, 5% of patients originally diagnosed with pancreatic pseudocysts on CT were found to have mucinous cystic neoplasms on EUS (
). Finally, in absence of a definitive episode of clinical pancreatitis, other types of cysts that can mimic PFCs such as duplication or mucinous cysts should be excluded. In one study, 5% of patients originally diagnosed with pancreatic pseudocysts on CT were found to have mucinous cystic neoplasms on EUS ( ![]() ).
).
EUS-guided drainage of PFCs should be performed in a thoughtful and systematic manner, adopting variations in endoscopic techniques according to the type, size, location of the PFC, and subsequent response to intervention. This approach, in conjunction with collaborative management with pancreatic surgeons and interventional radiologist, is especially important in the management of WON, where treatment success can be suboptimal at only 60% to 70%.
The single-gate technique consists of creation of a single transmural tract, through which plastic or metal stents are placed for PFC drainage. This is a multi-step process when plastic stents are being deployed ( ![]() ), but can be a single-step process when using lumen-apposing metal stents (LAMS) with an electrocautery-enhanced delivery system (
), but can be a single-step process when using lumen-apposing metal stents (LAMS) with an electrocautery-enhanced delivery system ( ![]() ).
).
Therapeutic linear array echoendoscope with a 3.7-mm working channel
Fluoroscopy
19-gauge fine-needle aspiration (FNA) needle
Syringe for aspiration of PFC contents for Gram stain and culture
0.025- or 0.035-inch guidewire
Two types of dilators: (1) 4.5-Fr tapered tip endoscopic retrograde cholangiopancreatography (ERCP) cannula or needle-knife catheter or cystotome catheter AND (2) Graded balloon dilator
Two or more 7-Fr diameter 4-cm length double pigtail plastic stents
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here