Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The prevalence of esophageal adenocarcinoma has risen dramatically over the past three decades. Esophageal cancer is the eighth most common cancer worldwide, and more than 480,000 new cases were diagnosed in 2008. The documented increase is approximately six- to sevenfold, with adenocarcinoma now contributing to approximately 80% of the esophageal cancer surgical workload in many surgical units. This increase is unprecedented and has occurred at a faster rate than for any other cancer.
Treatment of this disease continues to represent a formidable challenge. According to American Cancer Society's Cancer Facts & Figures, 2013, the overall 5-year survival rate for all patients is 19%. Despite the common practice of incorporating chemotherapy or chemoradiotherapy into the treatment paradigm, surgical resection remains the cornerstone of most treatment protocols for early-stage and locally advanced disease. Today patients treated with an esophagectomy have a 5-year survival rate close to 35%.
The first esophageal resection was performed more than 125 years ago, yet it remains one of the most formidable operations, carrying the highest morbidity rate of any commonly performed resection. The perioperative mortality rate has decreased from 40% to less than 3% in selected academic centers, largely because of advances in surgical and anesthetic technique, intensive care, and management of complications. The rate of morbidity for this surgery has not decreased as dramatically as has the rate for mortality. With average mortality rates at 10% and complication rates exceeding 50%, careful selection of patients, thoughtful surgical planning, meticulous technique, and timely management of complications are essential to ensure good outcome for the patient.
In general, the principle objective for the surgeon is to balance oncologic principles of clear surgical margins and adequacy of lymphadenectomy with the risks associated with the planned operative approach on a case-by-case basis. Patient and tumor-related characteristics should play a pivotal role in the surgical decision making rather than individual or institutional practice patterns.
The esophageal surgeon must be familiar with the anatomy of the neck, chest, and abdomen and be skilled in surgery of the entire alimentary tract to provide complete care of the patient requiring esophageal resection. Only with this knowledge can the surgeon fit the operation to the patient, not the patient to the operation. These issues are detailed in this chapter.
Ivor Lewis, writing in 1946, is credited with popularizing transthoracic resection of the esophagus. He initially performed the operation in two stages, first mobilizing the stomach through a laparotomy and second, several days later, resecting the esophagus and performing an intrathoracic anastomosis through a right thoracotomy. Lewis showed a sophisticated understanding of the challenges involved in esophageal surgery when he stated that “the oesophagus is a difficult surgical field for three reasons: its inaccessibility; its lack of a serous coat, and its enclosure in structures where infection is especially dangerous and rapid.” The Ivor Lewis and transhiatal approaches are the most commonly used techniques of esophageal resection today. In 1962, McKeown described a three-stage esophagectomy, with the addition of a cervical incision and anastomosis to allow better margins for proximal tumors. It is now called the three-hole or tri-incisional esophagectomy.
Open esophagectomy has up to 60% reported morbidity. For this reason, several approaches have been supplemented with thoracoscopic or laparoscopic modifications. Since Cuschieri and colleagues first introduced the concept of a minimally invasive approach to esophagectomy in 1992, there have been numerous descriptions in the literature.
The tri-incisional esophagectomy (modified McKeown) is a versatile technique that can be used for tumors at any level and for various benign and malignant conditions. It combines the advantages of the Ivor Lewis approach with those of a transhiatal technique. These include complete lymph node dissection in the chest, direct visualization of the intrathoracic dissection, avoidance of an intrathoracic anastomosis, maximal margins, and diminution of chances for postoperative gastroesophageal reflux disease. It is especially useful for tumors of the mid and upper esophagus and for tumors arising in a long Barrett segment where almost total excision of the esophagus is required.
Fusion of the right pleural space or inability to support ventilation with the left lung would make an approach through a right thoracotomy difficult. Relative contraindications include small gastric conduits secondary to tumor or limited vascular supply. Patients with tumor extending onto the stomach require more gastric resection than do those with tumor isolated to the esophagus. These patients are most suited for an Ivor Lewis or a jejunal reconstruction if there is not enough stomach.
A detailed history and physical examination are necessary to evaluate for coexistent comorbid disease. Computed tomography and positron emission tomography of the chest and abdomen should be performed to evaluate for metastatic disease, anatomic abnormalities, and location of the tumor adjacent to vital structures. Pulmonary function tests should be obtained. Poor forced expiratory volume in 1 second (FEV 1 ) is not a contraindication to a muscle-sparing limited thoracotomy, although increased risk of pulmonary complications may be expected. Computed tomography and positron emission tomography of the head are useful in ruling out metastatic disease. Preoperative bowel preparation is generally done in the rare and unfortunate case in which there needs to be a change in conduit.
Esophagogastroduodenoscopy is performed to identify the location of the tumor and to rule out disease of the stomach or duodenum. Retroflexion of the esophagogastroduodenoscopy is important for proper evaluation of the gastroesophageal junction. The carina is at the level of 25 cm from the incisor on esophagogastroduodenoscopy. Tumors in this area should be carefully evaluated by bronchoscopy for tumor invasion of the trachea. A double-lumen tube is placed, and the patient is positioned in a left lateral decubitus position. A right posterolateral thoracotomy incision is made wide enough to insert the surgeon's hand (approximately 10 cm). A portion of the latissimus is divided; the serratus is spared ( Fig. 38-1 ). Division of the intercostal muscle from the vertebral bodies posteriorly to the mammary vessels anteriorly usually provides enough working space without division of a rib. The chest is entered through the fifth or sixth interspace, depending on the location of the tumor.
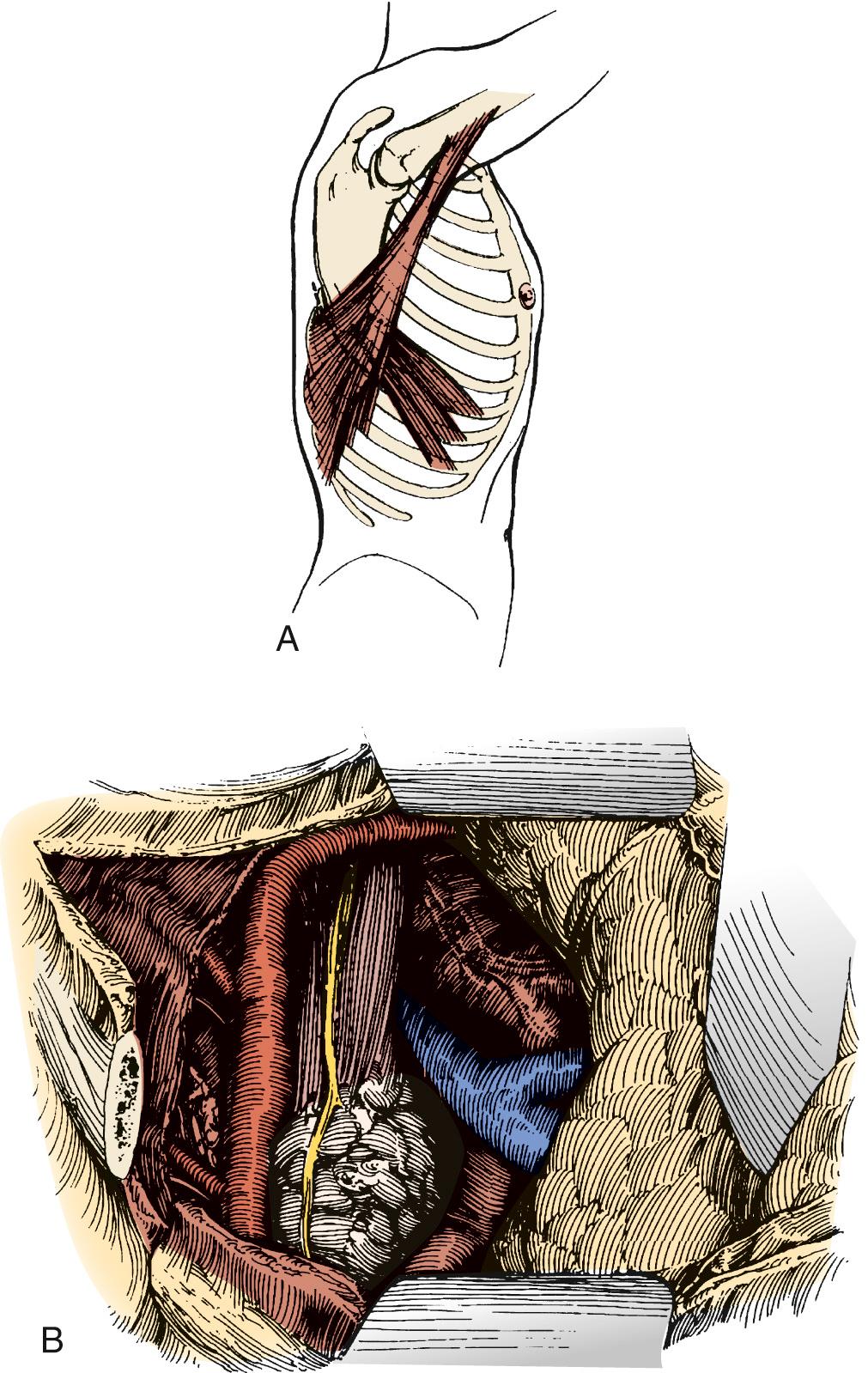
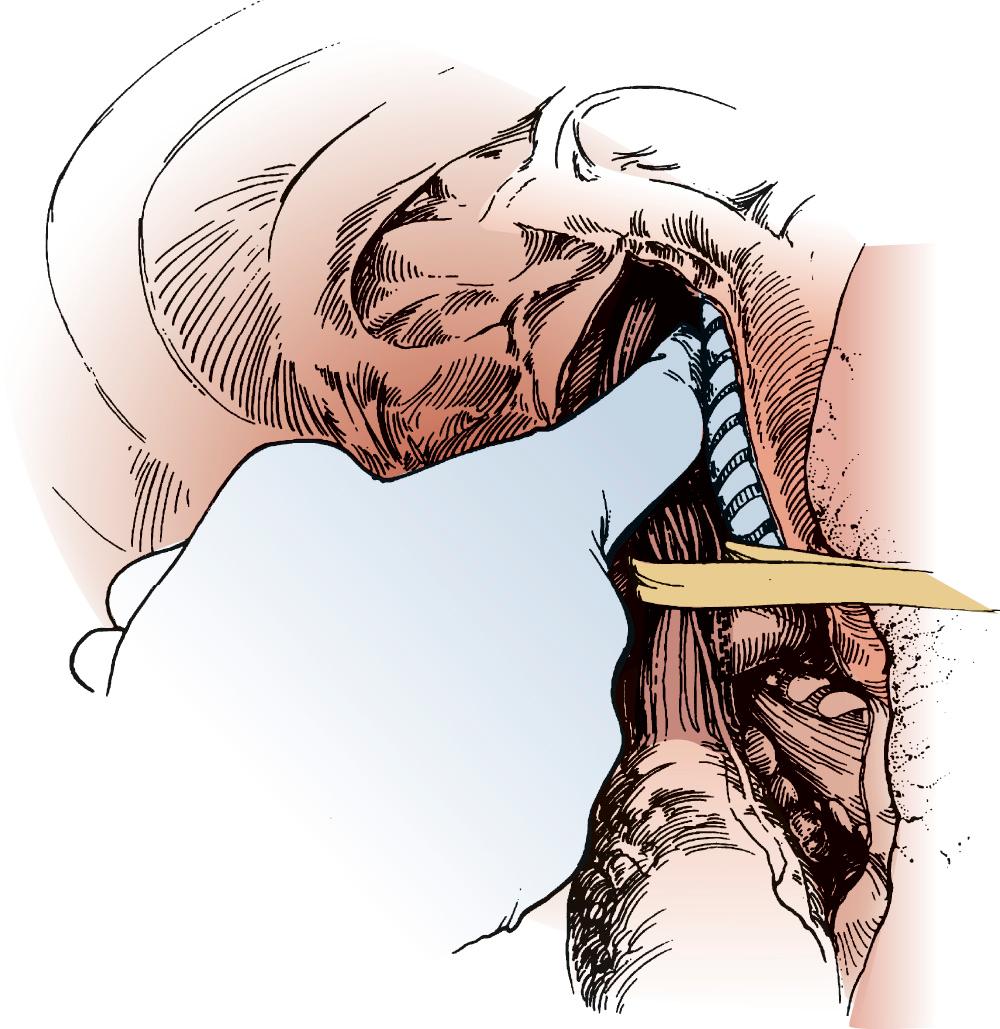
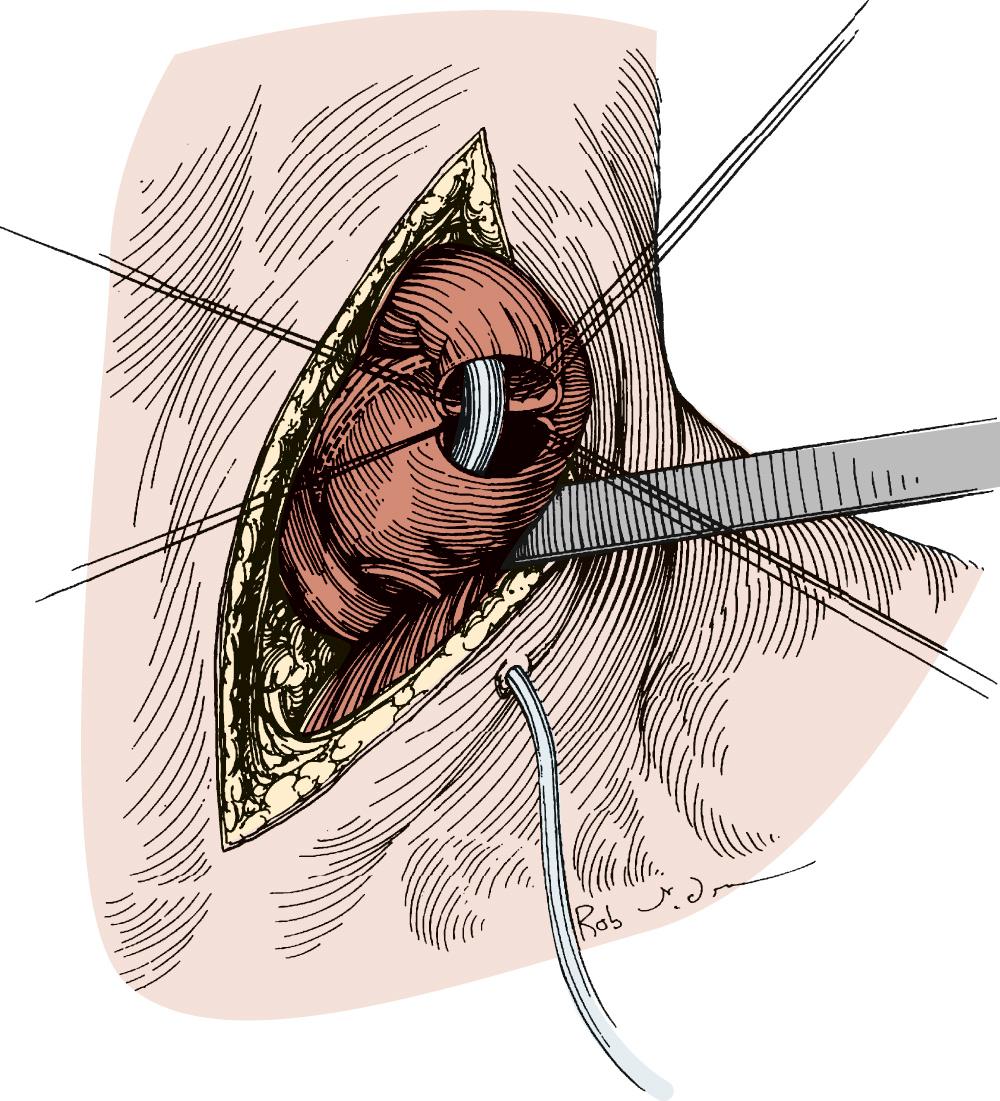
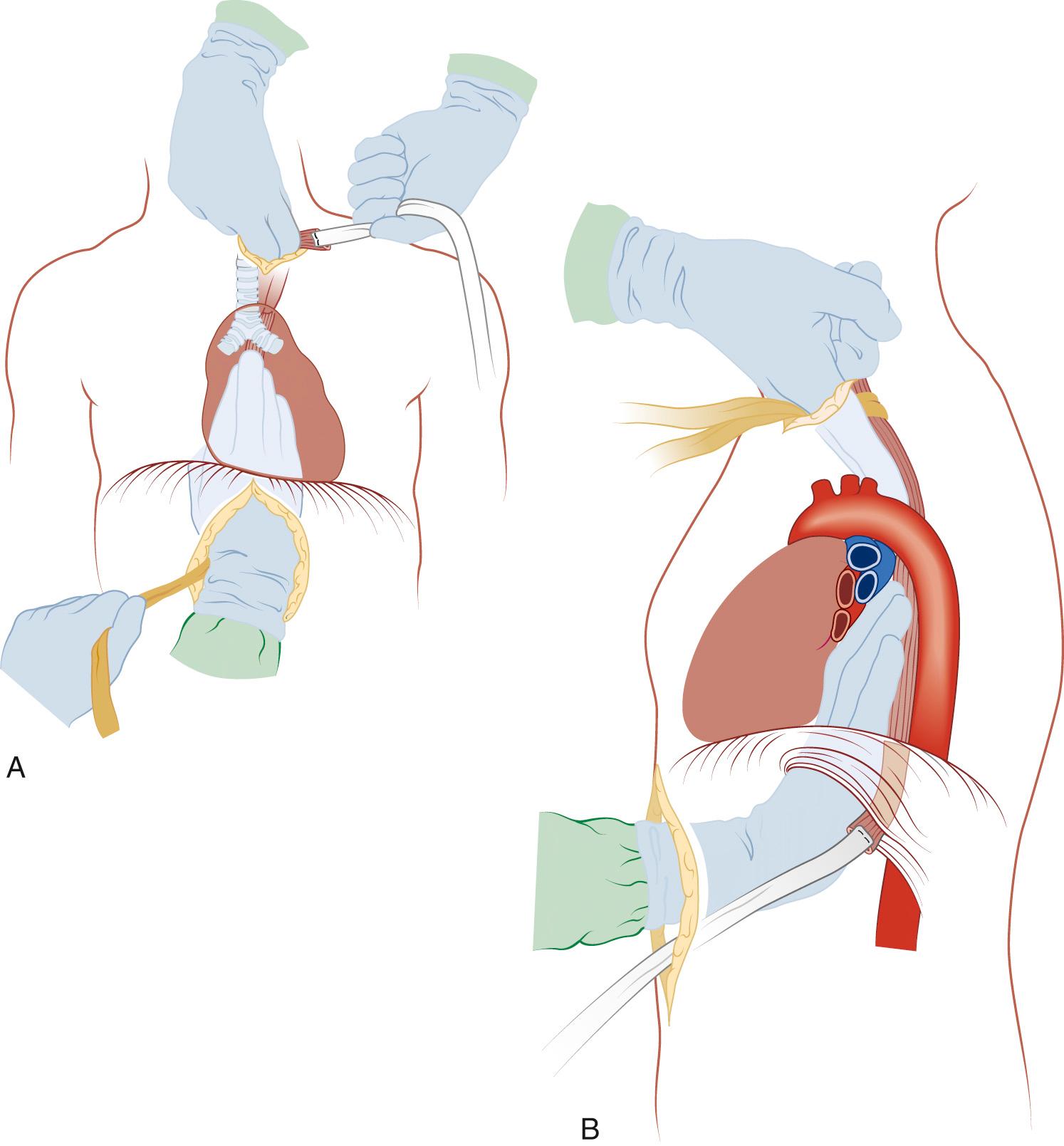
The lung is retracted anteriorly, and the inferior pulmonary ligament is divided with use of cautery. In a region away from the tumor and away from any scarring, the pleura overlying the esophagus is incised anteriorly and posteriorly and the esophagus is surrounded with a Penrose drain. With traction on the Penrose drain, the esophagus is retracted both anteriorly and posteriorly and is dissected by electrocautery, including all adjacent lymph node tissue. Arterial branches supplying the esophagus from the aorta are clipped before being divided. Any cautery performed in the region of the carina must be at low settings to avoid thermal injury to the trachea. The azygos vein is typically divided. At the level of the azygos vein, the vagus nerves are identified on the esophagus and divided between clips. Division of the vagal nerve is important because an intact vagus nerve can lead to a traction injury of the recurrent laryngeal nerve. Further cranial dissection proceeds with the vagus nerves away from the esophagus to avoid recurrent nerve injury. Blunt dissection is useful high in the right chest ( Fig. 38-2 ). Most dissection should be in the posterior plane to avoid injury to the recurrent laryngeal nerve, which is in the groove between the esophagus and trachea. A knotted Penrose drain is placed around the cervical esophagus and pushed into the posterior neck for retrieval during the cervical portion of the case ( Fig. 38-3 ). This Penrose drain, positioned inside the vagus nerves relative to the esophagus, permits isolation of the cervical esophagus without traction on the recurrent nerve. A sponge is packed high in the chest to assist in hemostasis and is removed before closure. The lower portion of the esophagus is encircled with a second Penrose drain and the remaining distal esophagus is dissected, including in the specimen all tissue lateral to the pericardium and medial to the aorta and spine.
If the tumor is near the gastroesophageal junction, a 2-cm rim of diaphragm is resected with the esophagus ( Fig. 38-4 ). The Penrose drain is knotted and left in the abdomen for retrieval during the abdominal portion of the operation. Mass ligature of the thoracic duct at the level of the hiatus is performed by encircling all tissue between the aorta and spine with a 0 silk suture. The chest is inspected for hemostasis, the sponge previously placed is removed, and a 28 Fr straight chest tube is inserted through a separate stab incision and directed to the apex of the chest. The ribs are approximated with interrupted #2 Vicryl sutures. The latissimus is closed with running 0 Vicryl suture. The subdermal fascia is closed with a running 2-0 Vicryl suture. The skin is closed with staples or suture. The patient is repositioned in the supine position and is reintubated with a single-lumen tube. A transverse roll is placed under the scapula, and the head is turned 45 degrees to the right. An upper midline laparotomy is performed from the umbilicus to the junction of the costal margin and the left side of the xiphoid; the xiphoid is occasionally resected for better visualization. The abdomen is explored for metastatic disease, which includes inspection of the liver, omentum, and peritoneal surfaces. The left lobe of the liver is mobilized by dividing the triangular ligament. Close to the triangular ligament are large diaphragmatic veins that should be avoided during this dissection. The Penrose drain surrounding the gastroesophageal junction is grasped, and the remaining phrenoesophageal ligament is divided ( Fig. 38-5 ). The gastroepiploic pulse is palpated. At a point 2 cm away from the gastroepiploic artery on the greater curvature of the stomach, the lesser sac is entered. Dissection along the greater curvature of the stomach toward the spleen proceeds with division of vascular branches by use of double clips, silk ties, or the harmonic scalpel. Short gastric vessels are taken in identical fashion. Displacement of the spleen anteriorly by placement of lap pads behind the spleen may aid in dissection of the short gastric vessels. Once the short gastric arteries are divided, additional dissection toward the pylorus is performed. The gastroduodenal artery travels behind the pylorus before branching into the right gastric epiploic and superior pancreaticoduodenal arteries. The duodenum is mobilized from its retroperitoneum connections by a Kocher maneuver. The duodenum is adequately mobilized when the pylorus can reach the esophageal hiatus.
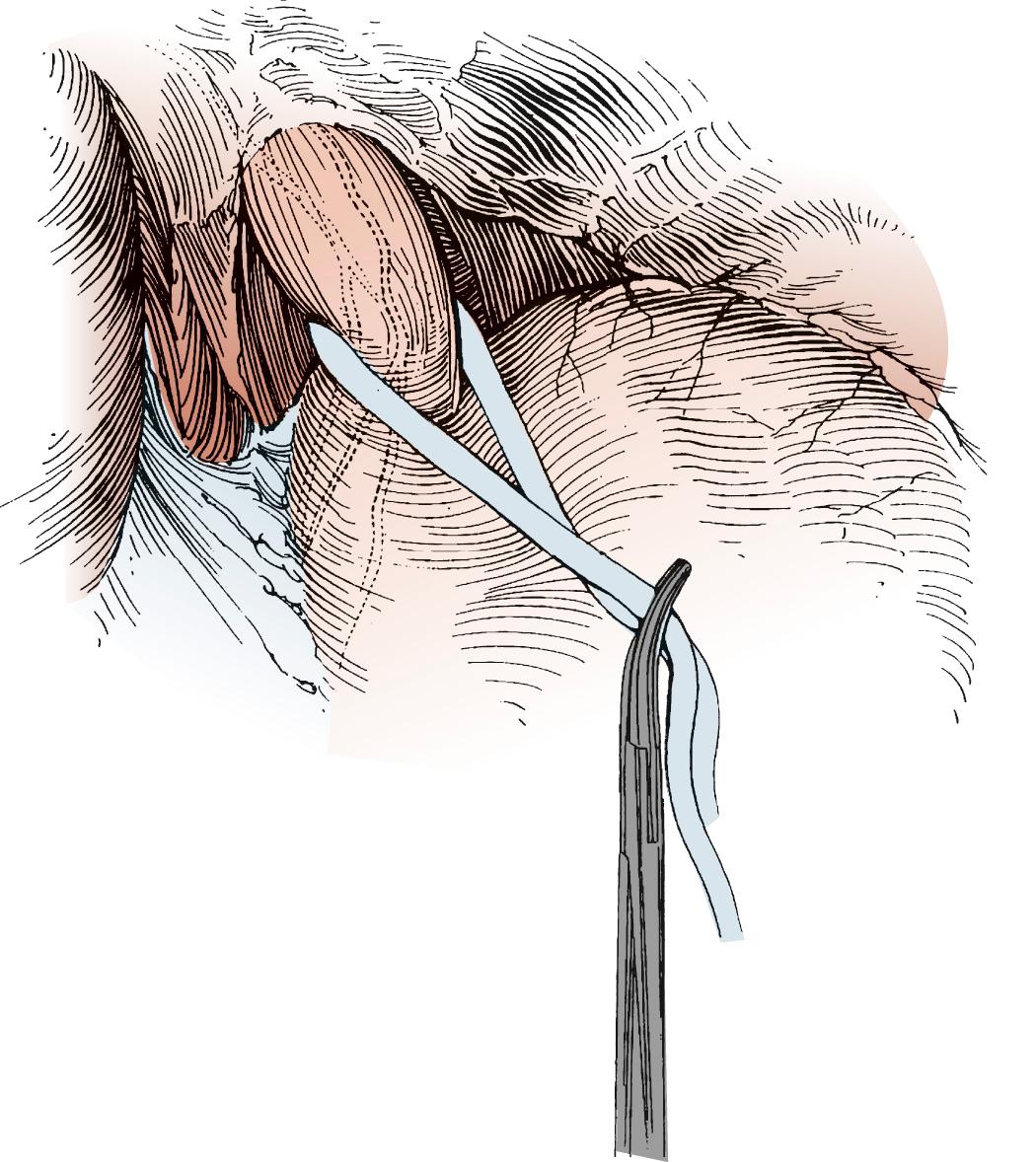
The stomach is then lifted anteriorly, and any adhesions between the stomach and pancreas are divided with electrocautery. The left gastric artery is identified and skeletonized, sweeping lymph node tissue onto the specimen. With a 30-mm vascular stapler, the base of the left gastric artery is clamped. A continued strong pulse in the gastroepiploic artery is confirmed, and the left gastric artery is divided ( Fig. 38-6 ). The gastrohepatic ligament is divided by a combination of cautery and the stapler. Either a pyloromyotomy or a pyloroplasty may be performed. If a pyloroplasty is performed, it should be a single layer with interrupted 3-0 silk sutures, carefully incorporating mucosa and muscular wall.
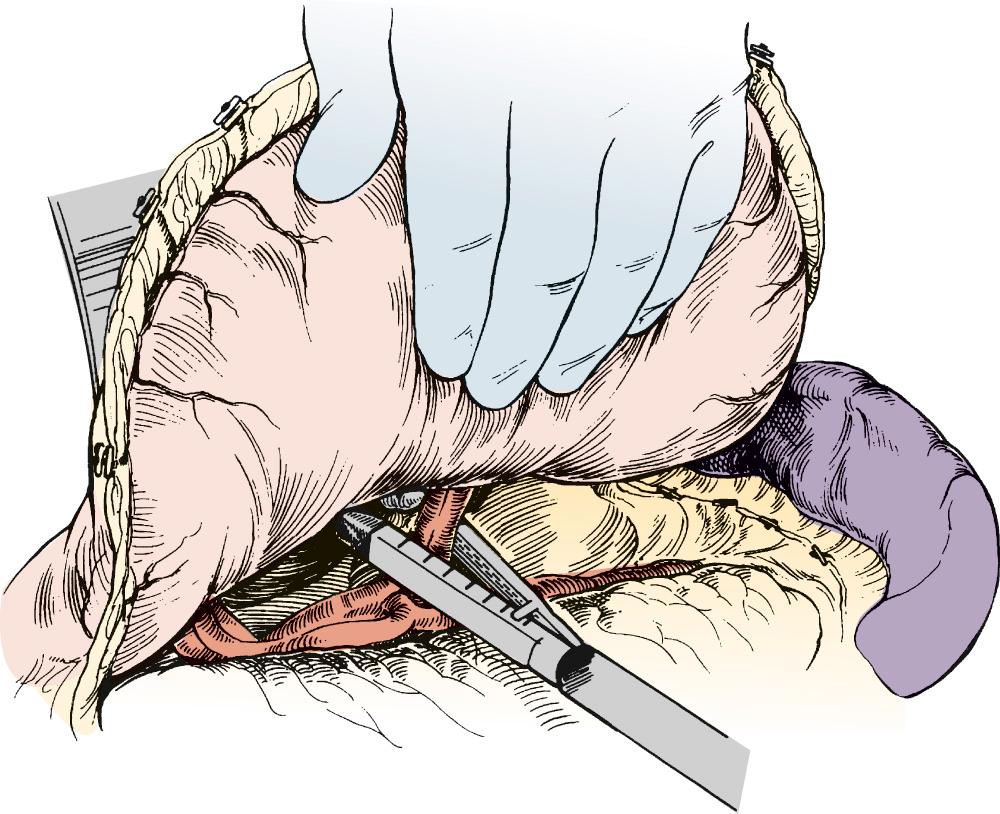
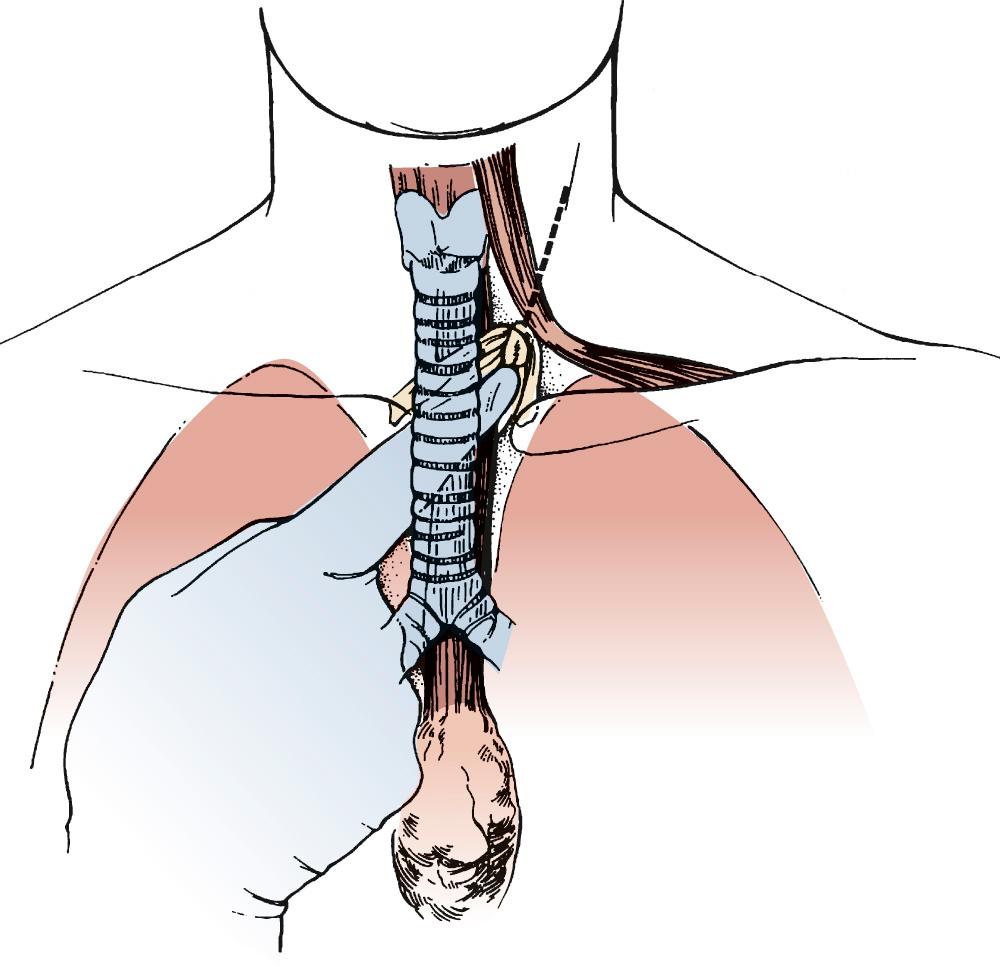
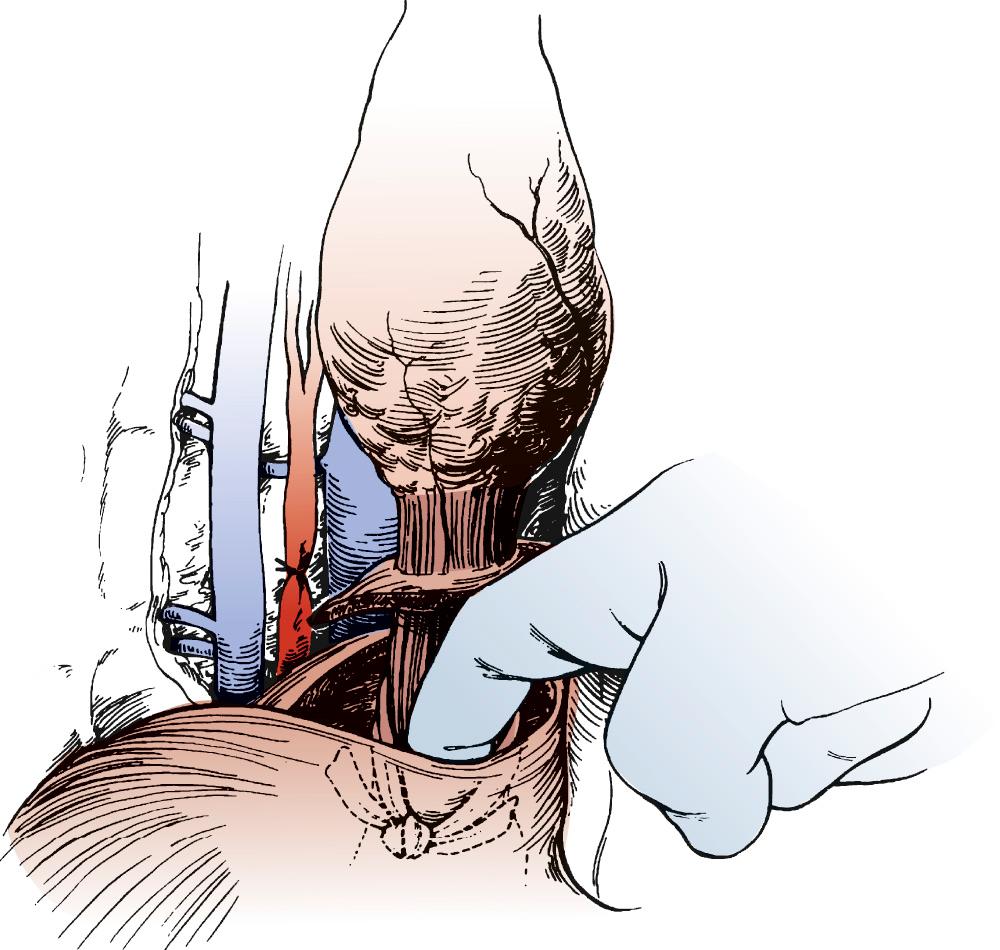
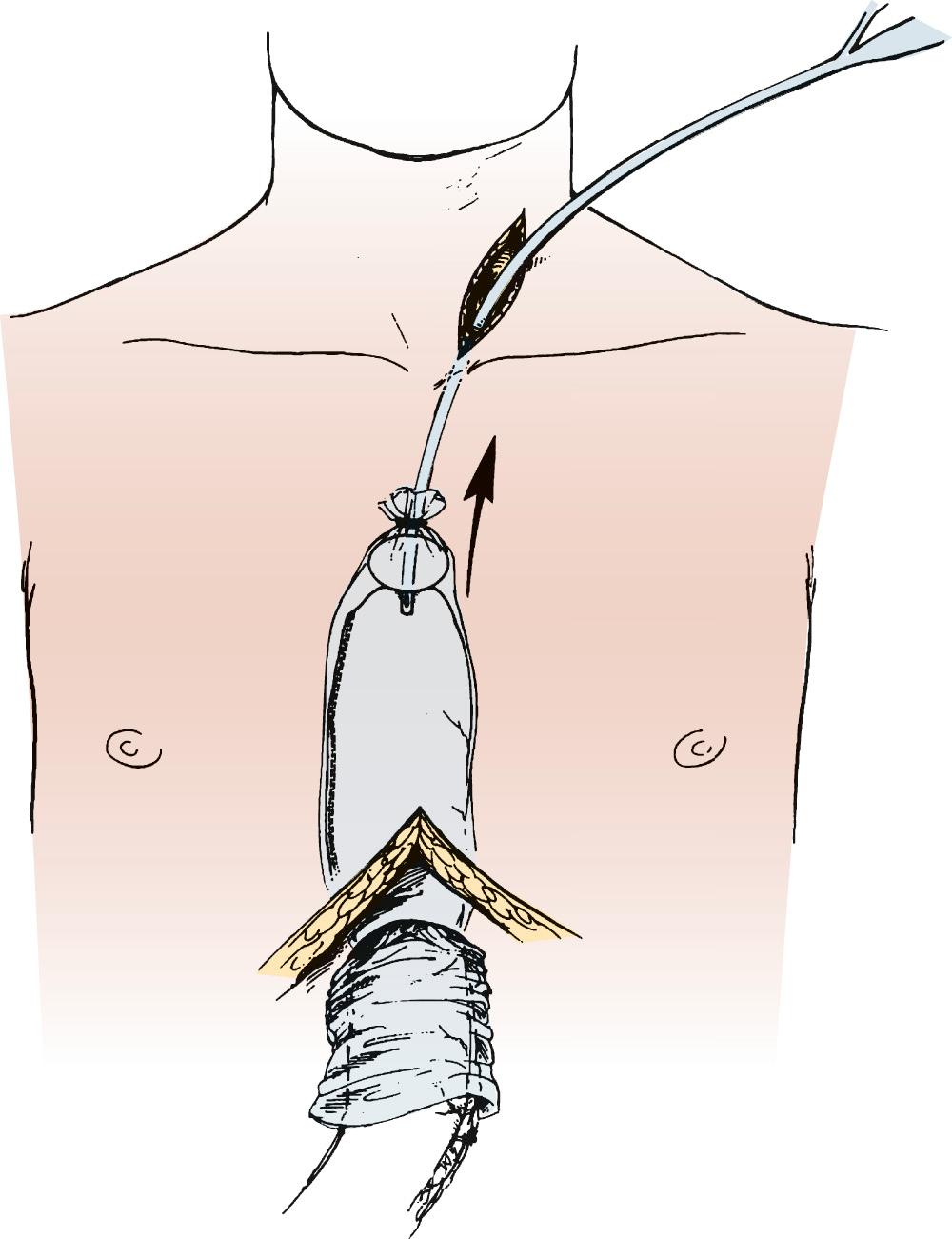
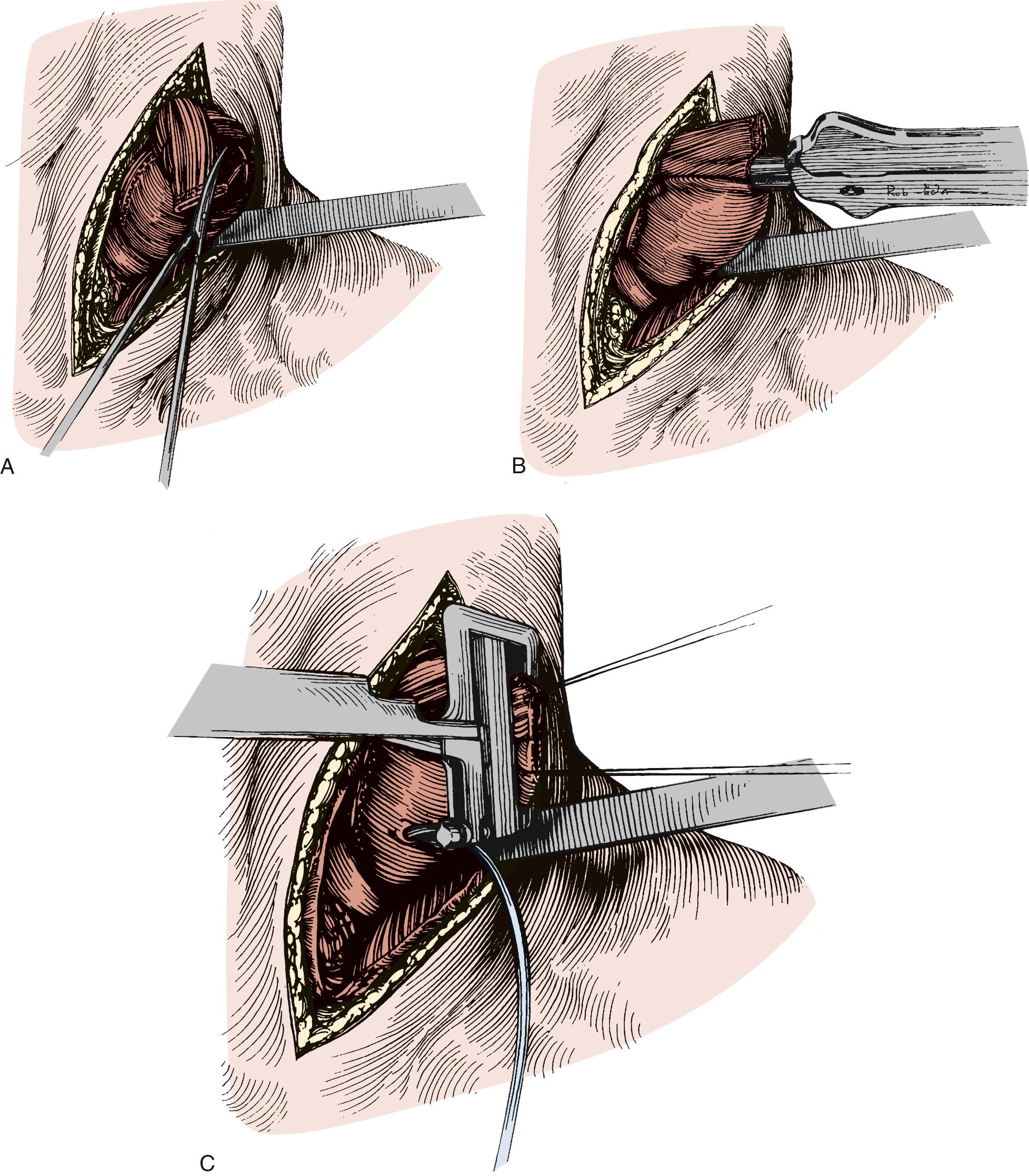
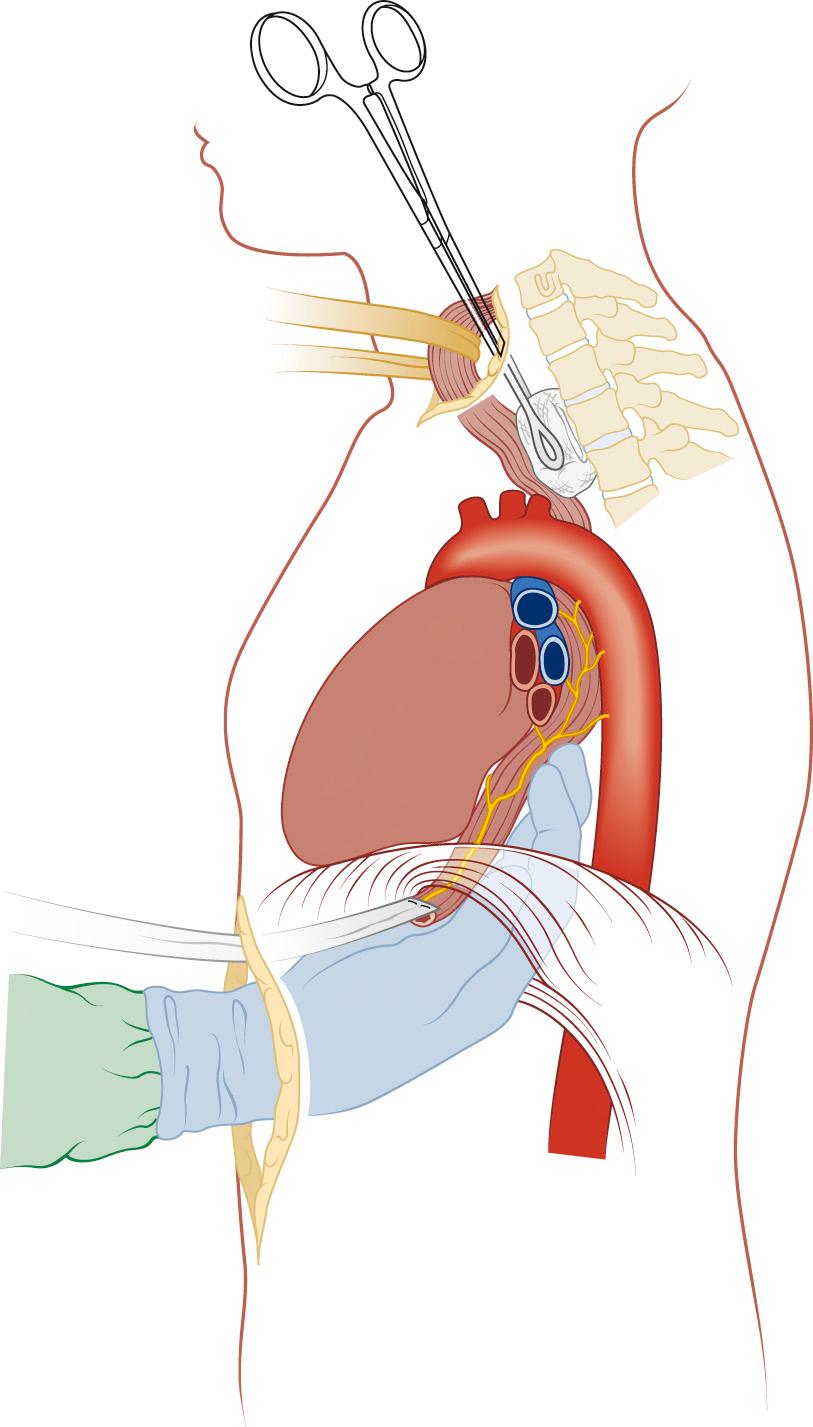
Attention is then turned to the neck, and a 6-cm incision is made from the sternal notch along the anterior border of the sternocleidomastoid muscle. The platysma is divided, and dissection proceeds between the carotid sheath laterally and the strap muscles medially. The omohyoid muscle may be divided. The knot of the Penrose drain should be palpable on the spine. The Penrose drain is grasped, and the esophagus is gently mobilized. The nasogastric tube is partially withdrawn, and the cervical esophagus is divided with a 75-mm linear stapler ( Fig. 38-7 ). A #2 silk suture ligature is fastened to the distal end of the divided esophagus, and the specimen is drawn into the abdomen with the attached silk suture. The cervical end of the silk suture is fastened to a clamp.
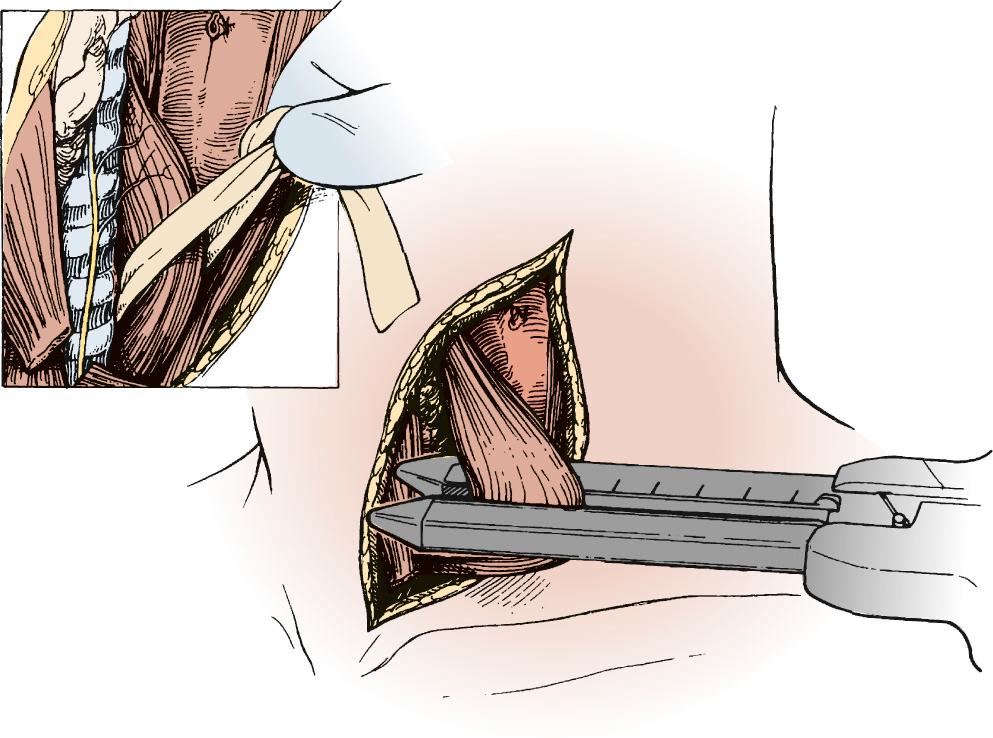
A gastric tube is fashioned by resecting the gastroesophageal junction and the lesser curve of the stomach with a series of 75-mm-thick tissue staples ( Fig. 38-8 ). A narrow gastric tube aids in gastric emptying. A diameter of less than 6 cm, however, can compromise venous and arterial blood supply. The line of division ends at a point on the lesser curve near the crow's-feet of veins. At this point along the lesser curve, the right gastric artery and associated tissue can be divided to maximize conduit length.
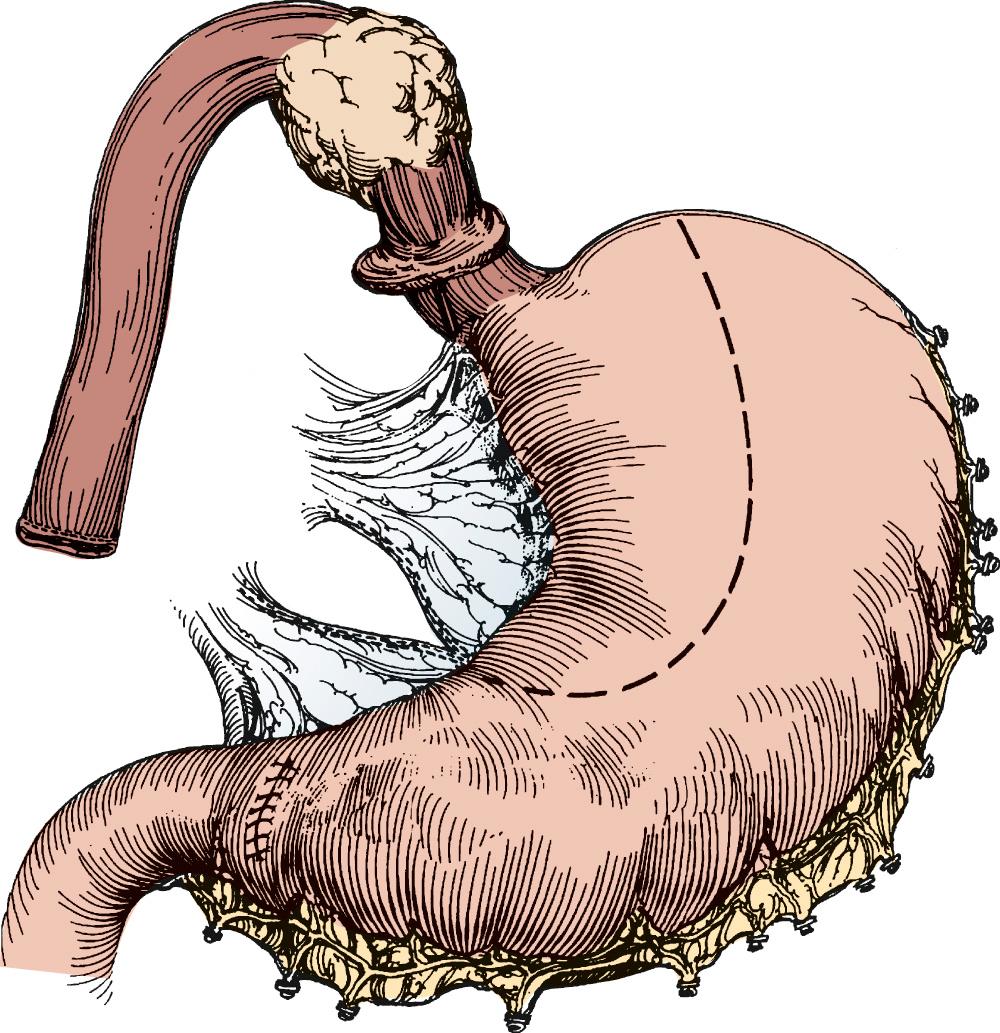
With the specimen removed, a final check for hemostasis is made in the bed of the stomach and spleen. Before the conduit is pulled into the neck, the esophageal hiatus should be dilated to four fingers. The gastric conduit may be pulled to the neck in relatively atraumatic fashion by the use of an endoscopic camera bag attached to a Foley catheter. The heavy silk tie that traverses the mediastinum from the neck is tied to the valved end of a 30-cm three-way Foley urinary catheter. The endoscopic bag is secured to the Foley balloon. The conduit is placed in the bag, and the valved end is drawn into the neck ( Fig. 38-9 ). The assistant must guide the conduit through the hiatus and up the lower mediastinum, ensuring that there is no torsion. The bag is cut away from the conduit in the neck, and the conduit is grasped with a Babcock instrument. The pylorus should sit at the hiatus. A side-to-side, functional end-to-end stapled anastomosis may be performed. The anastomosis is created with a 75-mm linear stapler followed by a 30-mm endoscopic stapler. Alternatively, the neck anastomosis can be hand sewn with interrupted circumferential full-thickness 3-0 silk sutures ( Fig. 38-10 ). The nasogastric tube is advanced across the anastomosis and positioned just proximal to the pylorus before closure of the remaining anastomotic defect ( Fig. 38-11 ). A drain is placed along the spine posterior to the anastomosis, and the platysma is run closed with 2-0 Vicryl sutures. The skin is closed with staples. A feeding tube is inserted in the jejunum at a point 40 cm distal to the ligament of Treitz. The abdominal fascia is closed with a running #2 monofilament suture, and the skin is closed with staples.
The indications are similar to those for a tri-incisional approach.
Tumors located in the upper third of the thoracic esophagus, above the carina, are better approached with resection of the cervical esophagus and anastomosis in the neck to ensure adequate margins. Long-segment Barrett esophagus with extension into the cervical esophagus is also a contraindication to an anastomosis in the right chest. A fused pleural space or severely compromised lung function should lead to reconsideration of a thoracotomy.
The patient is positioned supine. Preoperative bronchoscopy and esophagogastroduodenoscopy are performed. An upper midline incision is made extending from the umbilicus to the xiphoid. The abdominal portion of the operation is identical to that described for a tri-incisional esophagectomy, with complete mobilization of the stomach, kocherization of the duodenum, pyloroplasty, construction of the gastric tube, and placement of a J tube. The conduit is advanced into the chest as far as possible before the abdomen is closed. After placement of a double-lumen endotracheal tube, the patient is repositioned in the left lateral decubitus position, and a standard right posterolateral thoracotomy is performed. Entry into the chest is through the fourth or fifth interspace. The azygos vein is divided and the intrathoracic esophagus is dissected, including all adjacent areolar and lymphatic tissue. A margin of at least 5 cm and ideally 10 cm is desirable, and thus the anastomosis is usually performed high in the right chest. The esophagus is dissected free to a point only several centimeters above the proposed line of transection to preserve blood supply to the anastomosis. The esophagus is divided and the stomach is pulled up, and the gastric conduit is fashioned with a gastrointestinal anastomosis (GIA) stapler. Various techniques may be used to perform the anastomosis, including single-layer hand sewn, double-layer hand sewn, end-to-end stapled, and side-to-side functional end-to-end stapled.
With all techniques, wrapping of the anastomosis with omentum and passage of the nasogastric tube before completion of the final portion of the anastomosis are advised. A stapled anastomosis may be performed in a side-to-side, functional end-to-end manner with creation of the anastomosis by a GIA 75-mm or sequential 30-mm endoscopic stapler and closure of the defect with a thoracoabdominal (TA) 30-mm or 60-mm stapler. A similar technique involves lining up the gastric conduit and esophagus in a linear fashion and stapling the back wall of the anastomosis with a linear stapler or endostapler and sewing the front wall by either a one- or a two-layer technique. This type of approach is used in the neck as described by Orringer. The anastomosis can also be performed with an end-to-end anastomosis (EEA) stapler, although anastomoses created with EEA staplers smaller than 33 mm carry a significant risk of stricture. With the EEA stapler, passage of the nasogastric tube is, of course, performed after completion of the anastomosis.
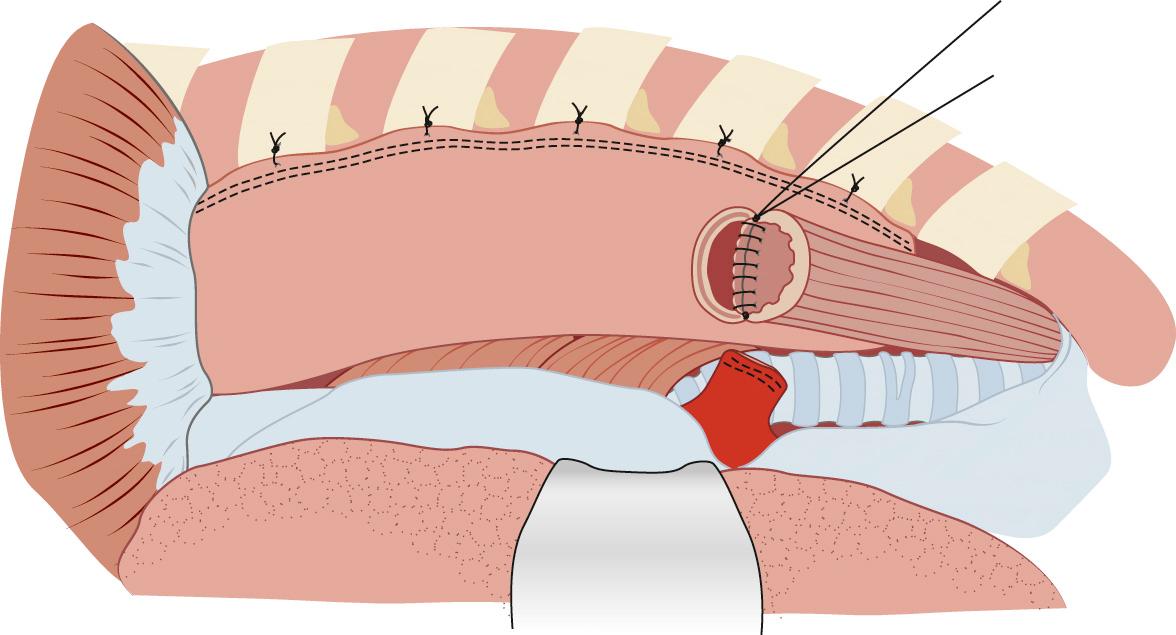
If a hand-sewn anastomosis is to be performed, the proximal esophagus is divided with a knife after clamping the esophagus proximally with a noncrushing bowel clamp. A single-layer anastomosis is performed with interrupted 3-0 silk stitches with full-thickness bites of the mucosa and muscularis and careful approximation of tissues. Knots on the posterior row may be tied inside the lumen ( Fig. 38-12 ). A double-layer anastomosis was described by Churchill and Sweet in 1942. At a point at least 2 cm beyond the staple line, a 2-cm-diameter area of gastric serosa is scored. Underlying vessels are ligated with interrupted silk sutures. The posterior layer of the anastomosis is created with interrupted 3-0 silk stitches. An inner, full-thickness layer is performed with a fine 4-0 absorbable suture, either catgut or Monocryl, by a running Connell stitch to invert the mucosa. Before completion of the inner layer, the nasogastric tube is passed to the hiatus. An anterior outer layer is then performed with interrupted 3-0 silk. Omentum is used to wrap the anastomosis. The distal portion of the stomach is reduced into the abdomen to avoid excess redundancy of the stomach in the chest, which might impair conduit emptying. Stitches are placed anchoring the conduit to the diaphragmatic hiatus. Some surgeons use additional stitches anchoring the intrathoracic conduit to the pleura, although the effectiveness of these stitches is debated.
Some physicians believe that transhiatal esophagectomy is ideally suited for benign esophageal disease and possibly Barrett esophagus with high-grade dysplasia, for which complete lymphadenectomy may not be necessary. Other factors, such as poor pulmonary function (FEV 1 <800 mL or <35% predicted) and pleural symphysis, would favor a technique that avoids a thoracotomy.
Bulky tumors of the midthoracic esophagus are difficult to visualize from the transhiatal approach, and injury to adjacent structures may occur. Scarring after neoadjuvant treatment of esophageal tumors may also make the transhiatal approach difficult. The need to perform a complete lymphadenectomy is a contraindication to this approach.
The presence of severe coronary or valvular disease makes the episodic drops in blood pressure associated with blunt dissection behind the heart especially treacherous. A transthoracic approach may be preferable in these cases.
The preparation is identical to preparation for a tri-incisional esophagectomy.
Bronchoscopy and esophagogastroduodenoscopy are performed as previously described. The abdominal portion of the procedure is performed as described in the previous section. An upper hand retractor aids in raising the xiphoid and sternum for visualization of the mediastinum. The esophagophrenic attachments are divided with cautery, the gastroesophageal junction is separated from the crura, and the distal esophagus is encircled with a Penrose drain. The hiatus is dilated to allow entry of the surgeon's hand. Various hand-held malleable retractors may be used in dissection of the mediastinum through the hiatus. With the use of the Penrose drain to retract the lower esophagus, the lower esophagus is dissected through the hiatus. Arterial branches supplying the lower esophagus from the aorta are clipped under direct vision. With the fingertips against the esophagus, the esophagus is dissected off the vertebral plane bluntly into the upper chest. The palmar aspect of the fingertips is kept directly against the esophagus. Arteries supplying the esophagus branch approximately 1 cm away from the esophagus, and dissection close to the esophagus disrupts only the smaller arterial branches directly entering the esophagus. Close dissection of the esophagus also avoids injury to adjacent structures. A cervical incision is made along the lower border of the left sternocleidomastoid muscle. Dissection proceeds as described in the previous section with the exception that the esophagus must be identified and encircled from the neck. An approach from the left side of the neck aids in avoiding injury to the right recurrent laryngeal nerve, which is farther from the esophagus than the left recurrent nerve at this level. Dissection is kept immediately on the esophagus to avoid recurrent nerve injury. Metal retractors should not be placed near the tracheoesophageal groove. A Penrose drain is placed around the cervical esophagus.
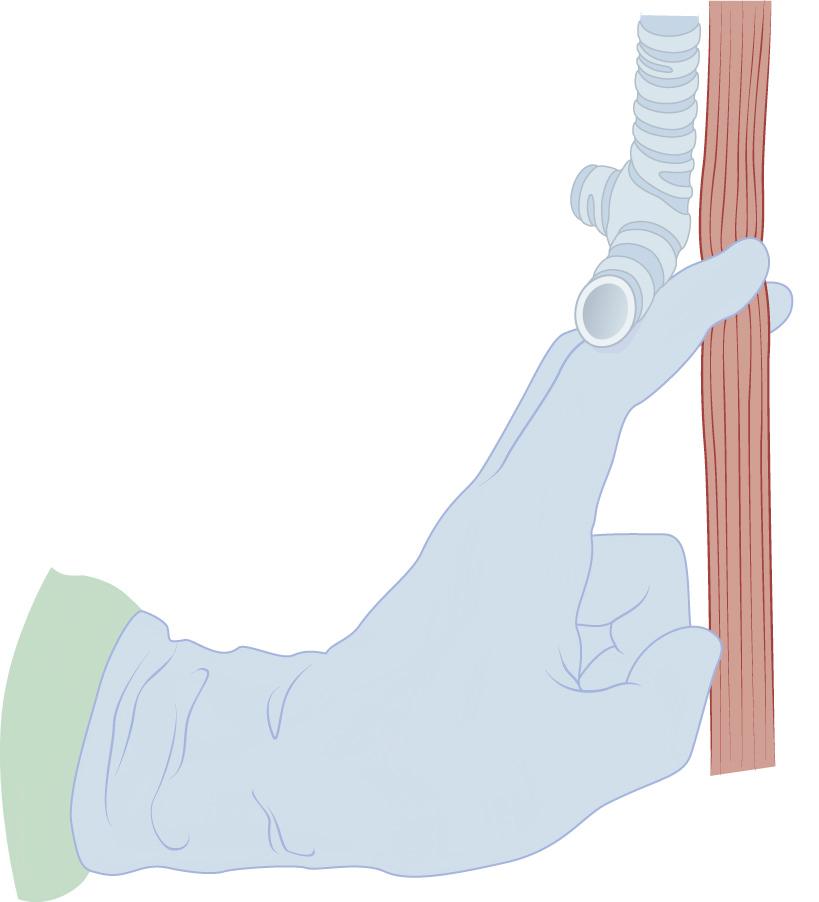
Dissection caudally is performed with two fingers against the esophagus in the posterior plane. Depending on the length of the surgeon's fingers and the width of the abdominal surgeon's hand, it should be possible for the two hands to contact directly. A thin plane of tissue remains, which must be torn for the two hands to touch. Alternatively, a sponge stick can be advanced posteriorly from the neck to touch the dissecting hand from the abdomen ( Fig. 38-13 ). Dissection then proceeds in a similar fashion on the anterior surface of the esophagus. Extreme care must be taken in the region of the trachea and especially the carina to avoid injury to the membranous trachea. (At this point in the operation, any difficulty in dissecting the esophagus from the trachea or any concern of adhesion to, or invasion of, the trachea should result in repositioning of the patient in the left lateral decubitus position, and a right thoracotomy should be performed for direct visualization and dissection.) The fingers from above and below should meet in the region of the carina ( Fig. 38-14 ).
As much dissection as possible should be performed under direct vision from the neck and from the abdomen. Portions of the lateral dissection near the lower aspect of the trachea often cannot be visualized, and lateral dissection must be performed bluntly. The surgeon's right hand is advanced from the abdomen high up in the chest to the point where circumferential dissection has been completed from the neck. The first and second fingers surround the esophagus, and with a raking motion, the lateral attachments are avulsed as the hand is drawn back into the abdomen. Care must be taken near the region of the azygos vein ( Fig. 38-15 ). After complete mobilization of the esophagus, it is divided in the neck, and the specimen is brought out into the abdomen. The mediastinum is packed for hemostasis. Before the gastric tube is drawn up into the neck, the mediastinal packing is removed and both pleural spaces are inspected for integrity. Entry into the pleural space is managed by chest tube placement before removal of the drapes.
Numerous nonrandomized, retrospective trials have been performed in an attempt to define differences in either perioperative complication rate or long-term survival between the transhiatal and transthoracic approaches ( Table 38-1 ). Most of these were limited by small study size and selection bias. A large meta-analysis review was performed by Rindani and coworkers in 1999. Forty-four trials published in the English literature between 1986 and 1996 consisting of 5483 patients undergoing either Ivor Lewis or transhiatal esophagectomy were reviewed. The overall incidence of pneumonia was 25%, and it was not appreciably different between the two techniques. The incidence of bleeding and cardiac complications was also not different. The most significant differences were seen in the anastomotic leak rate (16% transhiatal vs. 10% Ivor Lewis), stricture (28% transhiatal vs. 16% Ivor Lewis), and recurrent nerve injury (11% transhiatal vs. 5% Ivor Lewis). Perioperative mortality was higher in the Ivor Lewis population (9.5%) than in the transhiatal population (6.3%). Overall long-term (5-year) survival was similar in the two groups, 25%. Hulscher and colleagues also performed a meta-analysis of trials between 1990 and 1999 published in English language journals. Fifty publications were identified, some randomized, some comparing transthoracic with transhiatal approaches, and some evaluating only one technique. Overall, cardiac complications (20% vs. 7%), anastomotic leakage (14% vs. 7%), and vocal cord paralysis (10% vs. 4%) were higher in the transhiatal versus the transthoracic groups. Pulmonary complications (19% vs. 13%), in-hospital mortality (9% vs. 6%), and operative time (5.6 vs. 4.0 hours) were higher in the transthoracic versus the transhiatal group. Overall 5-year survival was similar among all the studies and patients evaluated (23% for transthoracic resections and 21.7% for transhiatal resections). These reviews are of historical and factual interest only, and little can be concluded about the relative merits of each technique in matched populations.
| Complication | Transthoracic | Transhiatal |
|---|---|---|
| Blood loss (mL) | 1001 | 728 |
| Operative time (hours) | 5.6 | 4.0 |
| Cardiac complications (%) | 6.6 | 19.5 |
| Pulmonary complications (%) | 18.7 | 12.7 |
| Anastomotic leak (%) | 7.2 | 13.6 |
| Vocal cord paralysis (%) | 3.5 | 9.5 |
| Chyle leak (%) | 2.4 | 1.4 |
| In-hospital mortality (%) | 9.2 | 5.7 |
Orringer and colleagues reviewed their 30-year experience with transhiatal esophagectomies in 2007 patients. The patients were divided into two groups: group I, those operated on between 1976 and 1998; and group II, those operated on from 1998 to 2006. Postoperative mediastinal bleeding requiring reoperation occurred in less than 1%. The incidence of recurrent laryngeal nerve injury was significantly lower in group II (2%) compared with group I (7%; P < 0.0001). They believed that this decrease in injury was secondary to an increase in operative volume. Chylothorax was seen in 1%. The incidence of respiratory complications that required a hospital stay beyond 10 days was 2%. They also reported that the routine use of side-to-side stapled cervical esophagogastric anastomosis after 1997 has led to a notable decrease in leak rate and need for postoperative dilation. The overall hospital mortality was 3%. The hospital mortality fell with increased volume (group I, 4%; group II, 1%). It is clear that the high volume at this center has produced impressive results with regard to transhiatal esophagectomy.
Wolff and associates retrospectively reviewed all esophagectomies at the Mayo Clinic between 1994 and 2004. Of the 517 esophagectomies performed, the type of surgery was as follows: transhiatal (68), Ivor Lewis (392), and extended Ivor Lewis (57). The mean lymph nodes retrieved for the Ivor Lewis and extended Ivor Lewis were 18.7 and 17.4, respectively. This was not statistically different. The transhiatal patients had a mean of 8.99 lymph nodes in the specimen. They found that the combined Ivor Lewis group and the transhiatal group were significantly different, showing on average 9.5 lymph nodes in the surgical specimen ( P < 0.001). The importance of the number of lymph nodes harvested at the time of surgery is discussed in a later section.
Orringer and associates reviewed outcomes after transhiatal and transthoracic surgery using the Surveillance, Epidemiology, and End Results (SEER) cancer registry. They identified patients between 1992 and 2002 undergoing esophagectomy. Of the 868 patients studied, 643 had a transthoracic approach and 225 underwent a transhiatal esophagectomy. They concluded that patients undergoing a transhiatal esophagectomy had a lower operative mortality rate than did those undergoing transthoracic operations (6.7% vs. 13.1%; P = 0.009). The need for anastomotic dilation was higher in the transhiatal group (43.1%) compared with the transthoracic patients (34.5%; P = 0.02). The 5-year survival rate was significantly higher in the transhiatal group; however, this benefit over the transthoracic group was diminished after adjustments were made for patient and hospital or provider characteristics, such as volume performed at the center and involvement of trainees.
Three randomized, prospective trials comparing transhiatal resection with transthoracic resection demonstrated that either technique can be performed safely in skilled hands and no clear differences have been shown between the two techniques, in large part because of an inadequate number of patients enrolled in the trials. Extrapolating from their retrospective meta-analysis, Rindani and colleagues estimated that 2360 patients would have to be randomized for a significant difference to be shown in perioperative mortality, and 6400 patients would be needed for a difference to be shown in long-term survival. Clearly, this is beyond the capacity of any prospective esophagectomy trial.
The first randomized, prospective trial was published in 1993 by Goldminc and coworkers. They randomized 67 patients younger than 70 years with squamous cell cancer of the esophagus to Ivor Lewis esophagectomy or transhiatal esophagectomy. Operative time was longer (6 vs. 4 hours) in the transthoracic group. No difference was found in incidence of pneumonia (20%), anastomotic leak, recurrent nerve injury, bleeding, perioperative mortality, or length of hospitalization. At a mean follow-up of 3 years, survival was not statistically different between the two groups. For those patients with nodal disease, however, none of the transhiatal patients was alive at 18 months, whereas 30% of the transthoracic patients were alive at 18 months.
Wong and colleagues reported a prospective, randomized series of 39 patients with lower third esophageal cancers treated with either an Ivor Lewis resection or a transhiatal resection. Patients undergoing neoadjuvant therapy or those with an FEV 1 below 70% were excluded from the study. There were no perioperative (30-day) deaths in either group, although the in-hospital mortality rate was 15% for the transhiatal group and 0% for the transthoracic group (not significantly different). Intraoperative hypotension occurred in 60% of transhiatal patients but in only 5% of transthoracic patients. There was no difference in blood loss, pneumonia, or recurrent nerve injury. Operating time was longer for the transthoracic group. The mean proximal margin was 3 cm longer in the transhiatal group. No significant difference was seen in tumor recurrence or survival. This group recently published their 5-year results from this study. After a complete 5-year follow-up, they concluded that overall survival rate was not significantly different between the two groups. However, they reported a trend toward improved survival in the extended transthoracic group over the transhiatal group in the patients with a type I esophageal cancer.
More recently, a randomized study was completed in the Netherlands comparing transhiatal resection with extended transthoracic en bloc resection for distal adenocarcinomas of the esophagus or cardia ; 106 patients had a transhiatal resection, and 114 patients had a transthoracic resection. The median follow-up was 4.7 years. The in-hospital mortality rate was 2% to 4% in each group. The incidence of respiratory complications, including atelectasis and pneumonia, was higher in the transthoracic group (57% vs. 27%), as was the incidence of chyle leak (10% vs. 2%). The high incidence of respiratory complications in the transthoracic group (57%) should be questioned, because it is much higher than that quoted in many previous transthoracic resection series. Although statistical significance was not reached, there was a trend toward improved survival at 5 years in the transthoracic group (39% vs. 29%).
Cuschieri is credited with first applying minimally invasive techniques to an esophagectomy. Since his initial description of subtotal endoscopic esophagectomy using thoracoscopic techniques for mobilization of the esophagus, there have been numerous descriptions with varying degrees of endoscopic involvement. The three most widely performed open procedures for esophageal resection—tri-incisional, Ivor Lewis, and transhiatal esophagectomy—have endoscopic counterparts. These are discussed.
Minimally invasive techniques can be used to perform a modified McKeown esophagectomy. All or part of the procedure may be replaced with thoracoscopic or laparoscopic dissection.
There are no finite indications for minimally invasive esophagectomy (MIE). There are relative indications advocated by those who specialize in this surgery. These reported benefits of MIE are discussed in a later section.
Fusion of the right pleural space or inability to maintain isolated lung ventilation is a contraindication to the approach. Unlike its open counterpart, video-assisted thoracoscopic esophagectomy requires almost perfect lung isolation to allow adequate room to thoracoscopically maneuver within the thoracic cavity. On occasion, continuous positive airway pressure to the right lung will aid in achieving adequate ventilation and oxygenation with minimal effect on exposure. Relative contraindications with respect to minimally invasive surgery are the same as those in the open sections. As with any endoscopically assisted surgery, the surgeon's comfort is a strong determining factor in use of this technology. Those not trained in minimally invasive surgery should consider performing this highly technical surgery with a surgeon who is facile with this procedure.
Video-assisted thoracoscopic esophageal mobilization uses four thoracoscopic incisions. After the patient is prepared and draped in the left lateral decubitus position, a 1-cm incision is made in the eighth intercostal space at the posterior axillary line. Ideally, the camera needs to be as posterior as possible for optimal visualization. However, this port will be used for the chest tube, and any position farther posterior will result in an ineffective chest tube because the patient will be lying on it.
With the thoracoscope, the right hemithorax is examined for pleural disease that may prohibit further surgery. Three access incisions are placed next, with two of the ports in line with the tip of the scapula, at the level of the sixth and ninth intercostal spaces. The final access incision will be in the fourth intercostal space in line with the camera. This is used mainly for lung retraction and suctioning of the operative field.
Most surgeons use a port only for the camera. Standard open instruments, such as a ring forceps, are passed through the other incisions without a port. Some surgeons prefer laparoscopic instruments, so they use 5-mm and 10-mm ports to allow easy passage of these surgical tools.
Some surgeons place a stitch in the central tendon of the diaphragm and bring it out through a separate intercostal space to retract the diaphragm. The authors of this chapter place an endoscopic Kittner (Ethicon, Somerville, NJ) alongside the camera to provide gentle caudal retraction on the diaphragm.
Through the posterior ports, the surgeon uses an ultrasonic scalpel, the harmonic scalpel (Ethicon, Somerville, NJ), to divide the inferior pulmonary ligament. The posterior mediastinal pleura is opened, and the lymph nodes are swept off the pericardium toward the esophagus. This dissection is carried to the azygos vein. The pleura above the azygos vein is opened close to the esophagus. An endovascular stapler is used to ligate the azygos vein. Once this is achieved, the pleura on the posterior aspect of the esophagus is opened at an area away from the tumor and carina. Once circumferentially around the esophagus, a Penrose drain is placed. The Penrose drain is stapled together with an endovascular stapling device. This Penrose drain can be used as a handle to hold the esophagus during dissection. The vagus is divided just above the azygos vein to prevent traction injury to the recurrent laryngeal nerve. The thoracic esophagus is circumferentially dissected for its entire length. The diaphragmatic hiatus is not opened in the chest to allow adequate pneumoperitoneum during the laparoscopic portion. After adequate mobilization, the Penrose drain is placed into the apex of the thoracic cavity to allow retrieval from the neck. A complete thoracic lymphadenectomy is an important aspect of this component of the operation and should be performed routinely. The chest tube is placed, and all thoracic incisions are closed. The patient is turned supine, and the double-lumen endotracheal tube is changed to a single-lumen tube. The patient is prepared and draped for laparoscopic surgery.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here