Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Epithelial neoplasms develop far less frequently in the small intestine than in the colon, despite the fact that the small intestine has a larger epithelial surface area and a higher rate of cellular turnover. Overall, only 2% of all malignant neoplasms of the gastrointestinal (GI) tract occur in the small intestine. In contrast, 57% of neoplasms arise in the colon. A number of hypotheses have been proposed to explain the relative rarity of small-bowel adenomas and carcinomas. First, the transit time of substances through the small intestine is relatively short compared with the colon, resulting in brief contact time between the mucosa and the luminal contents. Second, unlike the colon, the small intestine does not contain a large quantity of bacteria. Bacteria are known to convert bile salts into potential carcinogens. Third, the luminal contents are more liquid in the small intestine than in the colon. As a result, potentially carcinogenic luminal substances are diluted, and the risk of mechanical trauma is reduced. Fourth, the small intestine is rich in lymphoid tissue, which provides a potentially high level of immunosurveillance against neoplastic cells. Finally, the small intestine is equipped with many microsomal enzymes, some of which may help detoxify potentially carcinogenic substances in the luminal contents.
Epithelial tumors in the small intestine are most commonly located in the duodenum, usually in the vicinity of the ampulla of Vater. This finding suggests that biliary or pancreatic secretions play a role in their development, possibly as a result of the carcinogenic effect of bile. Alternatively, constant influx of alkaline bile or acidic pancreatic juice may cause cell damage. Epithelial neoplasms also occur in the jejunum and in the ileum, but much less commonly.
A number of diseases predispose individual patients to the development of small-intestinal adenomas and carcinomas, including familial adenomatous polyposis (FAP), MUTYH -associated polyposis (MAP), Lynch syndrome (LS), Crohn’s disease (CD), and celiac disease. , The risk for small-intestinal carcinomas may also be increased in individuals with hamartomatous polyposis syndromes such as Peutz-Jeghers syndrome (PJS) , or juvenile polyposis syndrome , and in patients with long-standing ileostomies. ,
Small-intestinal epithelial tumors are most commonly glandular, although other forms of neoplasia are also seen. Tumors arising in the ampulla of Vater are separately classified from those arising elsewhere in the small intestine because significant treatment and prognostic differences exist for this group of tumors. Box 26.1 summarizes the histological classification of epithelial tumors of the small intestine by the World Health Organization (WHO).
Adenomatous polyp, low-grade dysplasia
Adenomatous polyp, high-grade dysplasia
Intestinal type, low-grade adenoma
Intestinal type, high-grade adenoma
Serrated low-grade dysplasia
Serrated high-grade dysplasia
Noninvasive pancreatobiliary papillary neoplasm with low-grade dysplasia
Noninvasive pancreatobiliary papillary neoplasm with high-grade dysplasia
Intraampullary papillary-tubular neoplasm
Adenocarcinoma, NOS
Mucinous adenocarcinoma
Signet ring cell carcinoma
Medullary carcinoma
Adenocarcinoma, intestinal type
Pancreatobiliary type carcinoma
Tubular adenocarcinoma
FAP is associated with adenomatous polyps of the intestinal tract and fundic gland polyps of the stomach. In the small intestine, most FAP-associated lesions arise in the duodenum and tend to cluster around the ampulla of Vater. Small-intestinal adenomas are usually multiple and may be numerous (>20 to 50) in some patients. Adenomas are often small, sessile, and tubular, usually measuring less than 1 cm in diameter. Jejunal and ileal adenomas also occur in patients with FAP, especially in those with extensive duodenal polyposis.
The prevalence of duodenal adenomas in FAP patients is 50% to 90%, with a 5% lifetime risk for developing small-bowel adenocarcinoma. Patients with FAP who have multiple duodenal adenomas have a 100- to 300-fold increased lifetime risk for duodenal or periampullary cancer compared with the general population. , In fact, periampullary adenocarcinoma is the most common extracolonic malignant neoplasm in FAP. As a result, patients with known FAP undergo endoscopic surveillance with biopsy examination of grossly normal duodenal and ampullary mucosa to identify potential early precancerous lesions. Duodenal polyposis may be classified with Spigelman staging ( Table 26.1 ). The risk of developing duodenal adenocarcinoma increases with increasing Spigelman stage, and it is reported to be <5% in stages 0 to III and increases to 50% in stage IV. Patients should undergo surveillance based on their Spigelman stage.
| Criterion | 1 point | 2 points | 3 points |
|---|---|---|---|
| Polyp number Polyp size, mm Histology Dysplasia |
1-4 1-4 Tubular Mild |
5-20 5-10 Tubulovillous Moderate |
>20 >10 Villous Severe |
| Stage | Spigelman Score | Surveillance | |
| 0 I II III IV |
0 1-4 5-6 7-8 9-12 |
Repeat endoscopy every 4 years Repeat endoscopy every 2-3 years Repeat endoscopy every 1-3 years Repeat endoscopy every 6-12 months Surgical evaluation Expert surveillance every 3-6 months Complete mucosectomy or duodenectomy or Whipple procedure if ampulla is involved |
|
MAP is inherited as an autosomal recessive disorder. Affected patients carry biallelic missense mutations in the human DNA glycosylase base-excision repair gene MUTYH, located on chromosome 1. Affected individuals present clinically with features similar to patients with FAP, including multiple adenomatous polyps and early-onset colonic adenocarcinoma and upper GI neoplasms. , Patients with MAP are also at increased risk for colonic serrated adenomas and sessile serrated polyps (sessile serrated lesions) as well as ovarian, bladder, breast, and endometrial neoplasms. , , , Duodenal adenomas and adenocarcinomas also occur commonly in affected patients. In one study, duodenal adenomas were identified in 34% of MAP patients at a median of 50 years of age. Most patients (84%) had only a few small polyps without high-grade dysplasia or villous architecture (Spigelman stages I or II). The lifetime risk for duodenal cancer is estimated to be approximately 4%. , , Current surveillance recommendations for MAP patients are the same as for those with FAP.
LS (also called hereditary nonpolyposis colorectal cancer [HNPCC] syndrome ) is an autosomal dominant genetic disorder that confers a high risk for both colorectal and endometrial tumors. It occurs as a result of a germline mutation in one of four major DNA mismatch repair genes: MLH1, MSH2, MSH6, or PMS2 . MLH1 or MSH2 mutations make up 90% of LS patients. MSH6 mutations make up an additional 7% to 10%, and PMS2 mutations affect <5% of patients. Germline mutations in EPCAM (epithelial cell adhesion molecule) may result in inactivation of MSH2 in a small percentage of patients. Affected individuals have an increased risk for colorectal and endometrial cancer. These patients also exhibit a risk for tumors in other sites, albeit at a lower rate than for colorectal and endometrial cancers. These include urothelial cancers, biliary tumors, and gastric and small-intestinal adenocarcinomas.
LS accounts for approximately 3% to 5% of all colorectal carcinomas. The average age at which colonic malignancy develops in the setting of LS is 44 years, compared with an average age of 64 years in sporadic colorectal carcinoma. , The risk for small-bowel adenocarcinoma in LS patients increases with age. The lifetime risk for small-intestinal adenocarcinoma among these patients is 1% to 4%, approximately 100-fold that of the general population ; in one-fourth of cases, the small-bowel tumor is the presenting neoplasm of the syndrome. , Unlike patients with FAP, adenomas or adenocarcinomas at any location throughout the small bowel may develop in patients with LS, although a duodenojejunal predominance has been reported. Screening for gastric and duodenal cancer can be considered in individuals at risk for or affected with LS by baseline esophagogastroduodenoscopy (EGD) with gastric biopsy at 30 to 35 years of age. Data for ongoing regular surveillance are limited, but ongoing surveillance every 3 to 5 years may be considered if there is a family history of gastric or duodenal cancer.
PJS is an autosomal dominant inherited syndrome characterized by mucocutaneous pigmentation and GI polyposis. The disorder has been linked to germline mutations in the serine/threonine kinase gene STK11 (also called LKB1 ) located on chromosome 19p13.3. , The most common cancers in this group of patients are gastrointestinal in origin, arising from gastroesophageal, small-intestinal, colonic, and pancreatic sites. The risk for small-intestinal adenocarcinoma among patients with PJS is estimated to be as much as 400 times that of the general population. The lifetime risk for small-bowel adenocarcinoma is 1.7% to 13%, and the risk increases rapidly with advancing age. ,
Surveillance guidelines for PJS are empirical and based on the risk for GI complications and cancer. A consortium review group has recommended that upper GI endoscopy and video capsule endoscopy be performed first at 8 years of age. If polyps are found, the examination should be repeated every 3 years. If none are identified on the initial examination, a second baseline examination should be done at 18 years of age and then every 3 years thereafter.
Juvenile polyposis syndrome is an autosomal dominant hamartomatous polyposis syndrome caused by defects in either the SMAD4/DPC4 or BMPR1A gene. The polyps arising in association with juvenile polyposis are not limited to the colon and may also occur in the small intestine and stomach. , These patients exhibit an increased risk for carcinomas of the colon, small intestine, stomach, and pancreas. , , , The exact risk for small-intestinal adenocarcinoma is unknown given the rarity of the syndrome. However, upper endoscopy is recommended every 1 to 3 years beginning at 12 years of age (or earlier for symptoms) and should be repeated every 1 to 3 years, depending on severity, with removal of polyps ≥5 mm. The small bowel past the duodenum should be periodically surveilled, depending on initial polyp findings, by enteroscopy, capsule endoscopy, and/or computed tomographic (CT) enterography if duodenal polyposis is present or if there is unexplained anemia, protein-losing enteropathy, or other small-bowel symptoms.
The WHO classification for small-intestinal epithelial neoplasms is summarized in Box 26.1 . This new classification includes several important changes to the taxonomy of small-bowel neoplasia. As for the current nomenclature used for pancreatobiliary neoplasms, the term intraampullary papillary-tubular neoplasm is now used for preinvasive lesions that arise almost exclusively within the ampulla. These lesions represent intraampullary versions of the intraductal papillary and tubulopapillary lesions of the pancreas and bile ducts. Intestinal-type adenomas that arise predominantly on the duodenal surface of the ampulla remain classified as adenomas .
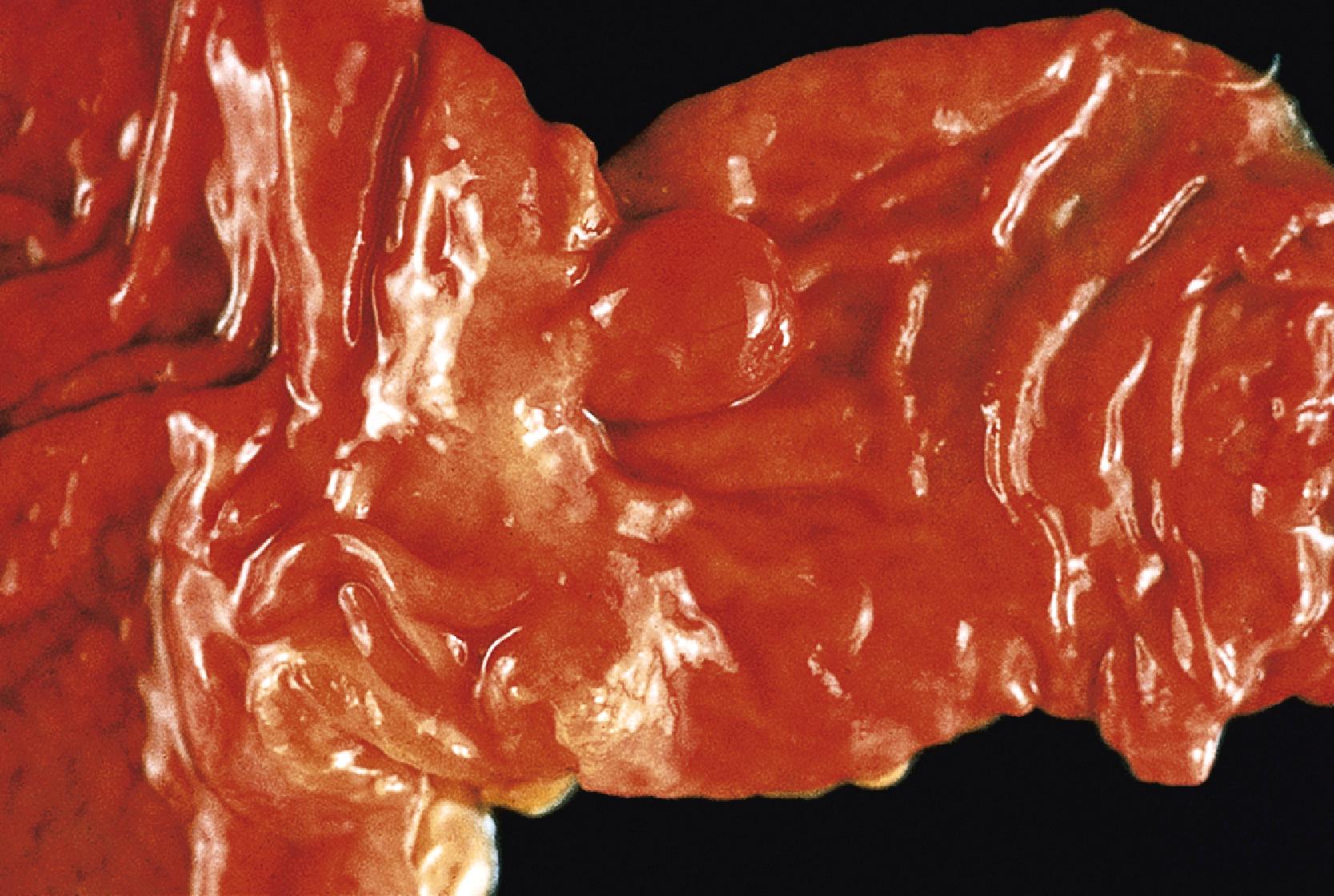
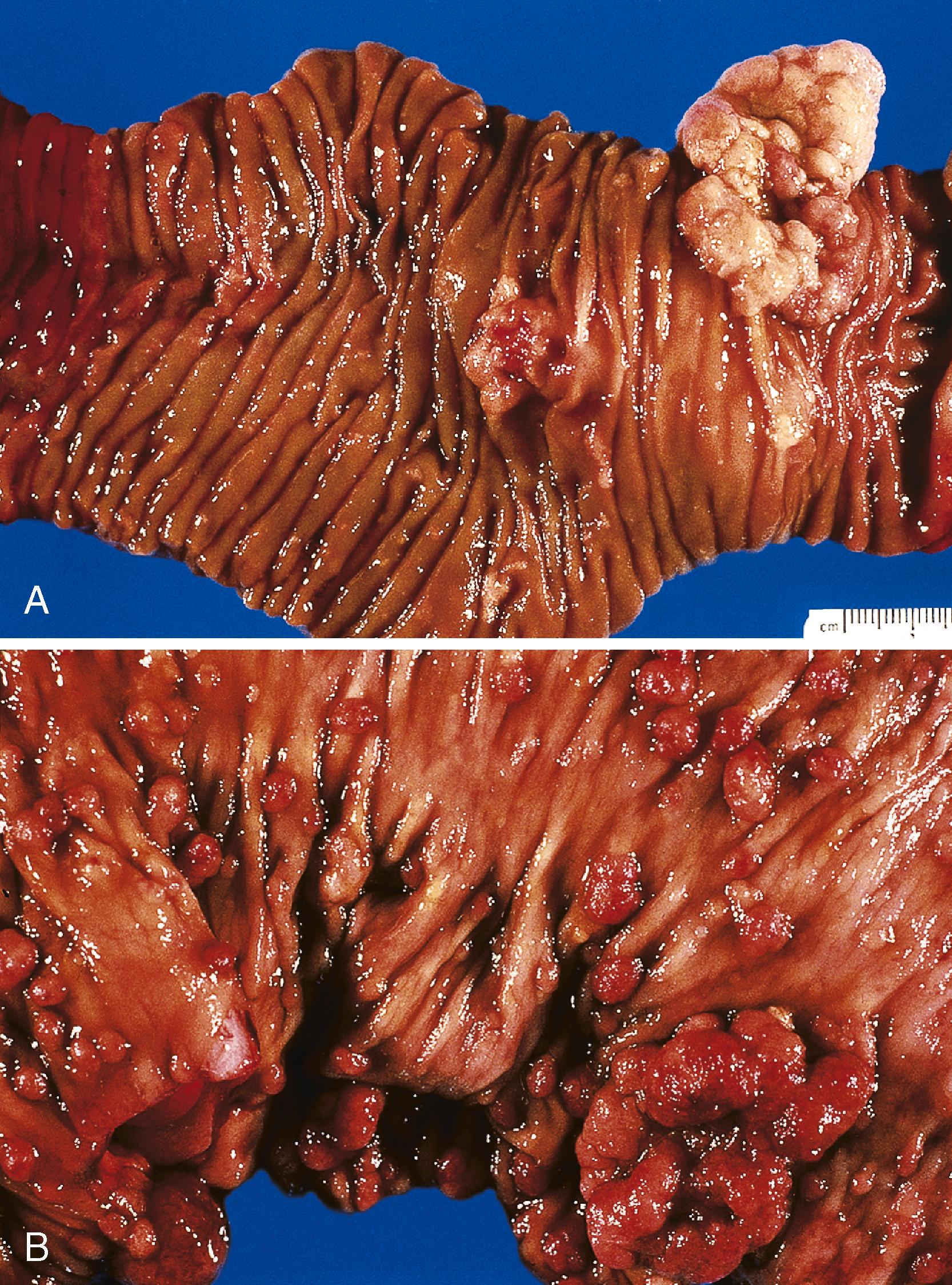
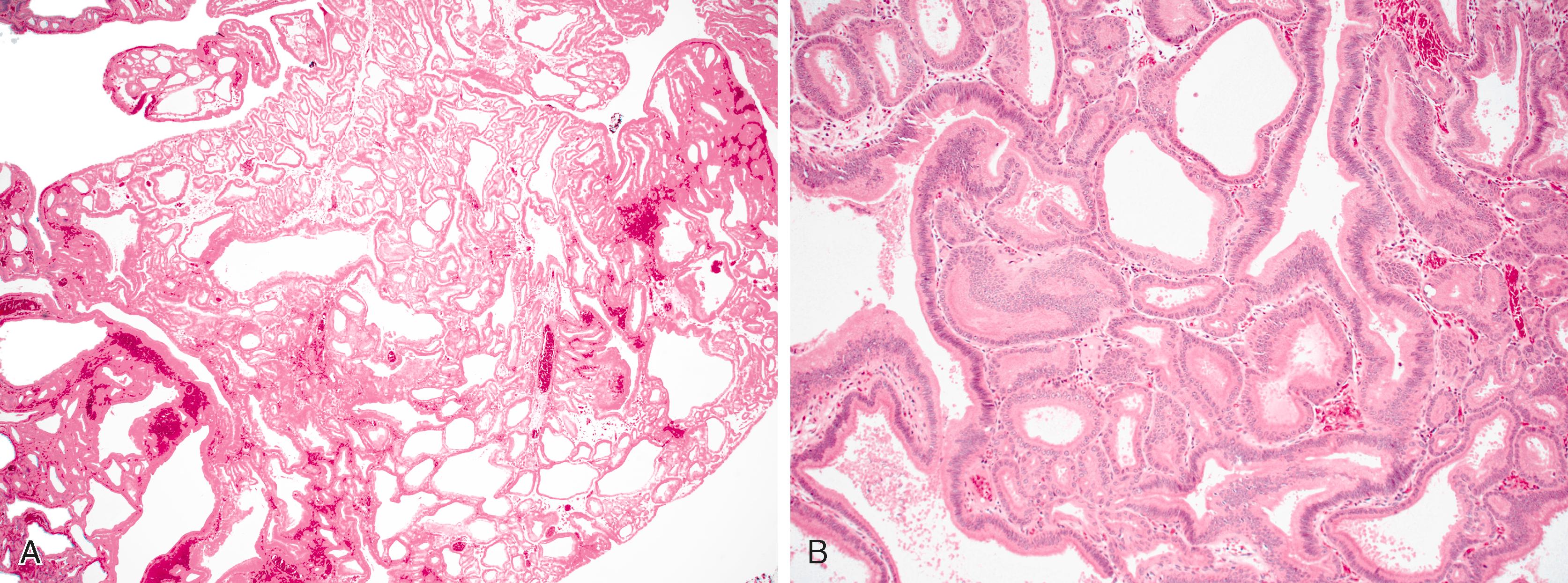
Small-bowel intestinal-type adenomas are rare, accounting for fewer than 0.05% of all intestinal adenomas. Adenomas peak in incidence in the seventh decade of life but may occur at any age. Most adenomas are asymptomatic. They are usually discovered incidentally in individuals who have undergone endoscopic examination for other reasons. Adenomas that are symptomatic typically involve the region of the ampulla of Vater and manifest with biliary colic and obstruction, acute cholangitis, or pancreatitis. Intestinal obstruction, bleeding, nausea, vomiting, anorexia, weight loss, pain, or intussusception may also develop, depending on the size and location of the lesion. Small-intestinal adenomas resemble those that arise in the colon in gross and microscopic characteristics ( Fig. 26.1 ). They are usually lobulated and soft, and they may be sessile, pedunculated, villous, or tubular. A higher proportion of small-intestinal lesions tend to be villous compared with adenomas of the colon; this is most likely a reflection of the underlying villous architecture of the small bowel. Tubular adenomas tend to be small, ranging from 0.5 to 3 cm in maximum diameter. Villous adenomas are often larger, sometimes reaching 8 cm or larger. Intestinal-type adenomas are usually single but can be multiple. The finding of multiple adenomas in the small intestine is rare in patients without a hereditary polyposis syndrome. As a result, identification of multiple lesions should raise suspicion of FAP ( Fig. 26.2 ).
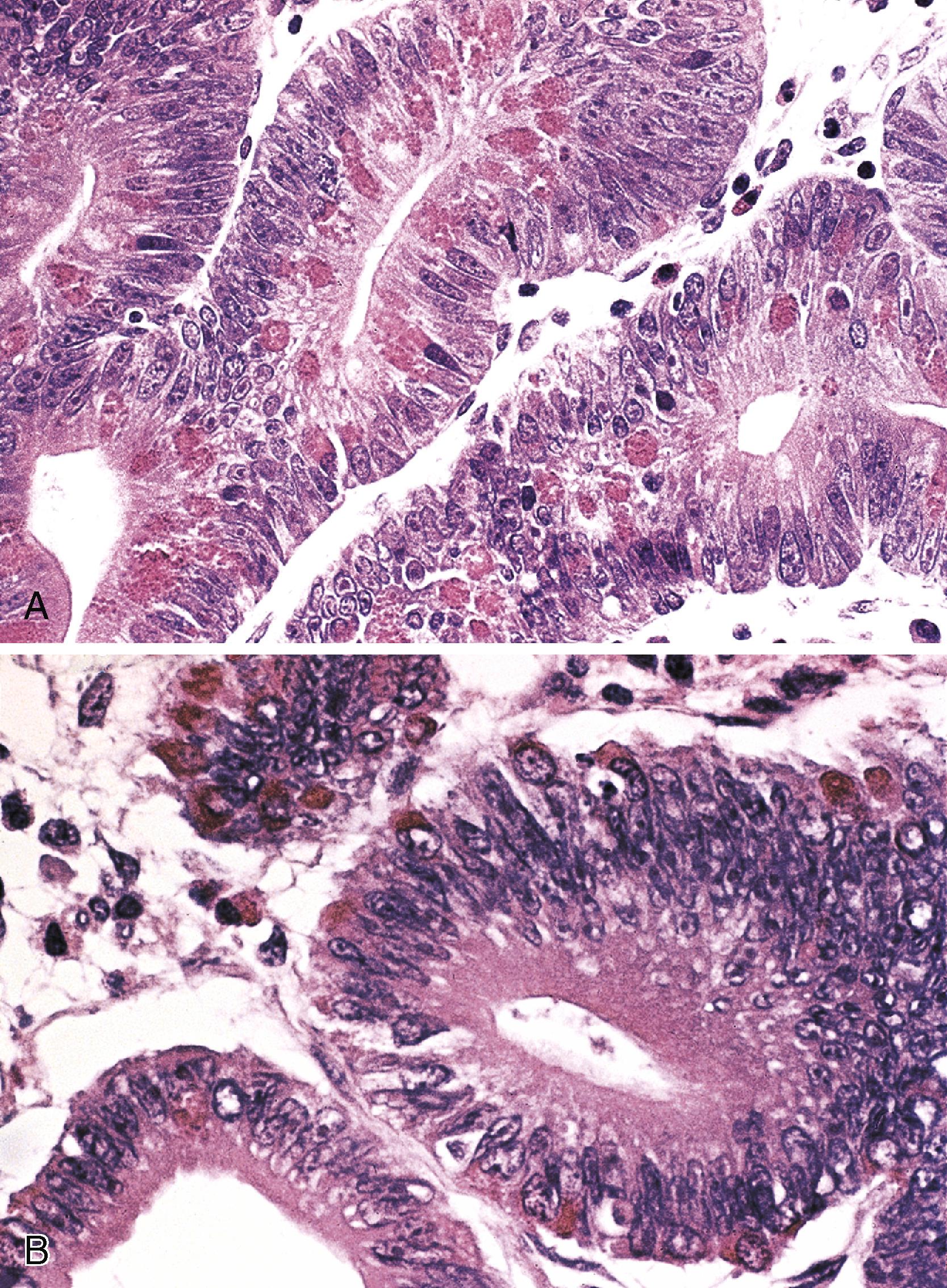
Histologically, intestinal-type adenomas may demonstrate tubular, tubulovillous, or villous growth patterns. They are composed of tall, columnar epithelial cells with elongated, crowded, hyperchromatic nuclei arranged in a “picket fence” pattern. Immature goblet cells may be present. In addition, endocrine cells, squamous cells, and particularly Paneth cells may be numerous ( Fig. 26.3 ). Mitoses, normally seen only in the base of the crypts, may occur at all levels of the adenomatous crypts and villi. A normal-appearing lamina propria is usually present between the neoplastic crypts.
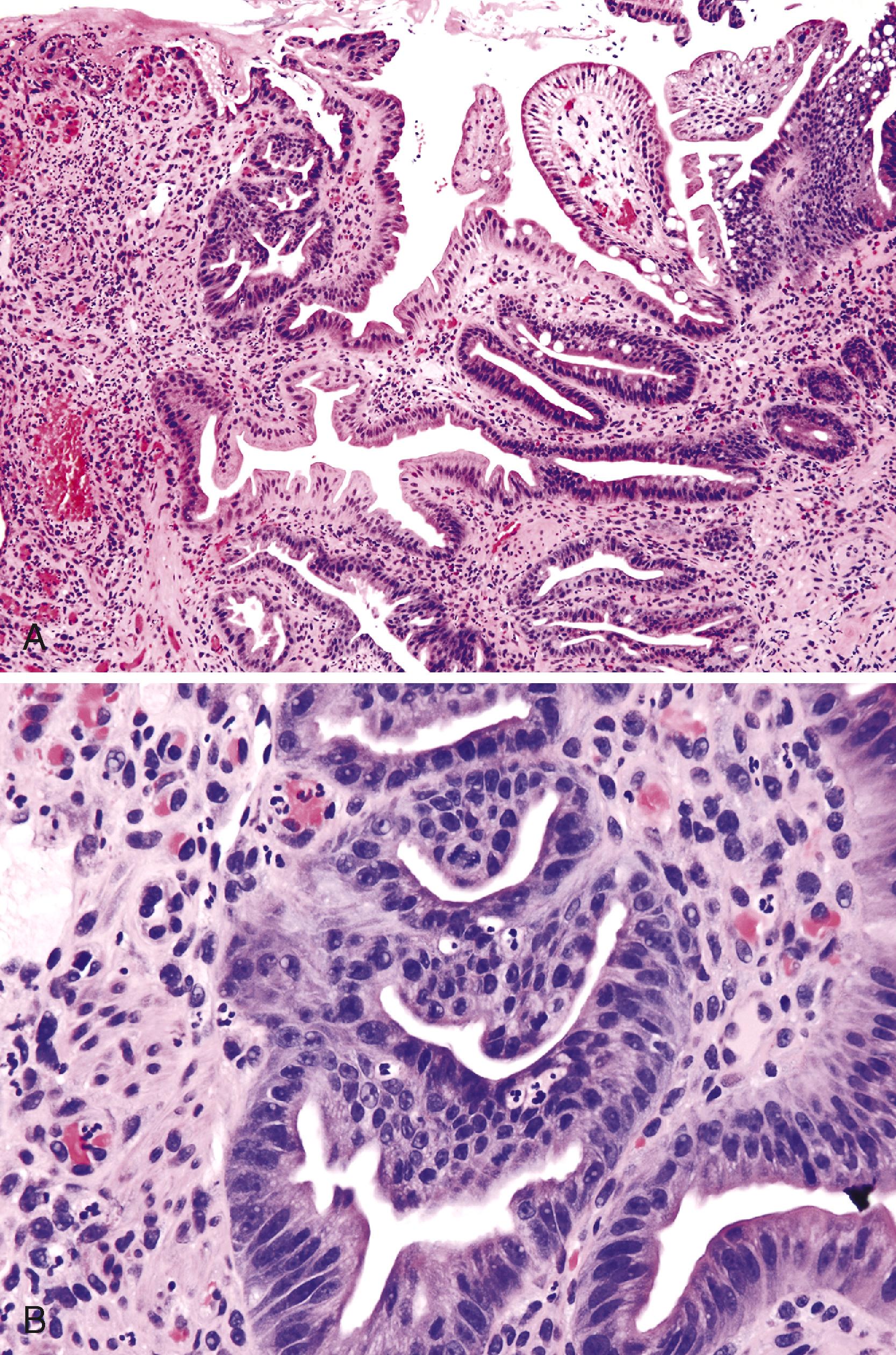
Intestinal-type adenomas can display varying degrees of dysplasia, ranging from low grade to high grade, and they may show intramucosal or associated invasive carcinoma. Although the degree of dysplasia increases, one also tends to see an increased ratio of nucleus to cytoplasm in the cells, loss of cell polarity, and an increased mitotic rate. Prominent crypt budding, nuclear stratification, and loss of mucinous differentiation may herald progression to malignancy.
It is important to distinguish regenerative atypia associated with surface erosion from an adenoma ( Fig. 26.4 ). Regenerating cells tend to mature toward the surface, whereas adenomas do not. The presence of Paneth or endocrine cells in the superficial portions of the lesion is almost always associated with a neoplastic alteration. Prominent acute inflammation with congested capillaries and fibrin deposition, especially when superficial, should alert the examiner to the possibility of regenerative atypia.
Adenomas arising in the small bowel may also be of the pyloric gland type. These lesions typically arise in the proximal duodenum and resemble pyloric gland adenomas arising elsewhere in the GI tract ( Fig. 26.5 ).
Most preinvasive neoplasms in the region of the ampulla of Vater represent intestinal-type adenomas, and approximately 80% of all small-intestinal adenomas arise at this site. Ampullary adenomas resemble their nonampullary counterparts, as described earlier.
Pancreaticobiliary-type ampullary adenocarcinomas are thought to arise from noninvasive papillary and flat intraductal neoplasia reminiscent of that seen in the bile duct and pancreas ( Fig. 26.6 ). The current WHO terminology for these lesions is intraampullary papillary-tubular neoplasm (IAPN). An IAPN is defined as a dysplastic, compact, exophytic lesion that is localized almost exclusively within the ampulla and grows predominantly within the ampullary channel, with minimal or no involvement of the bile duct, pancreatic duct, or duodenal papilla. These preinvasive lesions are encountered infrequently and are usually associated with a coexisting invasive carcinoma.
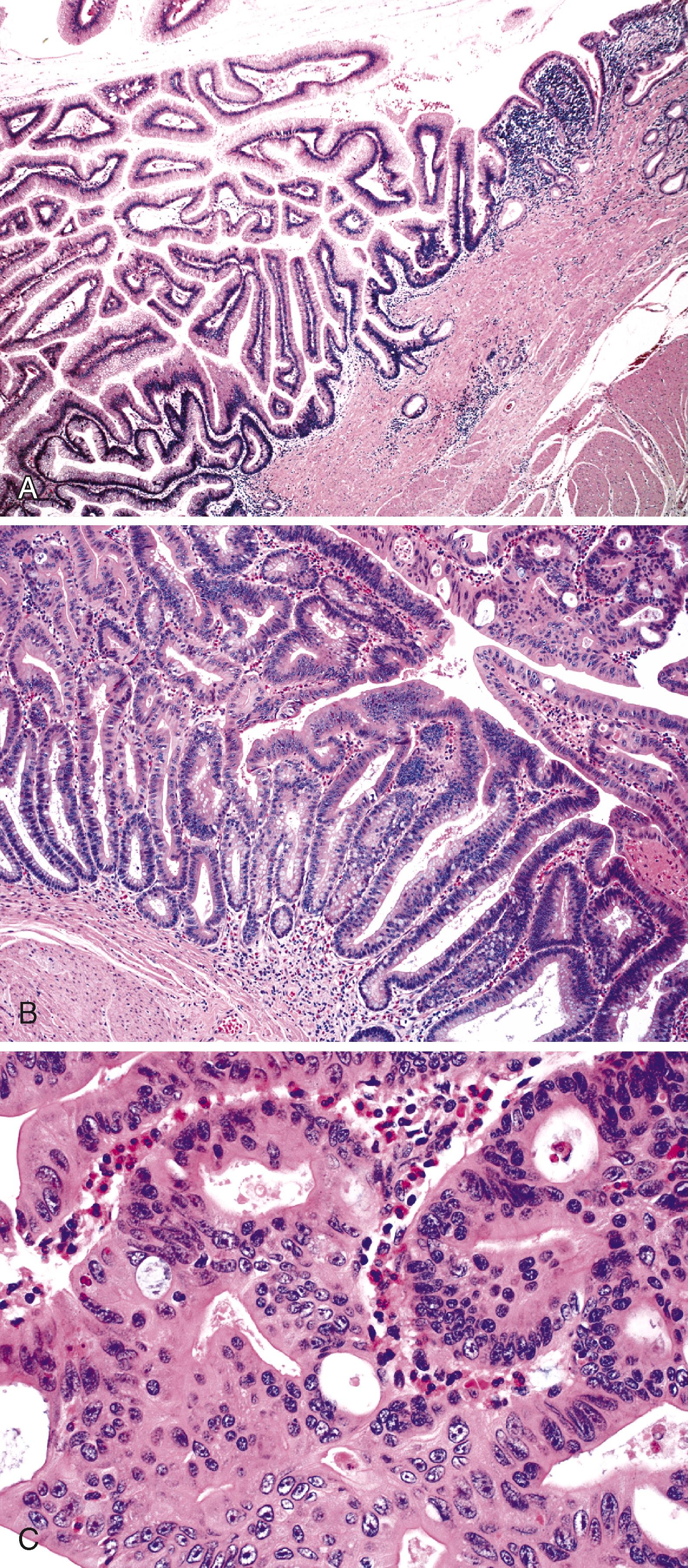
Histologically, these neoplasms consist of complex, arborizing papillary structures lined by variably atypical epithelial cells. Almost all pancreaticobiliary noninvasive papillary neoplasms have focal high-grade dysplasia, and many have an associated invasive carcinoma. The invasive component most commonly has a tubular growth pattern and is the pancreaticobiliary type, although intestinal-type adenocarcinomas may occasionally arise from these papillary precursors.
Duodenal serrated adenomas are rare lesions, with approximately 35 reported cases in the literature. Histologically, they resemble their large-intestinal counterparts. These adenomas have serrated lumens lined by eosinophilic-appearing cells that contain pseudostratified nuclei with prominent nucleoli. Goblet cells are typically not well developed in these lesions. In one study of 13 serrated duodenal lesions, almost one-half demonstrated high-grade dysplasia or progressed to adenocarcinoma.
More than one-half of all small-intestinal carcinomas arise in the duodenum, even though this organ constitutes only 4% of the entire length of the small intestine. , , The incidence of duodenal adenocarcinoma has increased in recent years, most likely because of the increased use of upper endoscopy and the development of newer techniques such as video capsule endoscopy, double-balloon enteroscopy, and CT enterography. , In the United States, the incidence of small-intestinal adenocarcinoma is estimated at 0.7 per 100,000 population. Most small-intestinal carcinomas arise in the region of the ampulla of Vater. A smaller percentage of tumors arise in the jejunum, particularly in the first 30 cm distal to the ligament of Treitz. Ileal carcinomas are the least common, except in patients with CD. Small-intestinal carcinomas occur more frequently in men than in women and affect Blacks more often than whites. ,
Some diseases (e.g., FAP) are associated with an increased incidence of small-intestinal carcinomas ( Box 26.2 ). Cancers that arise in the upper GI tract, and especially in the periampullary region, represent a major cause of death in these patients. As discussed previously, patients with hereditary polyposis and familial cancer syndromes including FAP, LS, and PJS are at increased risk for development of small-intestinal tumors. In addition, patients with celiac disease have an 80-fold increased incidence of small-intestinal adenocarcinomas compared with the general population. In one study of 175 patients with adenocarcinoma of the small bowel, 13% had celiac disease. The diagnosis of celiac disease preceded that of adenocarcinoma in 63% of these patients. Tumors in these patients often arise in the jejunum.
Sporadic adenomatous polyps
Congenital anomalies
Long-standing ileostomy
Crohn’s disease
Celiac disease
Alpha chain disease
Familial adenomatous polyposis
Gardner’s syndrome
MUTYH -associated polyposis syndrome
Peutz-Jeghers syndrome
Lynch syndrome
Juvenile polyposis syndrome
Ileal adenocarcinomas develop with increased frequency in individuals with long-standing CD (see later discussion). However, in these patients, adenocarcinomas typically arise in the setting of dysplasia (flat or polypoid) rather than in preexisting adenomas.
Most small-intestinal carcinomas manifest in patients between 60 and 70 years of age. , However, tumors that arise in the setting of a hereditary cancer syndrome are seen in younger individuals. Patients may have presenting symptoms of intestinal obstruction, bleeding, intussusception, or perforation. Ampullary carcinomas often manifest with bile duct obstruction, pancreatitis, and jaundice. Pancreatitis may also develop secondary to pancreatic outflow obstruction.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here