Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Aortic aneurysms result in significant morbidity and mortality, accounting for nearly 10,000 deaths and 69,000 hospital discharges per year in the United States. A wide variety of pathological states are associated with aortic aneurysms, including degenerative diseases, genetic disorders, infections, inflammatory conditions, and trauma. Although aneurysms may affect any part of the aorta from the aortic root to the abdominal aorta, the prognosis and outcome in patients with aortic aneurysms vary based on location, etiology, and comorbidities. Timely and appropriate intervention may improve the natural history of the disease process. This chapter reviews the epidemiology and prognosis of aortic aneurysms.
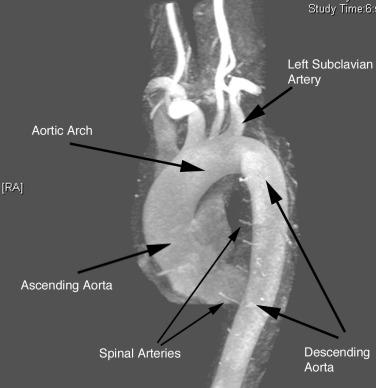
The aorta is the primary conduit vessel through which the heart delivers blood to the entire body. It courses from the heart through the thorax and abdomen, and ultimately bifurcates into the common iliac arteries (CIAs) in the lower abdomen. In the thorax, the aorta can be subdivided into three segments: ascending aorta (from the aortic valve to the innominate artery), transverse aorta or aortic arch (including the great vessels and extending to the left subclavian artery), and descending aorta (from the distal edge of the subclavian artery to the level of the diaphragm) ( Fig. 35.1 ). The abdominal aorta consists of the segment between the diaphragm and the iliac bifurcation.
Like other arterial structures, the aorta is composed of three layers: tunica intima, tunica media , and adventitia . The innermost surface of the tunica intima is lined by a single-cell-thick layer of endothelial cells (ECs). The intima is bound by the internal elastic lamina. The tunica media is composed of vascular smooth muscle cells (VSMCs) intertwined with collagen, fibroblasts, and elastin fibers, which together control vessel tone. The presence of elastin fibers in the media defines the aorta as an elastic artery and provides the tensile strength that permits the aorta to withstand high-pressure, pulsatile blood flow from the heart. Elastin content gradually decreases with greater distance from the heart. The outermost layer, the adventitia, is a thin layer that contains collagen fibers and fibroblasts, and the nutritive vasa vasorum. In addition to the vasa vasorum, nutrients are delivers to the vessel wall layers via trans-intimal diffusion.
In adults, the normal diameter of the aorta is approximately 3.5 to 4.0 cm in the aortic root to 3 cm in the ascending aorta, 2.5 cm in the descending thoracic aorta, and 1.8 to 2 cm in the abdominal aorta. Aortic aneurysm is defined as 50% increase in size compared with the normal proximal segment and varies by location in the aorta. Mild expansion that does not meet these criteria may be referred to as aortic ectasia . True aneurysms are classified into two major groups on the basis of morphology: (1) fusiform ( Fig. 35.2 ), defined as a circumferential expansion of the aorta, and (2) saccular , representing a focal outpouching of a segment of the aorta ( Fig. 35.3 ). Fusiform aneurysms are the most common form of aneurysmal disease. In contrast to true aneurysms, which involve expansion of all three layers of the aortic wall, a pseudoaneurysm , also known as a false aneurysm , results from a disruption of the aortic wall and essentially represents a contained aortic rupture.
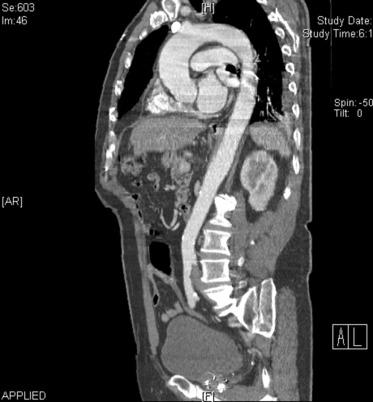
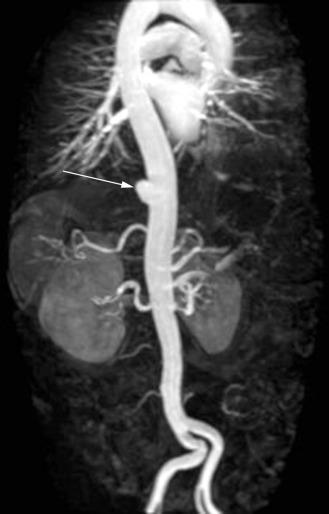
Aortic aneurysms ( Fig. 35.4 ) are typically defined as an increase in diameter of 50% compared with the adjacent normal segment of the aorta; the upper limit of normal for the abdominal aorta is 3 cm. The absolute size definition for abdominal aortic aneurysms (AAAs) is preferable, given that body size and baseline diameter may vary on the basis of height, sex, weight, and presence of a thoracoabdominal aortic aneurysm (TAAA). However, all these factors should be taken into consideration when considering risk in any given individual.
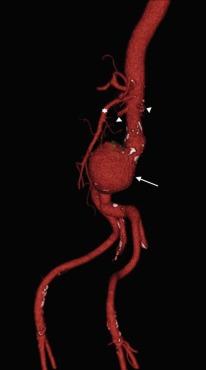
The prevalence of aneurysms of the abdominal aorta has been determined on the basis of several large screening studies and autopsy series ( Table 35.1 ). In an early series of 24,000 consecutive autopsies performed over 23 years, 1.97% of the subjects were found to have an AAA. Of the 473 aneurysms found, 58% were larger than 4 cm in diameter, nearly three quarters of the patients were men, and one-fourth of the aneurysms had ruptured. More recent large screening programs in targeted populations have further evaluated the prevalence of AAA. The largest screening program performed was the Aneurysm Detection and Management (ADAM) Study Screening Program, which studied 126,196 veterans 50 to 79 years of age. In this cohort of predominantly male American veterans, 3.6% of subjects had an infrarenal aortic diameter greater than 3 cm, and an AAA 4 cm or larger was found in 1.2%. The Multicentre Aneurysm Screening Study (MASS) also screened 27,147 of 33,830 invited men aged 65 to 74 and reported a 4.9% prevalence of AAA 3 cm or larger.
| Author | No. | Gender | Age (Years) | Aneurysm Frequency (%) | Nation |
|---|---|---|---|---|---|
| Pleumeekers | 5,419 | 42% M | > 55 | 4.1 M, 0.7 F | Netherlands |
| Lederle | 126,196 | 97% M | 50–79 | 1.3 | United States |
| Ashton | 27,147 | M | 65–74 | 4.9 | United Kingdom |
| Singh | 2,998 | F | 25–84 | 2.2 | Norway |
| Lederle | 3,450 | F | 50–79 | 1.0 | United States |
| Scott | 9,342 | F | 65–80 | 1.3 | United Kingdom |
Several studies have demonstrated a lower prevalence of AAA in women. The largest of these studies screened nearly 10,012 women (mean age, 69.6 years) and found an AAA prevalence rate of 0.7%, with only 4 of 74 detected aneurysms measuring larger than 5 cm. These low prevalence rates were consistent with findings from earlier studies. Among 4237 subjects aged 65 to 80 who participated in a screening study among general practitioners in West Sussex, United Kingdom, 2290 women agreed to undergo abdominal ultrasonography. Only 1.4% of the women had an AAA 3 cm or larger, and only 0.3% had an AAA 4 cm or larger. This dramatically lower prevalence in women has been confirmed in subsequent studies. In the Norwegian Tromso study, 2% of 2943 women aged 55 to 84 had an AAA 3 cm or larger, and 0.5% had an abdominal aneurysm 4 cm or larger. Finally, among female American veterans, the prevalence of an AAA 3 cm or larger was just 1%. However, an increasing number of cardiovascular risk factors does increase the risk of AAA in women, with a prevalence rate as high as 6.4% in this higher-risk group.
Three risk factors predict the vast majority of AAAs: age, sex, and cigarette smoking. Aneurysms usually affect the elderly, seldom occurring in those younger than 60 years of age, and there is a clear increase in incidence with increasing age, even when limiting the studies to older individuals. In a Norwegian population-based study of 6386 men and women aged 25 to 84, the incidence of AAA in men increased from 0% in those aged 25 to 44, to 6% in those aged 55 to 64, and to 18.5% in those aged 75 to 84. Large North American epidemiological studies have demonstrated an increase in AAA risk ranging from 58% to 300% with each additional decade of life. Sex is also an important predictor of AAA formation; in all age groups, risk of AAA is two- to sixfold higher in men than in women. Cigarette smoking is the most potent modifiable risk factor and increases the risk of AAA by 60% to 850%. In the ADAM study and the Edinburgh Artery Study, risk of an aneurysm increased threefold with any smoking history. Risk of AAA development further increases with number of cigarettes smoked, duration of smoking, and lack of filtration, indicating a dose-response relationship. In the Whitehall study of 18,403 male civil servants examined at age 40 to 64 years, aneurysm frequency increased from sixfold with manufactured cigarettes with filters to 25-fold with hand-rolled cigarettes. Smoking cessation can reduce the risk of aneurysm formation, with former smokers having a lower AAA risk than current smokers.
Risk factors for cardiovascular disease in general (e.g., hypertension, hyperlipidemia) also increase the risk of AAA formation but are less potent risk factors than age, gender, and smoking. In the REACH registry, there was a clear but modest association of both hypertension and hyperlipidemia with AAA. Earlier studies suggested a less consistent relationship with hypertension, but aneurysm formation seems to correlate best with diastolic blood pressure or use of an antihypertensive medication. Some data suggest that hypertension may be a greater risk factor for aneurysm rupture than for initial aneurysm formation. The association between cholesterol and AAA is clear, although hyperlipidemia is a less potent risk factor than those mentioned previously. Risk of AAA formation increased 30% per 40 mg/dL total cholesterol in the Chicago Heart Association Detection Project in Industry cohort. For specific components of the lipid profile, higher levels of low-density lipoprotein cholesterol (LDL-C) and lower levels of high-density lipoprotein cholesterol (HDL-C) are both associated with aneurysm formation. Similarly, the presence of atherosclerosis increases risk of aneurysm formation. In the ADAM study of more than 100,000 subjects, hypertension, elevated cholesterol, and presence of other vascular disease increased risk of aneurysm formation by 15%, 44%, and 66%, respectively. In contrast, diabetes and black race appear protective against formation of an aneurysm. Diabetes decreases risk of aneurysm formation by 30% to 50%.
A dramatic increase in frequency of AAA formation in relatives of patients with aortic aneurysm suggests a genetic component to the disease. Although cigarette smoking numerically accounts for the vast majority of AAAs in the population, the most potent risk factor for aneurysm formation is a history of aneurysm in a first-degree relative. Norrgård et al. identified an 18% incidence of aneurysms in first-degree relatives of patients with AAA. In the ADAM study, a family history doubled the risk of AAA, but was reported in only 5.1% of more than 100,000 participants. Investigations specific to the impact of family history demonstrate a larger risk. Several studies have demonstrated that a family history of AAA increases the risk of AAA four- to fivefold. Frydman et al. screened the siblings of 400 AAA patients and found an AAA in 43% of male siblings and 16% of female siblings. More specifically, the risk of AAA formation consistently rises above 20% for men older than 50 who have a first-degree relative with AAA. Using segregation analysis, Majumder et al. reported that the relative risk of developing an AAA is 3.97 and 4.03 with paternal and maternal history, respectively. Risk increases to nearly 10-fold with an affected male sibling and 23-fold when a female sibling is affected. In the Liege AAA Family Study, investigators found a lifetime prevalence of AAA of 32% among brothers. Twin studies further support a genetic component to AAA formation, with one study reporting an odds ratio (OR) of 71 (95% confidence interval [CI], 27–183) for monozygotic twins and 7.6 (95% CI, 3.0–19) for dizygotic twins. Overall estimates for the heritability of AAA are as high as 77%. Family history of AAA was also related to earlier AAA formation and rupture by nearly a decade. Rate of rupture was nearly fourfold higher in patients with a family history than in sporadic AAA patients. Similarly, in a study of 374 Japanese individuals with AAA, family history was an independent predictor of accelerated aneurysm expansion.
Despite the wealth of data supporting a genetic component to AAA formation, no clear mode of inheritance and no single candidate gene have been identified. Early studies suggested evidence of both sex-linked and autosomal dominant patterns of inheritance. Associations have been made with blood types, haptoglobin variations, α 1 -antitrypsin, and human leukocyte antigen class II (HLA-II) immune response genes. More than 100 reports on genetic associations with AAA have appeared in the literature. One study suggested an association between reduced AAA growth and five single-nucleotide polymorphisms (SNPs) in latent TGF-β binding protein (LTBP4), as well as an allelic variant of TGFB3. However, another study showed no association between genetic polymorphisms in the main receptors for TGF-β and AAA formation. Two genome-wide association studies (GWAS) have suggested an association between AAA and a SNP located on chromosome 3p12.3 in a region near the gene encoding contactin-3 (CNTN3) , a lipid-anchored cell adhesion molecule. Another GWAS in a population from Iceland and the Netherlands found an SNP on 9q33 associated with AAA with an OR of 1.21. The same SNP has been associated with coronary artery disease (CAD), peripheral artery disease (PAD), and pulmonary embolism (PE). The SNP resides in the gene encoding DAB2IP, a cell growth and survival inhibitor. In addition, the same gene variant associated with myocardial infarction (MI) at locus 9p21 has been associated with AAA. Additional GWAS and candidate gene studies have implicated genes involved in lipid pathways ( LDLR and SORT1 ), vascular integrity (LRP1) , and inflammation (IL6R) . More recently, a meta-analysis of GWAS results including 10,204 AAA cases identified four new loci that were not associated with CAD or other traditional cardiovascular risk factors. Finally, of interest but of unclear significance, is the association between telomere length and AAA in a small cohort in the United Kingdom. This may represent the association of aging, telomere length, and aneurysm formation, but the direct pathophysiological link remains unclear.
The natural history of AAA is one of silent, progressive expansion followed by sudden lethal rupture. In a large autopsy study performed over a quarter of a century, one-fourth of abdominal aneurysms were ruptured on postmortem examination. The frequency of rupture was dependent largely on size in this study population, ranging from 9.5% in aneurysms smaller than 4 cm to 45.6% in aneurysms 7.1 to 10 cm in diameter. In a comparison of patients with aortic aneurysms divided into two groups at a cutoff point of 6 cm, survival was markedly decreased in the patients with larger aneurysms. In a single-center study that included 60 ruptured AAAs over a 30-year period, only two occurred in patients with an aneurysm diameter smaller than 5 cm. Similarly, in a study in Rochester, Minnesota, no ruptures occurred in aneurysms smaller than 5 cm, whereas rupture occurred in 25% of AAAs larger than 5 cm. In 198 patients with aneurysms 5.5 cm in diameter or larger, but who were deemed too risky for surgery, 23% had presumed rupture over a mean follow-up of 1.6 years. Mortality rates as high as 53% to 66% are observed in patients who present with a ruptured aortic aneurysm.
On the basis of these data, two large trials have been conducted to determine whether early recognition and treatment can alter the natural history of AAA. The UK Small Aneurysm Trial randomized 1090 patients aged 60 to 76 years with asymptomatic AAAs 4 to 5.5 cm in diameter to undergo early elective open surgery or ultrasonographic surveillance. In the surveillance group, surgery was performed when the aneurysm reached 5.5 cm. Early surgery did not affect overall mortality. At 3 years, nearly 20% of both groups had died, although abdominal aneurysms accounted for only a quarter of the deaths in both groups. Cardiovascular mortality unrelated to the aneurysm accounted for 40% of total mortality, and cancer caused slightly more than 20% of the deaths. Even among the lowest risk individuals within the trial, there was no benefit to early intervention, and there was no long-term benefit seen after 12 years of follow-up. In a similar study performed by the US Veterans Administration, 1136 subjects aged 50 to 79 years with asymptomatic AAA 4 to 5.5 cm in diameter were randomized to undergo either early elective open surgery or ultrasonographic surveillance. After 5 years, there was no significant difference in survival between the groups, each with a near 25% mortality rate. In this study, aneurysm-related deaths accounted for only 3% of total mortality. More recently, the PIVOTAL study demonstrated no difference between surveillance and early endovascular repair in patients with small aneurysms (measuring 4 to 5 cm), and no difference in aneurysm-related death in the two groups after 3 years of follow-up. Together, these data suggest that early repair of small aneurysms does not alter outcomes.
Several factors can predict the likelihood of expansion and rupture of AAAs and can help identify which patients require intervention. The factor most predictive of rupture is initial size of the aneurysm. In one study of patients too ill for surgery, aneurysm rupture rates ranged from 9.4% for AAA of 5.5 to 5.9 cm to 32.5% for AAA of 7 cm or more. In the UK Small Aneurysm Study, the rate of rupture was 0.9% in aneurysms 3 to 3.9 cm, 2.7% in aneurysms 4 to 5.5 cm, and 27.8% in aneurysms 5.6 cm and larger. Larger aneurysm size also predicted a more rapid increase in diameter. Similar to factors that predispose to initial development of AAA, cigarette smoking and higher blood pressure increase the risks of rapid expansion and rupture. In contrast to the decreased risk of AAA development in women, being female actually increases risk of rupture and death after rupture in those with established AAA. In the UK Small Aneurysm Study, women had a threefold higher risk of AAA rupture than men. As mentioned previously, some have advocated use of biomechanical factors (wall stress, wall strength) to help with risk prediction. Prospective studies will be required to determine whether these factors can improve assessment of patients at high risk for aneurysm rupture and can improve selection of patients for aneurysm repair.
The most common location of involvement of thoracic aortic aneurysms (TAAs) is the ascending aorta and/or aortic root (noted in 60%), with the descending aorta affected in approximately 40% of cases and the aortic arch in 10%. The diagnosis of an aneurysm in the thoracic aorta depends on its location. At the aortic root, an aortic diameter of greater than 4.0 cm would be considered an aortic aneurysm. TAAAs are defined by contiguous involvement of the descending thoracic aorta and abdominal aorta, and account for 5% to 10% of all aortic aneurysms. The prevalence of isolated TAA is poorly defined but estimated at 6 persons per 100,000 per year. In autopsy records reported from 63% of 70,368 deaths between 1958 and 1985 in the city of Malmo, Sweden, TAAs were diagnosed in 205 of the deceased, 53% of whom were men. Of the 44,332 autopsies performed, 63 individuals (0.14%) had died of a ruptured TAA. The relative infrequency of TAA is confirmed by a retrospective analysis in Rochester, Minnesota. Over 30 years, of approximately 45,000 residents, 72 (0.16%) were diagnosed with a TAA; 61% were women, and 67 patients had thoracic aortic involvement only. Most studies, however, suggest that men are twice as likely to develop a TAA as women. TAAAs occur even more infrequently. Of the 44,332 people to undergo necropsy in Malmo, Sweden, a mere 10 had TAAA. In the Rochester, Minnesota, experience the incidence was 0.37 per 100,000 person-years of follow-up. Because of the relatively small sample sizes reported in the literature, risk factors for TAAA development are less well defined. Risk factors currently associated with development of TAAA include smoking, hypertension, and atherosclerotic vascular disease.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here