Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Exposures to environmental agents can result in a wide spectrum of pulmonary pathologic patterns ( Table 18.1 ). The most common reactions are chronic due to exposure to mineral(s) over a cumulative period and have latencies often exceeding several decades. Acute reactions are usually now the result of industrial accidents, where exposures are relatively brief but extremely high. Occupational histories need to be detailed and complete, because the offending exposure may be quite early in an individual’s career.
| Reactions | Agents |
|---|---|
| Asthma | Isocyanates, metals |
| Bronchiolitis | Nitrogen dioxide |
| Macular/nodular pneumoconiosis | Coal, silica, silicates |
| Diffuse interstitial fibrosis | Asbestos, hard metal |
| Granulomatous | Beryllium |
| Diffuse alveolar damage | Toxic fumes |
| Desquamative interstitial pneumonia | Silica, silicates, hard metal |
| Giant cell interstitial pneumonia | Hard metal |
| Alveolar proteinosis | Silica |
| Emphysema | Coal, cadmium |
| Pleural plaque | Asbestos |
| Lung cancer | Asbestos, nickel, arsenic, chromium compounds |
| Mesothelioma | Asbestos |
Some definitions are useful. Fumes are generated by vaporization of a metal, subsequent oxidation, and condensation to form very small particles ranging from 0.1 to 0.4 microns. Dusts are solid aerosols derived from mechanical manipulation of rocks such as grinding, drilling, blasting, and milling, and the particles are generally larger than those in fumes. Pneumoconiosis is a nonneoplastic lung disease caused by exposure to dust. In the macular and nodular pneumoconioses, there is a conventional and arbitrary subdivision into simple and complicated. If lesions larger than 1 cm in diameter are present (so-called progressive massive fibrosis ), then the process is referred to as complicated, whereas if lesions measure 1 cm or less, at most, the process is designated simple. The type of pathologic reaction to a material depends on the properties of the mineral, including size, shape, and durability (biopersistence) and on cumulative exposure. Exposures can result from direct handling of the material or via such activities as washing the contaminated work clothes of a directly exposed member of the household (paraoccupational).
Diffuse pulmonary interstitial fibrosis caused by asbestos exposure
Although there are no reliable data for the incidence of asbestosis in various countries, the incidence is decreasing in industrialized countries
Morbidity and mortality depend on the severity of fibrosis
In industrialized countries, death due to asbestosis is uncommon
More common in males because of occupation
Onset is usually in middle-aged or elderly people
Insidious presentation with shortness of breath and later cough
Fine crackles over the bases of the lungs and sometimes finger clubbing
Restrictive pattern on pulmonary function testing
Small, irregular opacities more prevalent in the lower zones
Honeycombing in advanced cases
Frequent coexisting pleural changes include thickening, plaques, and round atelectasis
Usually slowly progressive, if at all
No specific treatment
Increased risk of associated lung cancer
Contracted lung with bosselated surfaces in advanced cases
Cut sections reveal fine honeycombing that is most marked in the lower zones and subpleural regions
Fibrotic nonspecific interstitial pneumonia-like pattern with subpleural accentuation; various grades of interstitial fibrosis ranging from peribronchiolar to diffuse with linking up of adjacent foci and disorganization of lung architecture
Asbestos bodies—at least 2 per cm 2 area or elevated fiber count in asbestosis range by mineral analysis
Usual interstitial pneumonia representing idiopathic pulmonary fibrosis, a connective tissue disease, or another disorder
Nonspecific interstitial pneumonia, fibrotic, representing a connective tissue disease or another disorder
Langerhans cell histiocytosis, advanced
Chronic hypersensitivity pneumonitis, advanced
Asbestos minerals are naturally occurring fibrous silicates that have been used extensively in several thousand products, including textiles, insulation products, cement, friction material, and construction. Asbestos mineral comprises two separate mineralogical groups: serpentine and amphibole. The only member of the serpentine group is chrysotile (white) asbestos, which accounts for 90% to 95% of the asbestos used in the United States. The amphibole group includes the major commercial forms, amosite (brown) and crocidolite (blue), and the mostly noncommercial forms anthophyllite, actinolite, and tremolite. Amphiboles and chrysotile are distinct in their chemical, physical, and biological properties, and these factors translate into significant differences in fiber toxicity and potency to induce asbestos-related diseases. The amphiboles have much greater tendency to cause asbestos-related diseases than chrysotile, because they possess much greater biopersistence in the lungs than chrysotile, which appears to be cleared within weeks to months after exposure.
Exposure(s) to asbestos may result in pulmonary fibrosis (asbestosis), lung cancer, pleural effusion, parietal pleural plaques, rounded atelectasis, diffuse pleural fibrosis, and mesothelioma. It should be noted that the pleural sequelae can occur at much lower exposures than those required to produce lung parenchymal changes of asbestosis and asbestos-related lung cancers.
Asbestosis is diffuse pulmonary fibrosis caused by the excessive inhalation of asbestos. The pathologic criteria were most recently set out in the 2010 Report of the Asbestosis Committee of the College of American Pathologists and Pulmonary Pathology Society (CAP-PPS). In industrialized nations where control of dust emissions has been exercised, the incidence of symptomatic asbestosis has declined considerably. Clinical cases of asbestosis are usually seen in the context of prolonged substantial direct exposures several decades ago, and the latency from first exposure to presentation is typically over 20 years. The onset of symptoms is insidious, with breathlessness on effort, and any progression, if it occurs, takes place over many years. Cough may occur in the later stages, and finger clubbing develops in about half of those with advanced fibrosis. Fine inspiratory crackles are present bilaterally at the lung bases and extend to the middle and upper zones with disease progression. Finally, cor pulmonale and death may result. Lung function tests usually show a restrictive pattern. Asbestosis is a dose-responsive disease, and there is evidence that latency is inversely correlated with cumulative dose.
Asbestosis is typically associated with small, irregular opacities, initially at the lung bases. In the later stages, honeycombing may occur. In about one-third of cases there is also pleural thickening, with or without calcification. High-resolution computed tomography (HRCT) is more sensitive than standard chest radiographs for detecting the changes of interstitial lung disease, but the findings are not specific, with overlap between asbestosis and idiopathic pulmonary fibrosis/usual interstitial pneumonia.
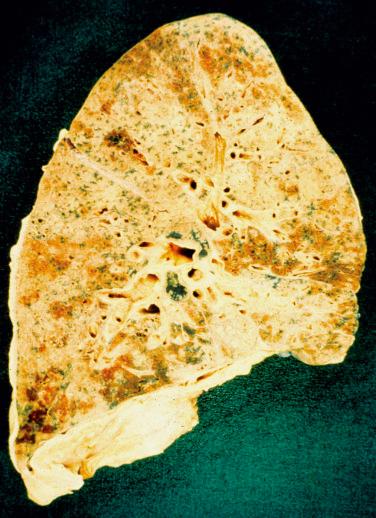
Asbestosis typically ranges in severity from macroscopically normal lungs through minor indurated ill-defined areas in the lower lobes with subpleural accentuation to advanced diffusely contracted lungs with surface pleural bosselation. Cut sections of the lung may reveal fine honeycomb changes ( Fig. 18.1 ). There is often some degree of visceral and, to a lesser extent, parietal pleural thickening and/or plaques. There is only a loose correlation of the extent of these pleural changes with the extent of the lung parenchymal abnormalities.
The CAP-PPS criteria for the microscopic diagnosis of asbestosis require the presence of interstitial fibrosis of an appropriate pattern (always paucicellular and collagenous rather than fibroblastic and inflammatory) and a sufficient number of asbestos bodies. An average rate of at least 2 per cm 2 should be present to make a confident diagnosis (based on Perls iron-stained routine thickness sections). Rarely, heavily exposed individuals with appropriate fibrosis have insufficient asbestos bodies in lung tissue to allow for a confident morphologic diagnosis. In such persons, mineral analysis may be performed to show elevated retained asbestos fibers and allows for a diagnosis of asbestosis ex asbestos bodies.
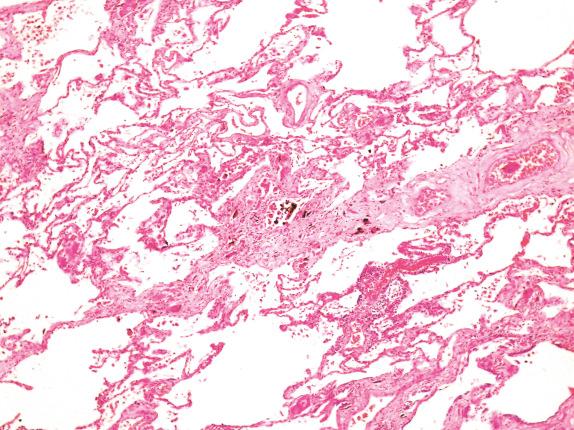
There is a commonly used light microscopic grading system ( Table 18.2 ). The earliest changes are seen around the respiratory bronchioles and first tier of alveoli ( Fig. 18.2 ), but as the disease progresses, the fibrosis extends along alveolar walls and alveolar ducts to link up with other lesions. Eventually, severe disorganization of lung architecture occurs, with honeycomb changes ( Fig. 18.3 ). Other features less commonly encountered are foreign body giant cells around the asbestos bodies, osseous metaplasia, and pulmonary blue bodies, but these are entirely nonspecific.
| Grade 1 | Fibrosis of the walls of respiratory bronchioles and first adjacent tier of alveoli |
| Grade 2 | Fibrosis around respiratory bronchioles that extends into adjacent alveolar ducts and alveoli but does not join up with fibrosis extending from other respiratory bronchioles |
| Grade 3 | Fibrosis that links up adjacent respiratory bronchioles but with little architectural distortion |
| Grade 4 | Honeycomb changes |
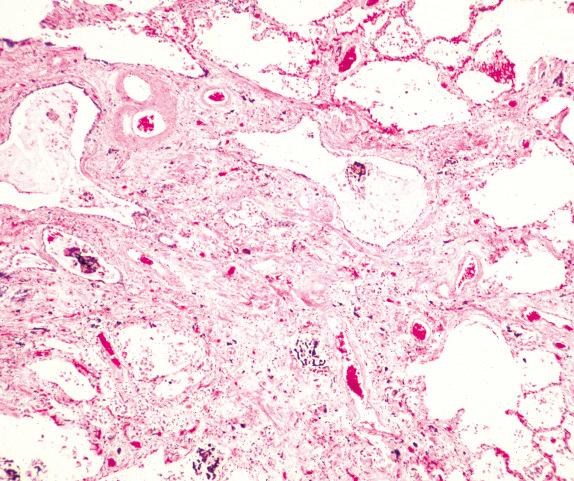
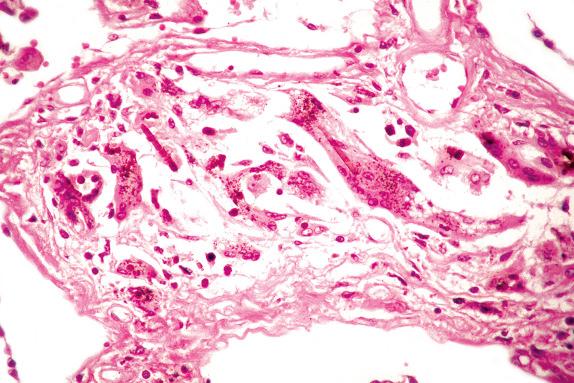
An asbestos body is characterized by a clear fibrous core coated by iron-protein-mucopolysaccharide material, which forms a golden-brown, beaded, dumbbell or drumstick-shaped structure ( Figs. 18.4 and 18.5 ). When these cores have been examined by analytical techniques, they are now almost always commercial amphibole fibers. Asbestos bodies rarely, if ever, form on chrysotile fibers. An iron stain can be used to highlight asbestos bodies. However, care has to be taken in identifying asbestos bodies because other material can be coated to form ferruginous bodies, but usually the core has a different appearance and it is not transparent ( Fig. 18.6 ). In persons with marked congestion and hemosiderin deposition in the lungs, the iron stain may be difficult to interpret.
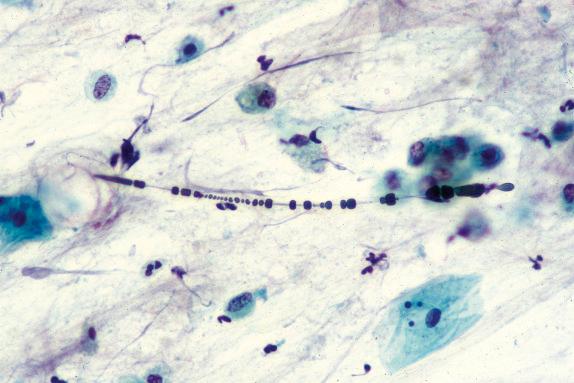
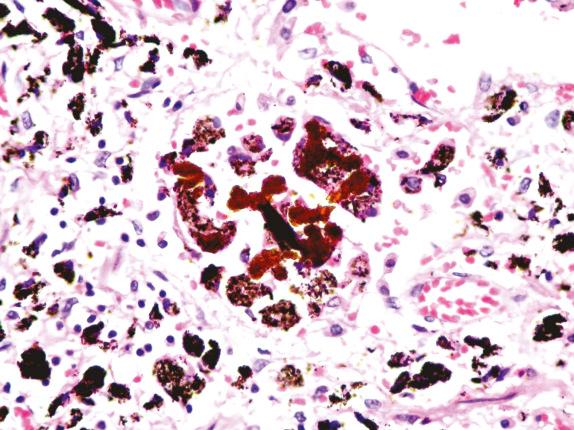
Mineral analysis of bronchoalveolar lavage (BAL) fluid or lung tissue shows high asbestos fiber/body burdens in cases of asbestosis. In a setting of asbestosis, the BAL usually shows a concentration greatly above 1 fiber per mL. It is distinctly unusual for a case of diffuse interstitial fibrosis without asbestos bodies on light microscopy to show lung tissue concentrations of asbestos fibers in the range usually associated with asbestosis.
Phase-contrast light microscopy is technically simple and inexpensive, although it has significant limitations, which include poor optimal resolution and lack of qualitative (fiber typing) information. Transmission electron microscopy (TEM) is the most sensitive method and can detect fibers across the spectrum of sizes. Scanning electron microscopy holds an intermediate position with capacity to provide fiber typing and fiber counting information, although it is less sensitive than TEM.
In a subject with diffuse interstitial fibrosis of appropriate pattern and a retained amphibole asbestos fiber count within the range observed for established asbestosis cases, based on morphologic criteria, the case should be regarded as asbestosis. The asbestosis range refers to the total retained amphibole asbestos fiber content in cases with asbestosis. The chrysotile content is not included because it does not correlate with the grade of fibrosis and reflects the low biopersistence of the fiber. A normal fiber count within the range for nonoccupationally exposed subjects provides strong evidence against a diagnosis of asbestosis.
In advanced cases, the major differential diagnoses include idiopathic pulmonary fibrosis, chronic hypersensitivity pneumonitis, sarcoidosis, Langerhans cell histiocytosis, and other mineral dust–induced pneumoconioses such as silicosis and siderosis. Asbestosis is usually associated with the presence of numerous asbestos bodies, and this separates it from idiopathic pulmonary fibrosis and the other conditions listed earlier. Also, fibroblast foci are rarely if ever seen in bona fide asbestosis. Temporal and spatial heterogeneity points to the alternative diagnosis of usual interstitial pneumonia, reflecting idiopathic pulmonary fibrosis, a connective tissue disease or hypersensitivity pneumonitis. Granulomas or Langerhans cell aggregates with mixed inflammation would be compatible with sarcoidosis and Langerhans cell histiocytosis, respectively. For diagnosis of the other mineral-induced pneumoconioses such as silicosis and siderosis, characteristic features should be present, such as silicotic nodules in silicosis and pseudoasbestos bodies with yellow or black cores in siderosis.
There are considerable problems in separating grade 1 and 2 lesions of asbestosis from peribronchiolar fibrosis due to cigarette smoking, urban pollution, and other mineral dust–induced lesions. Smoking-related lung fibrosis may be accentuated in subpleural regions and show “ropey” collagen without significant inflammation. These grades of fibrosis are frequent in nonasbestos-exposed individuals, appearing in some 40% of the general population. Such persons often have some degree of emphysema and respiratory bronchiolitis.
There is no specific therapy for asbestosis, and treatment is symptomatic. Progression is slow, but not invariable, and is dependent on cumulative exposure and fiber type. Today, it is uncommon to see fatal cases of asbestosis. However, there is an increased risk of mesothelioma in these patients, as well as lung cancer; the latter accentuated by the synergy between asbestos exposure and smoking.
Silicosis is a predominantly nodular pneumoconiosis caused by exposure to dusts containing a substantial amount of crystalline silica
Primarily seen in middle-aged or elderly males because of long latency and occupation
Insidious onset of shortness of breath and productive cough
Diffuse nodular opacities with a predominance in the upper zones and enlargement of mediastinal and hilar lymph nodes with peripheral calcification (“eggshell”)
Progression is typically slow
Treatment is symptomatic because there is no specific therapy
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here