Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Entrapment neuropathy is a condition wherein compression, or external pressure applied to a nerve, alters function. Multiple potential etiologies exist, including a space-occupying lesion, inflammatory processes, edema, or compression via anatomic structures. Lesions can present as either acute or chronic conditions. If untreated, the sequelae of prolonged compression can result in permanent compromise of function.
The median nerve at the wrist is the most common nerve compression in the upper extremity, but other sites of compression of the median, ulnar, and radial nerves in the arm, elbow, and forearm occur and can be problematic for athletes. Entrapment of a nerve as a result of any pathologic process can progress to neural injury and dysfunction. Initially, nerves experience edema secondary to compression, although this may be clinically silent or intermittent. Sensory fibers are typically affected first due to their lower threshold of injury because they are less invested in protective myelin sheath, with smaller dimensions. Prolonged compression results in an inflammatory response, microvascular changes, and Schwann cell degeneration. A greater degree of severity of compression can progress to include motor dysfunction and may ultimately result in irreversible changes.
The double crush phenomenon is a condition where the axons distal to the injury site are at increased risk of further injury due to affected axonal transport and myelin or Schwann cell injury that propagates distally. Patient recovery and relief of symptoms is dependent on release of all sites of compression along the course of the affected nerve.
Patients with entrapment neuropathy may present with motor dysfunction (weakness), sensory dysfunction (numbness), or both, with specific patterns dependent on the nerve and site of compression. Symptoms often follow typical patterns, given the anatomic distribution of the affected nerve, and range from intermittent to constant symptoms that may vary in severity. The duration and severity of symptoms may play a role in projecting outcomes following surgery.
Examination of an extremity must always begin proximally, especially with nerve-related complaints, to evaluate contributing or confounding factors such as cervical spine pathology or the aforementioned double crush phenomenon. In general, isolated motor neuropathies, such as anterior or posterior interosseous nerve (AIN; PIN) syndrome, will present with weakness or paralysis in the associated motor groups. Sensory findings follow anatomic distributions, presenting as diminished or altered sensation. Altered sensitivity to light touch (determined with Semmes-Weinstein monofilaments) is an early finding, whereas changes in two-point discrimination are a late finding. Entrapment also makes injured nerves susceptible to manual irritation or provocation of sensory symptoms, which can be reliably identified by the presence of a positive Tinel sign or distal paresthesias/ sensations, which occur when percussing a nerve in an area of irritation.
Radiographic imaging has a limited role in evaluation of entrapment neuropathy. In situations such as heterotopic ossification (HO) or posttraumatic elbow conditions, osseous anatomic anomalies may exist that result in nerve entrapment. Ultrasound is a relatively new modality and appears promising, but at this time there are limited data regarding its role. It can be used to help diagnose and classify cubital tunnel syndrome, as the ulnar nerve undergoes changes in cross-sectional area with entrapment at the elbow, and nerve subluxation can be visualized. There is both a diagnostic and therapeutic role in the management of carpal tunnel as well.
Magnetic resonance imaging (MRI) provides information regarding soft tissue anatomy and can occasionally be valuable in entrapment neuropathy. It can be helpful in evaluation of anomalous anatomic structures, space-occupying lesions. Cysts or tumors, such as a lipoma, can cause pressure on the nerve and can be particularly helpful in evaluating radial nerve in the forearm. MRI can also be used for evaluation of nerve integrity following trauma. It can also provide valuable information in the setting of motor dysfunction because degenerative changes in muscles can indicate level of injury or degree of involvement.
Further diagnostic information is available via electrophysiologic evaluation. Nerve compression results in demyelination along the length of the nerve, which progresses distally, impairing conduction velocity. More severe entrapment can progress to asynchronous conduction and even conduction block, with resultant weakness, atrophy, and eventual paralysis.
Electrodiagnostic studies have two components: nerve conduction velocities (NCVs) and electromyogram (EMG). NCV studies can potentially identify sites of slowing, which can help in diagnosing potential double crush injuries. Increased conduction latencies occur due to demyelination. Sensory nerves demonstrate changes earlier in the disease process due to their lower injury threshold and decreased myelin content in comparison to motor nerves. Amplitude of conducted impulses is proportional to the cross-sectional area of functional axons. Increased motor latency and decreasing amplitude indicate more advanced compression. EMG describes the muscles' response to stimulation, including motor recruitment. EMG or motor changes lag behind NCV changes in entrapment neuropathy, indicating more advanced disease. In addition, repeat studies provide useful information in monitoring nerve recovery after intervention, which is most useful in the context of motor function, with nerves such as the AIN and PIN. Early axonal denervation presents with sharp waves and fibrillation potentials, onset 1 to 4 weeks after nerve injury. Chronic injuries demonstrate these patterns but with diminished amplitude. A regenerating/healing nerve will have motor units with polyphasic patterns and nascent, low-amplitude potentials on EMG.
These studies are done with the limb in a resting position. Some athletes only have symptoms with provocation, such as ulnar nerve irritation in throwing athletes. Special circumstances, such as communications or connections with other major nerves, such as the anomalous Martin-Gruber connection, may be present, making it difficult to match the clinical and electrical findings. The Martin-Gruber connection is a motor nerve connection between the median and ulnar nerves and can result in retained distal function in the distribution of the transected nerve. In addition, temperature of the limb undergoing evaluation can influence outcomes, specifically EMG amplitude, due to corresponding variations in fluid distributions within muscle. Therefore these results should be interpreted within clinical context.
With early presentation, a trial of nonoperative treatment is warranted and typically pursued for 3 to 6 months, after which persistence or worsening of symptoms warrants surgical intervention. Severe entrapment or symptoms associated with trauma are best treated with early nerve decompression.
Nonoperative management should include activity modification, with avoidance of the offending position or movement, and use of an orthosis. Antiinflammatory medication can help with mild or moderate symptoms. Corticosteroid injections have a limited role in many entrapment neuropathies of the upper extremity other than carpal tunnel syndrome. Failure of nonoperative management or persistence or worsening of symptoms warrants surgical intervention.
Postoperatively, patients should be expected to return to full activity, with timing dependent upon the procedure and sport/position. This is typically a much shorter duration than with osseous or ligamentous injuries, which may be up to 6 months. The athletes must demonstrate the ability to safely take the affected limb through the necessary range of motion (ROM) and with the necessary strength and control to return to play (RTP).
Untreated entrapment neuropathy can eventually progress to chronic degeneration and associated permanent nerve dysfunction and muscle atrophy. Surgical intervention carries risks, including wound complications, iatrogenic nerve injury, inadequate or incomplete surgical decompression, and recurrence of symptoms. A rare but serious complication is the development of complex regional pain syndrome (CRPS), associated with chronic pain, early vasomotor changes, stiffness, and loss of function. It may be associated with chronic nerve entrapment, which can occur with nerve compressions, specific nerve injuries, or trauma in the extremity.
The median nerve is positioned along the anterior aspect of the arm, initially lateral to the brachial artery, although it crosses over to lie medially upon reaching the antecubital fossa. Approximately 1% of the population has an anomalous supracondylar humerus process that can give rise to a fibrous band that inserts at the medial epicondyle, named the ligament of Struthers. The median nerve travels beneath this structure, when present, and crosses the antecubital fossa, where it is bordered by the brachial artery (lateral), brachialis (posterior), pronator teres (PT) (medial), and lacertus fibrosus, or bicipital aponeurosis (anterior). The nerve then enters the anterior compartment of the forearm between the heads of the PT. The median nerve travels distally between the flexor digitorum superficialis (FDS) and flexor digitorum profundus (FDP). The AIN branches at a variable location within the proximal forearm. The median nerve becomes more superficial, running between the FDS and flexor pollicis longus (FPL), gives off the palmar cutaneous nerve branch and then enters the carpal tunnel at the wrist. The median nerve innervates the FDS, flexor carpi radialis (FCR), and palmaris longus (PL) muscles in the forearm ( Fig. 63.1 ).
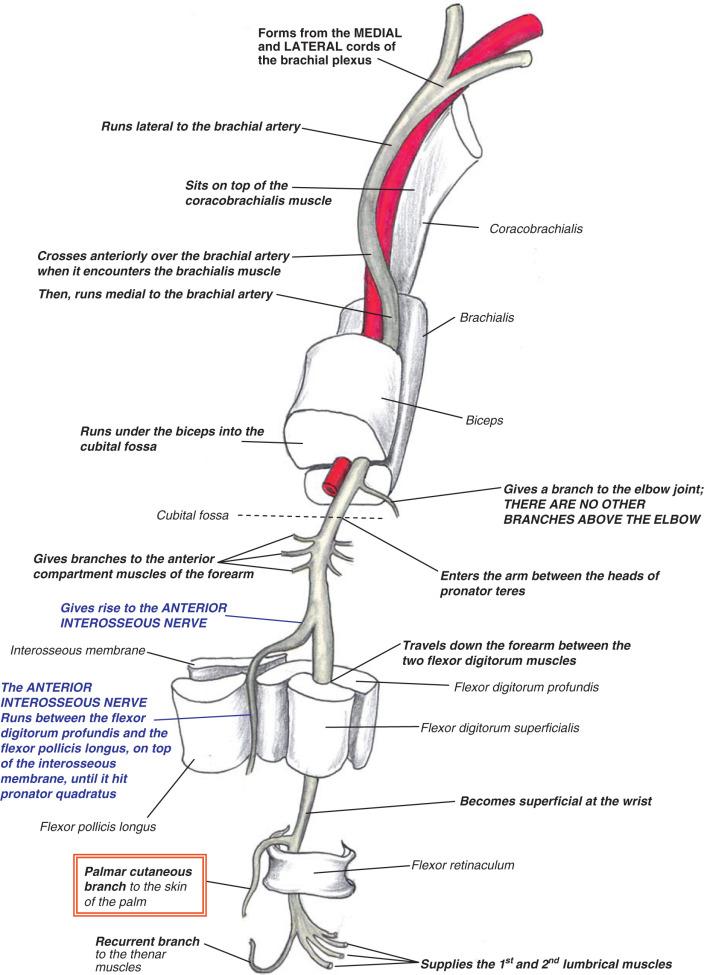
The AIN may divide from the median nerve as proximal as the two heads of the PT, typically in a radial direction. It passes through the FDS arch along with the median nerve and progresses distally volar to the interosseous membrane between the FDP and FPL. The AIN terminates at the pronator quadratus (PQ) muscle after innervating the FPL, variable portions of the radial FDP, and PQ muscles.
Of note, approximately 22% of the population can present with a connection between the AIN or median nerve and the ulnar nerve, known as the Martin-Gruber connection. A potential space-occupying anomaly is the Gantzer muscle, an accessory head of the FPL muscle, which can be present in up to 68% of patients.
Median nerve compression in the arm and forearm is typically associated with high stress, repetitive flexion through the elbow, and repetitive forceful pronation. Sports that rely on these motions include archery, baseball, and automobile racing.
Pronator syndrome is primarily attributed to median nerve compression between the two heads of the PT in the forearm. Other sites of entrapment include the proximal arch of FDS, the anomalous ligament of Struthers, or the lacertus fibrosus in the antebrachial fossa. Sources of potential external compression include tumors and the anomalous Gantzer muscle. Pronator syndrome has also been described with the presence of a persistent median artery. The most common sites of median nerve compression are at the deep head of the PT, the FDS arch, and lacertus fibrosus. There are surgeons who feel this is really entrapment at the lacertus fibrosus and isolated decompression at this level will resolve symptoms.
Pronator syndrome presents primarily with complaints of pain in the forearm, typically in the proximal, anterior region, with minimal weakness or sensory deficits. A notable differentiating factor from carpal tunnel syndrome is the absence of nocturnal symptoms. If sensory deficits are present, they can present as numbness or paresthesia in the thenar eminence, in the distribution of the palmar cutaneous branch of the median nerve, as well as the volar aspect of the thumb, index, middle, and radial aspect of the ring finger, following typical median nerve innervation.
As with other entrapment neuropathies, provocative maneuvers can help determine a site of compression. Reproducible pain with resisted forearm pronation while the elbow is extended suggests compression at the PT. When the site of compression is the fibrous arch of the FDS, resisted contraction of the middle finger proximal interphalangeal (PIP) joint will elicit pain in the volar forearm. Pain with resisted elbow flexion while the forearm is held in maximum supination is associated with median nerve compression under the lacertus fibrosus. Compression under the ligament of Struthers is suggested by pain with resisted elbow flexion at 120 to 130 degrees. The patient may also present with weakness of the FPL and FDP of the index finger, similar to findings with AIN neuropathy. The Gainor test involves direct compression of the PT in bilateral upper extremities while at rest. A positive test, which indicates PT compression, is defined as provocative symptoms only in the affected limb. The scratch-collapse test, which is positive with acute ipsilateral weakness in shoulder external rotation upon noxious stimulus to a site of nerve compression, has been previously described with sensitivity and specificity similar to other exam maneuvers. This is performed with the examiner in front of the patient and both arms flexed at the elbow and forearms in neutral rotation. The examiner attempts to bring the hands together while the patient resists. The nerve in question is then scratched, and the examiner again attempts to bring the hands together. A positive result occurs when the patient temporarily cannot resist the examiner's pressure and the extremity collapses due to the pressure. However, studies find this exam to be operator dependent with variable diagnostic outcomes, and although it may provide an adjunct to EMG or other exam findings, it is not independently diagnostic.
Vague anterior forearm pain is the primary and most reliable reproducible symptom, whereas history and exam maneuvers are not as specific as in other entrapment neuropathies. Electrodiagnostic studies are often normal. Imaging can provide some information because the anomalous supracondylar humerus process associated with the ligament of Struthers can be seen on x-ray. Further imaging, such as MRI or ultrasound, may be warranted with clinical concern for a space-occupying lesion, such as a cyst or tumor, or to evaluate anomalous anatomy.
Initial management should be nonoperative, including activity modification to avoid the offending action, immobilization, and antiinflammatory medications and can result in symptom resolution in 50% to 70% of cases.
Traditional surgical management requires exposure of the antecubital fossa to address all potential sites of compression of the median nerve. The skin incision typically begins 5 cm proximal to the elbow and extends distally to the mid-forearm. Incisions can either follow a lazy-S pattern with or without offset linear incision, or a transverse incision. Longitudinal incisions result in more noticeable scarring. Endoscopic assisted decompression has also been described.
The most common approach is via a lazy-S incision that progresses from the mid-arm along the medial aspect of the biceps, curving toward the supracondylar region medially and continuing distally over the flexor-pronator mass to the mid-forearm. Dissection should preserve the cutaneous nerve branches. Supracondylar exposure should be performed, with release of the ligament of Struthers, if indicated clinically or radiographically. The median nerve is identified and traced to identify potential sites of compression. The lacertus fibrosus should be divided and released. The two heads of the PT should be divided and adhesions released. Distally, the FDS arch should be released, with care to protect the AIN, which may have branched proximally. Any space-occupying masses should be excised and sent for pathologic evaluation.
Our preference is a complete decompression of the median nerve from the lacertus fibrosus through the fibrous arch of the FDS, as described previously.
Mobilization should take place within 1 week, with therapy directed at nerve gliding, motion, and strengthening. RTP is typically 6 to 8 weeks for most sports and is dependent upon adequate healing, motion, and return of grip strength but may be up to 6 months. Endoscopic surgery, or isolated decompression at a specific point, will result in a smaller incision and shorter recovery.
Surgical release has been shown to reliably relieve symptoms with good or excellent results in 60% to 80%+ of cases. This is supported with objective measures such as the Disabilities of the Arm, Shoulder, and Hand (DASH) score and some studies showing 60% of patients with near or complete resolution of symptoms. Outcomes are variable because Olehnik et al. report persistent symptoms in 25% of 39 patients.
Complications are unusual but include incomplete release, iatrogenic nerve injury, and scarring with recurrence of symptoms. Transient AIN palsy has been documented.
AIN syndrome is an isolated motor nerve dysfunction, often without associated trauma or injury. There has been no specific pattern of activity or sport that has been associated with this condition. It is often idiopathic, although entrapment is possible. Potential other etiologies include trauma, space-occupying lesions, and anomalous vasculature. If entrapment is the etiology of AIN palsy, the most common site of compression is under the FDS arch. Alternative sites of compression mirror those for pronator syndrome.
Clinical presentation often includes a dull ache or pain in the volar forearm. The diagnostic finding is motor dysfunction in the AIN distribution without sensory deficits. It is important to rule out Parsonage-Turner syndrome in these patients, or brachial neuritis. Parsonage-Turner syndrome is a prodrome of viral illness–like symptoms, followed by shoulder pain, several days to weeks prior to the onset of muscle weakness suggestive of AIN dysfunction. These patients typically respond to nonoperative management. In addition, sensory deficits may be associated with Parsonage-Turner or carpal tunnel syndrome, which is helpful in diagnosis differentiation.
Motor dysfunction in the AIN distribution is the hallmark of AIN syndrome. Patients often present with loss of flexion of the thumb interphalangeal (IP) joint (secondary to FPL dysfunction) and the index distal interphalangeal (DIP) joint (loss of FDP function). This results in loss of tip pinch between index and thumb (inability to make an “O” when opposing the tip of the thumb to the tip of the index finger). Dysfunction isolated to a single tendon must be evaluated to rule out tendon rupture, especially in patients with inflammatory arthropathies. This can be accomplished through tenodesis, or evaluation of resting passive tension of tendons, which allows for identification of tendon ruptures. When the wrist is flexed, the fingers should extend due to tension on the digital extensors. When the wrist is extended, the fingers should flex, with the ulnar digits flexing slightly more to maintain a normal digital cascade.
The diagnosis of an AIN syndrome is primarily clinical. However, further workup is required to determine a truly idiopathic AIN pathology from entrapment neuropathy that may possibly benefit from surgical intervention. Electrodiagnostic studies are often normal in idiopathic cases but do provide information regarding the severity of nerve compression in AIN syndrome. Advanced imaging such as MRI may be used to rule out space-occupying lesions and also evaluate potential atrophy in AIN-innervated muscle groups. The most reliable MRI finding in AIN syndrome diagnosis is increased signal intensity within the PQ.
Nonoperative management is the primary course of treatment. Rest, immobilization, and analgesia with antiinflammatory medications are the mainstays of early treatment. Avoiding offending actions, especially forceful gripping and repetitive pronation, and use of an orthosis or a sling to maintain rest with the elbow in flexion relieve tension on the AIN. Spontaneous resolution of symptoms often takes up to 9 months. Surgery is indicated for patients with compressive findings on electrodiagnostic testing that have failed nonoperative management, which can be defined as 3 to 6 months without improvement either clinically or via EMG studies.
Given that the sites of compression are the similar to pronator syndrome, surgical treatment follows the same approach. Anterior exposure of the distal arm, antecubital fossa, and proximal forearm is required to obtain access to all sites of compression. More extensive dissection at the proximal aspect of the incision helps to expose the origin of the AIN by releasing and reflecting the humeral head of the PT. Division of the lacertus fibrosus and release of the arch of FDS distally are carried out for decompression. The Gantzer muscle, if present, should be released. Innervating branches of the AIN to FPL and FDP must be preserved. Identification of vascular anomalies and mass lesions should be followed by appropriate excision or ligation.
Our technique mimics that used for pronator syndrome, because we prefer to release all potential sites of compression unless there is a localized point of compression confirmed on electrodiagnostic studies, in which case we will decompress only that area.
AIN syndrome secondary to a compressive etiology often results in progressive return of function postoperatively. RTP depends on return of strength in the affected motor groups, and similar to pronator syndrome, is typically 6 weeks but dependent on sport, position, and level of competition. Studies demonstrate recovery of strength within 6 to 12 months.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here