Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The enteroviruses, parechoviruses, and Saffold viruses are all members of the picornavirus family, a group of small, nonenveloped RNA viruses. Enteroviruses, including the polioviruses, coxsackieviruses, echoviruses, and numerically designated types, and parechoviruses constitute two distinct genera among the Picornaviridae, although Saffold viruses are members of the genus Cardiovirus, genetically related to Theiler murine encephalomyelitis virus. The enteroviruses, parechoviruses, and Saffold viruses exhibit clear differences in their physical and biochemical properties and mode of replication, but they are discussed together here because of shared features in their epidemiology, pathogenesis and clinical manifestations. Congenital and neonatal infections have been linked with many different enteroviruses and parechoviruses. At the present time, there are no data on congenital or neonatal infections with Saffold viruses, but the similarity of their clinical manifestations in older infants and children to those of both enteroviruses and parechoviruses suggests that reports will soon appear.
Historical recognition of enterovirus infection began with poliomyelitis. The earliest record is an Egyptian stele of the 18th dynasty (1580-1350 bc ), which shows a young man with a withered, shortened leg, the characteristic deformity of paralytic poliomyelitis. Underwood, a London pediatrician, published the first medical description in 1789 in his Treatise on Diseases of Children. During the 19th century, many reports appeared in Europe and the United States describing small clusters of cases of “infantile paralysis.” The authors were greatly puzzled about the nature of the affliction; not until the 1860s and 1870s was the spinal cord firmly established as the seat of the pathologic process. The contagious nature of poliomyelitis was not appreciated until the latter part of the 19th century. Medin, a Swedish pediatrician, was the first to describe the epidemic nature of poliomyelitis (1890), and his pupil Wickman worked out the basic principles of the epidemiology.
Poliovirus was first isolated in monkeys by Landsteiner and Popper in 1908. The availability of a laboratory animal assay system opened up many avenues of research that in the ensuing 40 years led to the demonstration that an unrecognized intestinal infection was common and that paralytic disease was a relatively uncommon event. In 1949, Enders and associates reported the growth of poliovirus type 2 in tissue culture, leading to the development of poliovirus vaccines in the 1950s and 1960s.
Coxsackieviruses and echoviruses have had a shorter history. Epidemic pleurodynia was first clinically described in northern Germany in 1735 by Hannaeus, more than 200 years before the coxsackieviral cause of this disease was discovered. In 1948, Dalldorf and Sickles first reported the isolation of a coxsackievirus by using suckling mouse inoculation. However, the development of tissue culture methods by Enders and his colleagues paved the way for the recovery of a large number of other cytopathic viruses. Most of these “new” viruses failed to produce illness in laboratory animals. Because the relationships of many of these newly recovered agents to human disease were unknown, they were called orphan viruses. Later, several agents were grouped together and called e nteric c ytopathogenic h uman o rphan viruses, or echo viruses. Enteroviruses were first categorized together and named in 1957 by a committee sponsored by the National Foundation for Infantile Paralysis ; the human enteric tract was believed to be the natural habitat of these agents, giving rise to the group nomenclature.
Subsequently, analysis of the replication, genome and protein content of echoviruses 22 and 23 found that they were distinctly different from most of the agents designated as enteroviruses, and they were placed in the genus Parechovirus . More recently, using molecular detection methods, Saffold viruses were identified in human infections. Extensive seroepidemiologic studies indicate that infections with Saffold virus types 2 and 3 are very common in young children. Some clinical associations have been noted, but it would seem that most infections are asymptomatic. As noted above, no data on congenital or neonatal infections with Saffold viruses have as yet been presented.
Inactivated polio vaccines (IPV) and live-attenuated oral poliovirus vaccines (OPV) became available in the late 1950s and early 1960s, and there has been a dramatic reduction in worldwide poliomyelitis because of immunization with IPV and OPV and the efforts of the global immunization initiative. The last case of confirmed paralytic polio in the Western Hemisphere caused by a nonvaccine type occurred in 1991. Aside from the polio immunization successes, there have been few major advances or new modes of prevention or treatment for enteroviral diseases. However, the use of nucleic acid detection systems has progressed over the past 2 decades, and rapid diagnosis of meningitis and other enteroviral and parechoviral illnesses has become possible.
Enteroviruses, parechoviruses and Saffold viruses are RNA viruses belonging to the family Picornaviridae (pico = small). They are grouped together because they share certain physical, biochemical, and molecular properties. In electron micrographs, the viruses are seen as 27- to 30-nm particles that consist of naked (nonenveloped) protein capsids, constituting approximately 70% to 75% of the mass of particles, and dense central cores containing the single-stranded message-sense genomic RNA.
The original classification of human enteroviruses is shown in Table 25-1 . The enteroviruses were originally distributed into four groups based on their different effects in tissue culture and pattern of disease in experimentally infected animals: polioviruses (causal agents of poliomyelitis in humans and nonhuman primates); coxsackie A viruses (coxsackievirus A), associated with herpangina, human central nervous system (CNS) disease, and flaccid paralysis in suckling mice); coxsackie B viruses (coxsackievirus B) (human CNS and cardiac disease, spastic paralysis in mice); and the echoviruses (nonpathogenic in mice and not initially linked to human disease).
| Cytopathic Effect | Illness and Pathology | ||||
|---|---|---|---|---|---|
| Virus | Serologic Types † | Monkey Kidney Tissue Culture | Human Tissue Culture | Suckling Mouse | Monkey |
| Polioviruses | 1-3 | + | + | — | + |
| Coxsackieviruses A | 1-24 ‡ | — | — | + | — |
| Coxsackieviruses B | 1-6 | + | + | + | — |
| Echoviruses | 1-34 § | + | ± | — | — |
∗ Many enterovirus strains have been isolated that do not conform to these categories, leading to the revised classification scheme shown in Table 25-2 .
† Newer types were eventually assigned enterovirus type numbers instead of coxsackievirus A, coxsackievirus B, or echovirus numbers. Types 68 through 71 (EV68-EV71) were initially identified.
‡ Coxsackievirus A23 was found to be the same as echovirus 9.
§ Echovirus 10 was reclassified as a reovirus: Echoviruses 22 and 23 were made the first members of the Parechovirus genus of Picornaviridae, and echovirus 28 was reclassified as a rhinovirus.
Although this scheme was initially useful, many strains were subsequently isolated that do not conform to such rigid specificities. For example, several coxsackievirus A strains replicate and have a cytopathic effect in monkey kidney tissue cultures, and some echovirus strains cause paralysis in mice. For this reason, and to simplify the nomenclature, subsequent enteroviruses were assigned sequential numbers. After this convention, the prototype enterovirus strains Fermon, Toluca-1, J 670/71, and BrCr (identified from 1959-1973) were numerically designated enterovirus (EV) 68 through 71, respectively. Additional enteroviruses continued to be identified that could not be identified using antisera specific for the classic serotypes. More than 50 additional such EV types have been assigned, although not all have been linked to human disease.
Complicating matters somewhat, studies of echoviruses 22 and 23 found that they exhibited genomic and proteomic differences from other enteroviruses, and hence they were reclassified in the new genus Parechovirus as parechoviruses types 1 and 2. Similarly, hepatitis A virus was initially assigned the designation of EV72 but was reclassified as the sole member of the Hepatovirus genus within the picornavirus family because of marked genetic and biologic distinctions from the enteroviruses.
In recent years, genetic, biologic and molecular properties have been used to revise picornavirus taxonomy, leading to a reorganization of the human enteroviruses into four alphabetically designated human enterovirus species (HEV-A, -B, -C, and -D) ( Table 25-2 ). Determining the nucleotide sequence encoding the viral VP1capsid protein now plays a major role in the approach to taxonomy and predictably identifies viruses originally classified by serologic means, leading to the term “molecular serotyping.” This approach will, no doubt, dominate future phylogenetic studies. Of interest, the application of molecular phylogenetic approaches has also revealed that recombination between circulating enteroviruses is a frequent event and is likely to increase their genetic diversity. This propensity for recombination has played a role in recent outbreaks of paralytic diseases involving vaccine-derived stains and in well-documented cases of HEV-B infections.
| Species Designation | Types |
|---|---|
| Human enterovirus A (HEV-A) | Coxsackievirus A2-8, A10, A12, A14, A16 |
| Enterovirus A71, A76, A89, A90, A91, A114, A119 | |
| Human enterovirus B (HEV-B) | Coxsackievirus A9 |
| Coxsackievirus B 1-6 | |
| Echovirus 1-9, 11-21, 24-27, 29-33 | |
| Enterovirus B69, B73-B75, B77-B88, B93, B97, B98, B100, B101, B106, B107 | |
| Human enterovirus C (HEV-C) | Poliovirus 1-3 Coxsackievirus A1, A11, A13, A17, A19-22, A24 |
| Enterovirus C95, C96, C99, C102, C104, C105, C109, C113, C116-C118 | |
| Human enterovirus D (HEV-D) | Enterovirus D68, D70, D94, D111 |
Similarly, molecular analysis has led to the identification of 16 distinct types of human parechoviruses. Of these, types 1 and 3 have most often been associated with human illness. Substantial recombination among the parechoviruses has also been noted, allowing rapid genetic diversification.
The genome of picornaviruses is a single-stranded, positive-sense RNA molecule approximately 7.4 to 8 kb in length. It consists of a 5′ noncoding region, followed by a single long open-reading frame, a short 3′ noncoding region, and a polyA tail. The 5′ noncoding region folds into highly conserved structures that are thought to play a role in the initiation of the replication of the viral genome and contain an internal ribosome entry site, which is essential for the initiation of translation. Similarly, the 3′ noncoding region is well conserved within each picornavirus genus and is thought to be involved in replication of the viral genome. The viral genome is packaged into naked capsids that exhibit icosahedral symmetry with 20 triangular faces and 12 vertices.
Enterovirus replication begins with the adsorption of virions to cell surface receptors, which are typically integrins or immunoglobulin-like proteins ( Table 25-3 ). The virions penetrate the surface of the cell, uncoat, and the viral genome functions as messenger RNA for the viral polyprotein. This polypeptide contains three domains, P1 to P3, which are cleaved into three to four proteins each. The P1 region is liberated from the polyprotein by the viral 2A protein, a chymotrypsin-like protease. P1 is initially split into three proteins—VP0, VP1, and VP3—by the viral 3C protease. VP0 is then further processed into two smaller proteins, VP4 and VP2. Portions of VP1, VP2, and VP3 are exposed at the surface of the virion, whereas VP4 is entirely internal. VP1, VP2, and VP3 have no sequence homology but share the same topology. Specifically, they form an eight-stranded antiparallel β-barrel that is wedge shaped and composed of two antiparallel β-sheets. The amino acid sequences in the loops that connect the β-strands and the N- and C-terminal sequences that extend from the β-barrel domain of VP1, VP2, and VP3 give each enterovirus its distinct antigenicity.
| Virus | HEV Species | Receptor | Cofactor for Infection ∗ |
|---|---|---|---|
| Enterovirus 71 | HEV-A | PSGL-1, SCARB2 | |
| Coxsackieviruses B1-6 | HEV-B | CAR | Some coxsackieviruses B may use CD55 (DAF) or heparin for attachment |
| Echovirus 9 | HEV-B | α v β 3 integrin (vitronectin receptor) | MAP-70 |
| Echoviruses 1, 8 | HEV-B | VLA-2 (α 2 β 1 integrin) | Heparin sulfate |
| Coxsackieviruses A13, 17, 20, 21, 24 | HEV-C | ICAM-1 | |
| Polioviruses 1-3 Enterovirus 70 |
HEV-C HEV-D |
CD155 (PVR) CD55 (DAF) |
∗ The cofactors generally facilitate adhesion to cells, but their sole expression is insufficient to permit infection to occur.
The replication of enteroviruses occurs in the cytoplasm in membrane-associated replication complexes, and is completed rapidly (5-10 hours). Studies of polioviruses and coxsackieviruses have shown that enteroviral replication is associated with disruption of cellular protein secretion, and host cell protein synthesis is suppressed because of cleavage of eIF4G by the enteroviral 2A protein. The coxsackievirus 2A protein also cleaves dystrophin, a cytoskeletal protein; this activity has been hypothesized to play a role in damage to the myocardium.
The parechoviruses replicate in a similar fashion. Integrins (α v β 3 and perhaps α v β 1 ) have been identified as key receptors. In contrast to the enteroviruses, the parechovirus 2A protein does not function as a protease but may function in genome replication. Moreover, parechoviral capsids are composed of three proteins: VP1, VP3, and an uncleaved VP0 protein. In addition, parechovirus replication occurs in small, discrete foci in the cytoplasm, rather than in large accumulations of membranous vesicles like the enteroviruses. Moreover, transcription and translation do not appear to be disrupted by the parechoviruses, perhaps explaining their relatively mild and delayed cytopathic effect when grown in tissue culture.
The replication of Saffold viruses has received little specific study thus far, but they are likely to exhibit features of other members of the Cardiovirus genus.
Enteroviruses and parechoviruses are relatively stable viruses in that they retain infectivity for several days at room temperature and can be stored indefinitely at ordinary freezer temperatures (−20° C). They are inactivated quickly by heat (>56° C), formaldehyde, chlorination, and ultraviolet light but are refractory to ether, ethanol, and isopropanol.
Enterovirus strains grow rapidly when adapted to susceptible host systems and cause cytopathology in tissue culture in 2 to 7 days. The typical tissue culture cytopathic effect is shown in Figure 25-1 ; characteristic pathologic findings in mice are shown in Figures 25-2 and 25-3 . Final titers of virus recovered in the laboratory vary markedly among different viral strains and the host system used; typically, concentrations of 10 3 to 10 7 infectious doses per 0.1 mL of tissue culture fluid or tissue homogenate are obtained. Unadapted viral strains frequently require long periods of incubation. In both tissue culture and suckling mice, evidence of growth usually is visible. Blind passage occasionally is necessary for the cytopathology to become apparent.
Although many different primary and secondary tissue culture systems support the growth of various enteroviruses, primary rhesus monkey kidney cultures generally are accepted to have the most inclusive spectrum. Other simian kidney tissue cultures, however, also have the same broad spectrum. Tissue cultures of human origin have a more limited spectrum, but several echovirus types have shown more consistent primary isolation in human than in monkey kidney. A satisfactory system for the primary recovery of enteroviruses from clinical specimens would include primary rhesus, cynomolgus, or African green monkey kidney tissue cultures; a diploid, human embryonic lung fibroblast cell strain; rhabdomyosarcoma cell line tissue cultures; and intraperitoneal and intracerebral inoculation of suckling mice younger than 24 hours.
Human parechovirus types 1 to 6 can be isolated in several commonly used tissue culture systems. Human parechovirus types 1, 4, and 6 can be recovered in HT29 (primary colorectal adenocarcinoma) cells. Types 1 and 4 can be isolated in Vero (African green monkey kidney), A549 (human lung adenocarcinoma), and RD (rhabdomyosarcoma) cells. Human parechovirus 3 grows in Vero and A549 cells.
Although some minor cross-reactions exist between several coxsackievirus and echovirus types, common group antigens of diagnostic importance have not been defined well. Heat treatment of virions and the use of synthetic peptides have produced antigens with broad enteroviral reactivity. These antigens have been used in enzyme-linked immunosorbent assay (ELISA) and complement-fixation tests to determine IgG and IgM enteroviral antibodies and for antigen detection. In one study, Terletskaia-Ladwig and colleagues reported the identification of patients infected with enteroviruses by the use of an immunoglobulin M (IgM) enzyme immunoassay (EIA). This test used heat-treated coxsackievirus B5 and echovirus 9 as antigens, and it identified patients infected with echoviruses 4, 11, and 30. The sensitivity of the test was 35%. In another study involving heat-treated virus or synthetic peptides, the respective sensitivities were 67% and 62%. However, both tests lacked specificity. Intratypic strain differences are common findings, and some strains (prime strains) are neutralized poorly by antisera to prototype viruses. However, in animals, these prime strains induce antibodies that neutralize the specific prototype viruses.
The identification of polioviral, coxsackieviral, and echoviral types by neutralization in suckling mice or tissue culture with antiserum pools is relatively well defined. Neutralization is induced by the epitopes on structural proteins VP1, VP2, and VP3; in particular, several epitopes are clustered on VP1. Prime strains do cause diagnostic difficulty because frequently they are not neutralized by the reference antisera, which is a particular problem with echoviruses 4, 9, and 11 and enterovirus 71. If these types are suspected, in some instances, this problem can be overcome by using antisera in less diluted concentrations or antisera prepared against several different strains of problem viruses. Recently, Kubo and associates have been able to type enteroviral isolates not identified through neutralization by nucleotide sequence analysis of the VP4 gene. They specifically identified prime strains of echovirus 18 and enterovirus 71. Sequence analysis of the VP1 gene also is useful for typing enteroviral prime strains not identified by neutralization.
It was long believed that humans were the only natural hosts of enteroviruses. Enteroviruses have also been recovered in nature from sewage, flies, swine, dogs, a calf, a budgerigar (i.e., small Australian parakeet), a fox, mussels, clams, and oysters. Serologic evidence of infection with enteroviruses similar to human strains has been found in chimpanzees and other nonhuman primates, cattle, rabbits, a fox, a chipmunk, and a marmot. It is possible that infection in some of these reports was the result of their direct contact with infected humans or infected human excreta. However, genetically distinct enteroviruses have been identified in cattle, possums, domesticated pigs, sheep, and nonhuman primates.
Although enteroviruses do not multiply in flies, they appear to be a possible significant vector in situations of poor sanitation and heavy human infection. The contamination of shellfish is also intriguing because, in addition to their possible role in human infection, they offer a source of enteroviral storage during cold weather. Contaminated foods are another possible source of human infection.
Enteroviruses and parechoviruses are spread from person to person by fecal-oral and possibly by oral-oral (respiratory) routes. Swimming and wading pools may serve as a means of spread of enteroviruses during the summer. Children are the main susceptible cohort; they are immunologically susceptible, and their unhygienic habits facilitate spread. Spread is from child to child (by feces to skin to mouth) and then within family groups. Recovery of enteroviruses is inversely related to age; the prevalence of antibodies to common enteroviruses and parechoviruses increases with age. The incidence of infections and the prevalence of antibodies do not differ between boys and girls. Oral-oral transmission by way of the contaminated hands of health care personnel and transmission by fomites have been documented on a long-term care pediatric ward. Echovirus 18 was isolated from human breast milk, and it was possible that enterovirus transmission to the baby occurred through the breast milk. Chang and colleagues detected coxsackievirus B3 in breast milk of two symptomatic mothers, and their babies both suffered severe illnesses with hepatic necrosis and meningitis caused by coxsackievirus B3.
Enteroviruses and parechoviruses have a worldwide distribution. ∗
∗ References .
Neutralizing antibodies for specific viral types have been found in serologic surveys throughout the world, and most strains have been recovered in worldwide isolation studies. In any one area, there are frequent fluctuations in predominant types. Epidemics probably depend on new susceptible persons in the population rather than on reinfections; they may be localized and sporadic and may vary in cause from place to place in the same year. Pandemic waves of infection also occur.
In temperate climates, enteroviral infections occur primarily in the summer and fall, but in the tropics, they are prevalent all year. A basic concept in understanding their epidemiology is the far greater frequency of unrecognized infection than that of clinical disease. This is illustrated by poliomyelitis, which remained an epidemiologic mystery until it was appreciated that unrecognized infections were the main source of contagion. Serologic surveys were instrumental in elucidating the problem. In populations living in conditions of poor sanitation and hygiene, epidemics do not occur, but wide dissemination of polioviruses has been confirmed by demonstrating the presence of specific antibodies to all three types in nearly 100% of children by the age of 5 years.
Epidemics of poliomyelitis first began to appear in Europe and the United States during the latter part of the 19th century; they continued with increasing frequency in the economically advanced countries until the introduction of effective vaccines in the 1950s and 1960s. The evolution from endemic to epidemic follows a characteristic pattern, beginning with collections of a few cases, then endemic rates that are higher than usual, followed by severe epidemics with high attack rates.
The age group attacked in endemic areas and in early epidemics is the youngest one; more than 90% of paralytic cases begin in children younger than 5 years. After a pattern of epidemicity begins, it is irreversible unless preventive vaccination is carried out. Because epidemics recur over a period of years, there is a shift in age incidence such that relatively fewer cases are in the youngest children; the peak often occurs in the 5- to 14-year-old group, and an increasing proportion is in young adults. These changes are correlated with socioeconomic factors and improved standards of hygiene; when children are protected from immunizing infections in the first few years of life, the pool of susceptible persons builds up, and introduction of a virulent strain often is followed by an epidemic. Extensive use of vaccines in the past 5 decades has resulted in elimination of paralytic poliomyelitis from large geographic areas, but the disease remains endemic in various parts of the world. Although seasonal periodicity is distinct in temperate climates, some viral activity does take place during the winter. Infection and acquisition of postinfection immunity occur with greater intensity and at earlier ages among crowded, economically deprived populations with less efficient sanitation facilities.
Molecular techniques have allowed the study of genotypes of specific viral types in populations over time. For example, Mulders and colleagues studied the molecular epidemiology of wild poliovirus type 1 in Europe, the Middle East, and the Indian subcontinent. They found four major genotypes circulating. Two genotypes were found predominantly in Eastern Europe, a third genotype was circulating mainly in Egypt, and the fourth genotype was widely dispersed. All four genotypes were found in Pakistan.
The epidemiologic behavior of nonpolio enteroviruses and parechoviruses parallels that of polioviruses; unrecognized infections far outnumber those with distinctive symptoms. The agents are disseminated widely throughout the world, and outbreaks related to one or another type of virus occur regularly. These outbreaks tend to be localized, with different agents being prevalent in different years. In the late 1950s, however, echovirus 9 had a far wider circulation, sweeping through a large part of the world and infecting children and young adults. This behavior has been repeated occasionally with other enteroviruses; after a long absence, a particular agent returns and circulates among the susceptible persons of different ages who have been born since the previous epidemic occurred. Other agents remain endemic in a given area, surfacing as sporadic cases and occasionally as small outbreaks. Multiple types are frequently active at the same time, although one agent commonly predominates in a given locality.
There are no available data on the incidence of symptomatic congenital and neonatal enteroviral infections. From the frequency of reports in the literature, it appears that severe neonatal disease caused by enteroviruses decreased slightly during the late 1960s and early 1970s and then became more common again. In 2007, there was an increase in the detection of severe neonatal disease caused by coxsackievirus B1 infection, which has subsequently persisted as the most common enterovirus type reported to the National Enterovirus Surveillance System.
Although more than 100 nonpolio enterovirus types and 16 parechovirus types have been identified, only 24 different virus types have been noted in the 48 years from 1961 to 2005. The five most prevalent nonpolio enterovirus isolations per year in the United States are shown in Table 25-4 . Most patients from whom viruses were isolated had neurologic illnesses. It is possible that other enteroviruses were also prevalent but did not produce clinical disease severe enough to cause physicians to submit specimens for study. Many coxsackievirus A infections, even in epidemics, have probably gone undiagnosed because suckling mouse inoculation was not performed. The use of molecular detection methods may better reveal these in the future.
| Five Most Common Viral Types per Year | |||||
|---|---|---|---|---|---|
| First | Second | Third | Fourth | Fifth | |
| 1961 | Coxsackievirus B5 | Coxsackievirus B2 | Coxsackievirus B4 | Echovirus 11 | Echovirus 9 |
| 1962 | Coxsackievirus B3 | Echovirus 9 | Coxsackievirus B2 | Echovirus 4 | Coxsackievirus B5 |
| 1963 | Coxsackievirus B1 | Coxsackievirus A9 | Echovirus 9 | Echovirus 4 | Coxsackievirus B4 |
| 1964 | Coxsackievirus B4 | Coxsackievirus B2 | Coxsackievirus A9 | Echovirus 4 | Echovirus 6, coxsackievirus B1 |
| 1965 | Echovirus 9 | Echovirus 6 | Coxsackievirus B2 | Coxsackievirus B5 | Coxsackievirus B4 |
| 1966 | Echovirus 9 | Coxsackievirus B2 | Echovirus 6 | Coxsackievirus B5 | Coxsackievirus A9, A16 |
| 1967 | Coxsackievirus B5 | Echovirus 9 | Coxsackievirus A9 | Echovirus 6 | Coxsackievirus B2 |
| 1968 | Echovirus 9 | Echovirus 30 | Coxsackievirus A16 | Coxsackievirus B3 | Coxsackievirus B4 |
| 1969 | Echovirus 30 | Echovirus 9 | Echovirus 18 | Echovirus 6 | Coxsackievirus B4 |
| 1970 | Echovirus 3 | Echovirus 9 | Echovirus 6 | Echovirus 4 | Coxsackievirus B4 |
| 1971 | Echovirus 4 | Echovirus 9 | Echovirus 6 | Coxsackievirus B4 | Coxsackievirus B2 |
| 1972 | Coxsackievirus B5 | Echovirus 4 | Echovirus 6 | Echovirus 9 | Coxsackievirus B3 |
| 1973 | Coxsackievirus A9 | Echovirus 9 | Echovirus 6 | Coxsackievirus B2 | Coxsackievirus B5, echovirus 5 |
| 1974 | Echovirus 11 | Echovirus 4 | Echovirus 6 | Echovirus 9 | Echovirus 18 |
| 1975 | Echovirus 9 | Echovirus 4 | Echovirus 6 | Coxsackievirus A9 | Coxsackievirus B4 |
| 1976 | Coxsackievirus B2 | Echovirus 4 | Coxsackievirus B4 | Coxsackievirus A9 | Coxsackievirus B3, echovirus 6 |
| 1977 | Echovirus 6 | Coxsackievirus B1 | Coxsackievirus B3 | Echovirus 9 | Coxsackievirus A9 |
| 1978 | Echovirus 9 | Echovirus 4 | Coxsackievirus A9 | Echovirus 30 | Coxsackievirus B4 |
| 1979 | Echovirus 11 | Echovirus 7 | Echovirus 30 | Coxsackievirus B2 | Coxsackievirus B4 |
| 1980 | Echovirus 11 | Coxsackievirus B3 | Echovirus 30 | Coxsackievirus B2 | Coxsackievirus A9 |
| 1981 | Echovirus 30 | Echovirus 9 | Echovirus 11 | Echovirus 3 | Coxsackievirus A9, echovirus 5 |
| 1982 | Echovirus 11 | Echovirus 30 | Echovirus 5 | Echovirus 9 | Coxsackievirus B5 |
| 1983 | Coxsackievirus B5 | Echovirus 30 | Echovirus 20 | Echovirus 11 | Echovirus 24 |
| 1984 | Echovirus 9 | Echovirus 11 | Coxsackievirus B5 | Echovirus 30 | Coxsackievirus B2, A9 |
| 1985 | Echovirus 11 | Echovirus 21 | Echovirus 6, 7 † | Coxsackievirus B2 | |
| 1986 | Echovirus 11 | Echovirus 4 | Echovirus 7 | Echovirus 18 | Coxsackievirus B5 |
| 1987 | Echovirus 6 | Echovirus 18 | Echovirus 11 | Coxsackievirus A9 | Coxsackievirus B2 |
| 1988 | Echovirus 11 | Echovirus 9 | Coxsackievirus B4 | Coxsackievirus B2 | Echovirus 6 |
| 1989 | Coxsackievirus B5 | Echovirus 9 | Echovirus 11 | Coxsackievirus B2 | Echovirus 6 |
| 1990 | Echovirus 30 | Echovirus 6 | Coxsackievirus B2 | Coxsackievirus A9 | Echovirus 11 |
| 1991 | Echovirus 30 | Echovirus 11 | Coxsackievirus B1 | Coxsackievirus B2 | Echovirus 7 |
| 1992 | Echovirus 11 | Echovirus 30 | Echovirus 9 | Coxsackievirus B1 | Coxsackievirus A9 |
| 1993 | Echovirus 30 | Coxsackievirus B5 | Coxsackievirus A9 | Echovirus 7 | Coxsackievirus B3 |
| 1994 | Coxsackievirus B2 | Coxsackievirus B3 | Echovirus 6 | Echovirus 30 | Enterovirus 71 |
| 1995 | Echovirus 9 | Echovirus 11 | Coxsackievirus A9 | Coxsackievirus B2 | Echovirus 30, coxsackievirus B5 |
| 1996 | Coxsackievirus B5 | Echovirus 17 | Echovirus 6 | Coxsackievirus A9 | Coxsackievirus B4 |
| 1997 | Echovirus 30 | Echovirus 6 | Echovirus 7 | Echovirus 11 | Echovirus 18 |
| 1998 | Echovirus 30 | Echovirus 9 | Echovirus 11 | Coxsackievirus B3 | Echovirus 6 |
| 1999 | Echovirus 11 | Echovirus 16 | Echovirus 9 | Echovirus 14 | Echovirus 25 |
| 2000 | Coxsackievirus B5 | Echovirus 6 | Coxsackievirus A9 | Coxsackievirus B4 | Echovirus 11 |
| 2001 | Echovirus 18 | Echovirus 13 | Coxsackievirus B2 | Echovirus 6 | Echovirus 4 |
| 2002 | Echovirus 7 | Echovirus 9 | Coxsackievirus B1 | Echovirus 11 | Coxsackievirus B5 |
| 2003 | Echovirus 9 | Echovirus 30 | Coxsackievirus B1 | Coxsackievirus B4 | Coxsackievirus A9 |
| 2004 | Echovirus 30 | Echovirus 9 | Coxsackievirus A9 | Coxsackievirus B5 | Coxsackievirus B4 |
| 2005 | Coxsackievirus B5 | Echovirus 6 | Echovirus 30 | Echovirus 18 | Coxsackievirus B3 |
| 2006 | Echovirus 6 | Echovirus 9 | Coxsackievirus A9 | Coxsackievirus B5 | Coxsackievirus B3 |
| 2007 | Coxsackievirus B1 | Echovirus 18 | Echovirus 9 | Coxsackievirus B4 | Echovirus 11 |
| 2008 | Coxsackievirus B1 | Echovirus 30 | Echovirus 6 | Echovirus 9 | Echovirus 11 |
∗ The majority of patients from whom viruses were isolated had neurologic illnesses.
An analysis of the Centers for Disease Control and Prevention (CDC) nonpolio enterovirus data for 14 years found that early isolates in a particular year were predictive of isolates for the remainder of that year. The six most common isolates during March, April, and May were predictive of 59% of the total isolates during July through December of the same year. Khetsuriani and associates at the CDC presented an extensive report on enterovirus surveillance in the United States for the period 1970 to 2005. During this period, the five most common enterovirus isolates, in order, have been echovirus 9, echovirus 11, echovirus 30, coxsackievirus B5, and echovirus 6. During the most recent period (2006-2008), the most common isolates, in order, have been coxsackievirus B1, echovirus 6, echovirus 9, echovirus 18, and coxsackievirus A9. Similar data are available for the most common enteroviral isolates in Spain from 1988 to 1997 and in Belgium from 1980 to 1994. The most common enterovirus isolated in both countries was echovirus 30. In 1997 and 1998, major epidemic disease caused by enterovirus 71 occurred in Taiwan, Malaysia, Australia, and Japan Similar outbreaks have since continued in other countries such as Australia and in Southeast Asia.
Live-attenuated trivalent polioviral vaccine was used until 2000 in the United States and has eliminated epidemic poliomyelitis in the Western Hemisphere. It is unclear if circulation of the polio vaccine strains had any effects on enteroviral ecology. In 1970, polioviruses accounted for only 6% of the total enteroviral isolations from patients with neurologic illnesses. Although the figures are not directly comparable, more than one third of the enteroviral isolations in 1962 from similar patients were polioviruses. However, Horstmann and associates studied specimens from sewage and asymptomatic children during the vaccine era and found that the number of yearly polioviral isolations (presumably vaccine strains) was greater than the number of nonpolio enteroviruses. However, the prevalence of oral polio vaccine viruses did not seem to affect the seasonal epidemiology of other enteroviruses.
Poliovirus infections in pregnancy can result in abortion, stillbirth, neonatal disease, or no evidence of fetal involvement. Gresser and associates have shown that the human amniotic membrane in organ culture can be infected, resulting in a persistent low-grade infection. It has been observed on many occasions that maternal poliomyelitis occurring late in pregnancy has resulted in transplacental transmission of the virus to the fetus in utero. The evidence that transplacental passage of virus occurs in early pregnancy is meager. Schaeffer and colleagues were able to recover virus from the placenta and the fetus after a spontaneous abortion in a 24-year-old woman with poliomyelitis.
Although attenuated poliovirus vaccines have been given to pregnant women, there has never been a search for the transplacental passage of vaccine virus. Viremia occurs after oral administration of polio vaccine, and, on occasion, this virus probably is passed transplacentally to the fetus.
Several investigators have studied coxsackievirus infections in pregnant animals and the transplacental passage of virus to the fetus. Dalldorf and Gifford studied two strains of coxsackievirus B1 and one of coxsackievirus A8 in gravid mice. In only one instance (coxsackievirus B1) were they able to recover virus from a fetus. They thought that this result was inconclusive because they were unable to recover virus in five other instances. Berger and Roulet observed muscle lesions in the young of gravid mice infected with coxsackieviruses A1 and B1. Selzer studied several viruses in gravid mice; coxsackievirus A9 was found in the placentas of two mice but in no fetuses, and coxsackievirus A18 was not recovered from fetuses or placentas. Selzer found that coxsackieviruses B3 and B4 passed the placental barrier. Soike also observed that in the last week of pregnancy, coxsackievirus B3 reached fetal mice transplacentally. Modlin and Crumpacker reported that infection in late gestational mice was more severe than that occurring in early pregnancy and that transplacental infection of the fetus occurred transiently during the maternal infection. Flamm observed that coxsackievirus A9, when injected intravenously in rabbits, reached the blastocyst early in pregnancy and the amniotic fluid later in pregnancy. He also demonstrated congenital infection in mice with coxsackievirus A1.
Palmer and coworkers studied the gestational outcome in pregnant mice inoculated intravenously with the Theiler murine encephalomyelitis virus, a murine enterovirus. In early gestational infections, they found a high rate of placental and fetal abnormalities. The rates of fetal abnormalities and placental infection were greater than the rate of fetal viral infection, suggesting that the adverse effects of the viral infections were direct and indirect. Gestational infection could result in virus passage to the fetus and fetal damage or in placental compromise with indirect fetal damage.
In another study, using the same murine model with the Theiler murine encephalomyelitis virus, Abzug found that maternal factors (i.e., compromised uteroplacental blood flow, concomitant infection, and advanced age) increased the risk of transplacental fetal infection.
In humans, the transplacental passage of coxsackieviruses at term has been documented on several occasions. Benirschke studied the placentas in three cases of congenital coxsackievirus B disease and could find no histologic evidence of infection. In 1956, Kibrick and Benirschke reported the first case of intrauterine infection with coxsackievirus B3. In this instance, the infant was delivered by cesarean section and had clinical evidence of infection several hours after birth. Brightman and colleagues recovered coxsackievirus B5 from the placenta and rectum of a premature infant. No histologic abnormalities of the placenta were identified. Konstantinidon and associates described the transplacental infection with coxsackievirus B3, which they confirmed using molecular techniques. At fetal autopsy they found mild arthrogryposis, necrotic meningoencephalitis with vascular calcifications, interstitial pneumonitis, mild myocardial hypertrophy, and chronic monocytic placental villitis. Coxsackievirus RNA was detected in placental tissue of six babies who had severe respiratory failure and subsequent nervous system sequelae. Other evidence of intrauterine infection has been presented for coxsackieviruses A4 and B2 through B6. More recently, a number of life-threatening cases of coxsackievirus B1 were noted to have their onset during the first day of life, suggesting intrauterine infection.
Evidence for intrauterine infection during the first and second trimesters of pregnancy with coxsackieviruses is less clear. Burch and coworkers reported the results of immunofluorescent studies of two fetuses of 5 months of gestation and one fetus of 6 months of gestation; the 6-month-old fetus had evidence of coxsackievirus B4 myocarditis, one 5-month-old fetus showed signs of coxsackievirus B3 infection, and the other 5-month-old fetus showed evidence of coxsackievirus B2, B3, and B4 infections. Basso and associates recovered coxsackievirus B2 from the placenta, liver, and brain of a fetus after a spontaneous abortion at 3 months of gestation. Plager and coworkers found no evidence of intrauterine viral transmission of coxsackievirus B5 infections during the first and second trimesters of pregnancy.
Euscher and associates detected coxsackievirus RNA in placental tissue from six of seven newborn infants with respiratory difficulties and other manifestations at birth. Of these infants, one died shortly after birth, and the other six suffered neurodevelopmental delays. The placentas of 10 normal infants were examined for coxsackievirus RNA, and results of these studies were negative. Three of the placentas from the affected infants showed focal chronic villitis, two showed focal hemorrhagic endovasculitis, and one showed focal calcifications. In addition to respiratory distress, two neonates had rashes, two had seizures, two had thrombocytopenia, and one had intraventricular hemorrhage.
Less is known about transplacental passage of other enteroviruses than about that of coxsackieviruses and polioviruses. Women in all stages of pregnancy are frequently infected with echoviruses, and viremia is commonly seen in these infections. In particular, epidemic disease related to echovirus 9 has been studied epidemiologically and serologically. In these studies, a search for teratogenesis was performed, but no definitive virologic investigations have been carried out; asymptomatic transplacental infection might have occurred. Echoviruses 6, 7, 9, 11, 19, 27, 30, and 33 have been identified in cases of transplacentally acquired infections. Otonkoski and coworkers reported the occurrence of neonatal type 1 diabetes after a possible maternal echovirus 6 infection. Hughes and colleagues reported a newborn with echovirus 14 infection who had a markedly elevated level of IgM (190mg/dL) on the sixth day of life; it seems likely that this infant was also infected in utero.
Despite these reports, transplacental echoviruses infections seem to be rare: Cherry and colleagues cultured samples from 590 newborns during a period of enteroviral prevalence without isolating an echovirus. Antepartum serologic study of a group of 55 mothers in this study showed that 5 (9%) were actively infected with echovirus 17 during the 6-week period before delivery. In two other large nursery studies, there was no suggestion of intrauterine echovirus infections.
Newer, numbered enteroviruses have also been associated with transplacental infection. Chow and associates described a 1300-g fetus, which was stillborn after 26 weeks of gestation, with unilateral hydrocephalus, hepatosplenomegaly, fibrotic peritonitis, and meconium staining. Enterovirus 71 was isolated from the amniotic fluid, and the same virus was identified by polymerase chain reaction (PCR) assay in the cord blood and by immunohistochemical staining in the fetal midbrain and liver.
Berkovich and Smithwick described a newborn without clinical illness and who had specific IgM parechovirus 1 antibody in the cord blood, suggesting intrauterine infection with this virus. In three studies of human parechovirus illnesses in neonates, symptoms have been noted on the first and second days of life, suggesting ascending infection or contact infection during birth.
Definitive evidence is lacking for ascending infection or contact infection with enteroviruses during birth. In prospective studies of genital herpes simplex and cytomegaloviral infections, there have been no enteroviral isolations. These results suggest that ascending infections with enteroviruses, if they occur at all, are rare. However, Reyes and associates recovered coxsackievirus B5 from the cervix of four third-trimester pregnant women. Three of the four positive cultures were obtained 3 weeks or more before delivery. In the fourth case, the cervical culture was obtained the day before delivery, and the child was delivered by cesarean section. All of the infants were healthy, but unfortunately, culture for virus was possible only from the infant delivered by cesarean section; the result was negative. In an earlier study, Reyes and colleagues reported a child who died of a disseminated echovirus 11 infection. The illness had its onset on the third day of life, and the virus was recovered from the mother’s cervix at that time.
Enteroviral infection during the birth process seems probable. The fecal carriage rate of enteroviruses in asymptomatic adult patients varies between 0% and 6% or higher in different population groups. Cherry and associates found that in 2 (4%) of 55 mothers, enteroviruses were present in the feces shortly after delivery. Katz, in a discussion of a child with neonatal coxsackievirus B4 infection, suggested that the infant might have inhaled maternally excreted organisms during birth. The fact that this child had pneumonia tends to support the contention. Infections occurring 2 to 7 days after birth could have been acquired during passage through the birth canal.
Neonatal infections and illnesses from enteroviruses are relatively common. Transmission of enteroviruses to newborns is similar to that for populations of older people. The main factor in the spread of virus is human-to-human contact. During the summer and fall of 1981 in Rochester, New York, 666 neonates were cultured for enteroviruses within 24 hours of birth and then weekly for 1 month. The incidence of acquisition of nonpolio enteroviral infections during this period was 12.8%. Two risk factors were identified: lower socioeconomic status and lack of breastfeeding.
Clinical poliomyelitis is rare in neonates, but the infection rate before the vaccine era was never determined. It is probable that the rarity of neonatal poliomyelitis was not related to lack of viral transmission but reflected the protection against clinically evident disease offered by specific, transplacentally transmitted antibodies directed against poliovirus. From experience gained in vaccine studies, it is apparent that infants with passively acquired antibody can be regularly infected.
In 1955, Bates reviewed the literature on poliomyelitis in infants younger than 1 month. He described six infants who apparently were not infected by their mothers and who had had other likely contacts. A neighbor was the contact in one case, siblings in two cases, nursery nurses in two cases, and an uncle in the sixth case. In most other infants, the mother had had poliomyelitis shortly before the child was born and probably was the contact. The mode of transmission—intrauterine, during birth, or postnatal contact—is unknown.
Bergeisen and colleagues reported a case of paralytic poliomyelitis from a type 3 vaccine viral strain. They suggested that the source of this virus might have been the child of the neonate’s babysitter, who was vaccinated about 2 weeks before the onset of the illness.
Several epidemics with coxsackieviruses B in newborn nurseries have been studied. Brightman and coworkers observed an epidemic of coxsackievirus B5 in a premature nursery. Their data suggested that the virus was introduced into this nursery by an infant with a clinically inapparent infection who had been infected in utero. Secondary infections occurred in 12 infants and two nurses. The timing of the secondary cases suggested that three generations of infection had occurred and that the nurses had been infected during the second generation. The investigators suggested that the infection had spread from infant to infant and from infant to nurse.
Javett and colleagues documented an acute epidemic of myocarditis associated with coxsackievirus B3 infection in a Johannesburg maternity home. Unfortunately, no epidemiologic investigation or search for asymptomatic infected infants was performed. However, analysis of the onset dates of the illnesses indicated that single infections occurred for five generations, and then five children became ill within a 3-day period.
Kipps and colleagues carried out epidemiologic investigations in two coxsackievirus B3 nursery epidemics. In the first epidemic, the initial infection was probably transmitted from a mother to her child; this infant was then the source of five secondary cases in newborns and one illness in a nurse. Infants with four of the five secondary cases were located on one side of the nursery, but only one cot was close to the cot of the index patient, and this cot did not adjoin the cots of the three other infants with contact cases. In the second outbreak, an infant who also was infected by his mother probably introduced the virus into the nursery. Infants with the three secondary cases were geographically far removed from the one with the primary case of infection.
There have been many other instances of isolated nursery infections and small outbreaks with coxsackieviruses, and it seems that the most consistent source of original nursery infection is transmission from a mother to her child, but introduction of virus into the nursery by personnel also occurs.
Although many outbreaks of echovirus infections have been observed in newborn nurseries, information on viral transmission is incomplete. Cramblett and coworkers reported an outbreak of echovirus 11 disease in four infants in an intensive care nursery. All infants were in enclosed incubators, and three patients became ill within 24 hours; the fourth child became ill 4 days later. Echovirus 11 was recovered from two members of the nursery staff. These data suggest that transmission from personnel to infants occurred because of inadequate hand washing. In another outbreak in an intensive care unit (ICU), the initial patient was transferred to the nursery because of severe echovirus 11 disease. After transfer, infection occurred in the senior house officer and a psychologist in the unit. It was inferred by the investigators that spread by respiratory droplets to nine other infants occurred from these infected personnel.
In a maternity unit outbreak of echovirus 11 involving six secondary cases, infection spread through close contact between the infected newborns and the nurses. In another reported nosocomial echovirus 11 outbreak, infants in an intermediate care unit for more than 2 days were more likely to become infected than those who were there for less than 2 days. Illness was also associated with gavage feeding, mouth care, and being a twin.
Modlin reviewed reports of 16 nursery echovirus outbreaks involving 206 ill infants. In only 4 of the 16 outbreaks was the source identified, and in all 4, the primary case was an infant who acquired infection vertically from its mother. After introduction of an infected newborn into a nursery, spread to other infants by personnel is common. Risk factors for nursery transmission as described by Rabkin and coworkers were “lower gestational age or birth weight, antibiotic or transfusion therapy, nasogastric intubation or feeding, proximity in the nursery to the index patient, and care by the same nurse during the same shift as the index patient.”
Wilson and associates reported an intensive care nursery epidemic in which respiratory syncytial virus and echovirus 7 infections occurred concurrently. This epidemic persisted from January to June 1984 despite an aggressive isolation cohorting program. A major factor in persistence was asymptomatic infections with both viruses.
Sato and associates reported a point-source outbreak of echovirus 33 infection in nine newborns related to one nursery over a 10-day period. The primary case was born to a mother who was febrile and who had a high echovirus 33 neutralizing antibody titer in a convalescent-phase serum specimen.
Jack and colleagues observed the endemic occurrence of asymptomatic infection with parechovirus 1 in a nursery during an 8-month period. A total of 44 infants were infected during this time, and nursery infection occurred when there was no known activity of parechovirus 1 in the community at large. The investigators believed that the endemic viral infection was spread by fecal contamination of hands of nursery personnel.
Nakao and colleagues and Berkovich and Pangan also documented parechovirus 1 infections in nurseries. Like Jack and colleagues, they observed that the infections seemed to be endemic to the nurseries rather than related to community epidemics. More recently in July 2009, an outbreak of human parechovirus type 1 occurred in a neonatal unit in Croatia. This involved seven neonates with respiratory and/or gastrointestinal symptoms. The source of this outbreak was not discussed.
Congenital infections with enteroviruses result from transplacental passage of virus to fetus. The method of transport from mother to fetus is poorly understood. Maternal viremia during enteroviral infections is common, and because virus has been recovered from the placenta on several occasions, it is probable that active infection of the placenta also occurs. Benirschke found no histologic evidence of placental disease in three cases of established transplacentally acquired coxsackievirus B infections. Batcup and associates found diffuse perivillous fibrin deposition with villous necrosis and inflammatory cell infiltration of the placenta in a woman who 2 weeks earlier, at 33 weeks of gestation, had coxsackievirus A9 meningitis. The woman was delivered of a macerated, stillborn infant. At birth, virus was recovered from the placenta but not from the stillborn infant.
It is assumed that infection in the fetus results from hematogenous dissemination initiated in the involved placenta. It is also possible that some in utero infection results from the ingestion of virus contained in amniotic fluid; in this situation, primary fetal infection involves the pharynx and lower alimentary tract. The portal of entry of infection during the birth process and the neonatal period is similar to that for older children and adults.
Figure 25-4 shows a schematic diagram of the events of pathogenesis. After initial acquisition of virus by the oral or respiratory route, implantation occurs in the pharynx and the lower alimentary tract. Within 1 day, the infection extends to the regional lymph nodes. On about the third day, minor viremia occurs, resulting in involvement of many secondary infection sites. In congenital infections, infection is initiated during the minor viremia phase. Multiplication of virus in secondary sites coincides with the onset of clinical symptoms. Illness can vary from minor to fatal infections. Major viremia occurs during the period of multiplication of virus in the secondary infection sites; this period usually lasts from the third to the seventh days of infection. In many echovirus and coxsackievirus infections, CNS involvement apparently occurs at the same time as other secondary organ involvement. This occasionally appears to happen with polioviral infections; however, more commonly, the CNS symptoms of poliomyelitis are delayed, suggesting that seeding occurred later in association with the major viremia.

Cessation of viremia correlates with the appearance of serum antibody. The viral concentration in secondary infection sites begins to diminish on about the seventh day. However, infection continues in the lower intestinal tract for prolonged periods.
The pathogenesis and pathology of enterovirus and parechovirus infections depend on the virulence, tropism, and inoculum concentration of virus, as well as on many specific host factors. Enteroviruses have marked differences in tropism and virulence. Although some generalizations can be made in regard to tropism, there are marked differences even among strains of specific viral types. Differences in virulence of specific enteroviral types may be the result of recombination among enteroviruses or point mutations.
Enterovirus infections of the fetus and neonate are thought to be more severe than similar infections in older individuals. This is undoubtedly true for coxsackievirus B infections and probably also true for coxsackievirus A, echovirus, and poliovirus infections. Although the reasons for this increased severity are largely unknown, several aspects of neonatal immune mechanisms offer clues. The similarity of coxsackievirus B infections in suckling mice to those in human neonates has provided a useful animal model. Heineberg and coworkers compared coxsackievirus B1 infections in 24-hour-old suckling mice with similar infections in older mice. They observed that adult mice produced interferon (IFN) in all infected tissues, whereas in suckling mice, only small amounts of IFN were identified in the liver. They thought that the difference in outcome of coxsackievirus B1 infections in suckling and older mice could be explained by the inability of the cells of the immature animal to elaborate IFN. Additional studies of abnormalities of innate immunity in neonates may enhance our understanding of the severity of enterovirus infections in newborns.
Others thought that the increased susceptibility of suckling mice to severe coxsackievirus infections was related to the transplacentally acquired, increased concentrations of adrenocortical hormones. Kunin suggested that the difference in age-specific susceptibility might be explained at the cellular level. He showed that a variety of tissues of newborn mice bound coxsackievirus B3, whereas tissues of adult mice were virtually inactive in this regard. It has been suggested that the progressive loss of receptor-containing cells or of receptor sites on persisting cells with increasing age might be the mechanism that accounts for infections of lesser severity in older animals. Supporting this suggestion, Ito and colleagues showed that expression of the coxsackie and adenovirus receptor (CAR) (see Table 25-3 ) decreases as rats age. Teisner and Haahr suggested that the increased susceptibility of suckling mice to severe and fatal coxsackievirus infections might be from physiologic hypothermia and poikilothermia during the first week of life.
In the past, it was assumed that specific pathology in various organs and tissues in enteroviral infections was caused by the direct cytopathic effect and tropism of a particular virus. However, a large number of studies using murine myocarditis model systems have suggested that host immune responses contribute to the pathology. These studies suggest that T-cell–mediated processes and virus-induced autoimmunity cause acute and chronic tissue damage. Other studies suggest that the primary viral cytopathic effect is responsible for tissue damage and that various T-cell responses are a response to the damage, not the cause. A review of various murine myocarditis model systems suggests that the genetics of the hosts and of the viral strains determine the likelihood of autoimmune, cell-mediated cellular damage. †
† References .
However, none of the model systems is appropriate for the evaluation of the pathogenesis of neonatal myocarditis. Although available studies suggest that enterovirus-induced myocarditis in older children and adults occasionally may have a delayed cell-mediated component, the short incubation period and fulminant nature of neonatal disease, as well as the similar infection in suckling mice, suggest that autoimmune factors are not major in the pathogenesis of acute enteroviral myocarditis in neonates.
During the last 45 years, the clinical manifestations caused by several enteroviral serotypes have changed. For example, echovirus 11 infection initially was noted in association with an outbreak of upper respiratory infection in a day nursery more than 50 years ago. Then in the 1960s, it was found to be related to exanthem and aseptic meningitis. After this, and occurring presently, is the association of echovirus infection and severe sepsis-like illnesses with hepatitis in neonates. ‡
‡ References .
Another example relates to enterovirus 71 infections. Initially, this virus was noted in association with aseptic meningitis, with only a small number of cases also having exanthem. During the last several decades, severe epidemic disease with enterovirus 71 has occurred in Taiwan, Singapore, Australia, Malaysia, Japan, and other countries in Southeast Asia. In these epidemics, hand-foot-and-mouth syndrome is a major finding, and the neurologic disease is more severe than in the past.
These phenotypic changes could be the result of point mutations or the result of recombination among enteroviruses. §
§ Reference .
Chan and AbuBakar have presented evidence indicating that a recombination event occurred between enterovirus 71 and coxsackievirus A16.
Great variations in the clinical signs of congenital and neonatal enterovirus infections are paralleled by wide variations in pathology. Because pathologic material usually is available only from patients with fatal illnesses, the discussion in this section considers only the more severe enteroviral manifestations. It is worth emphasizing, however, that these fatal infections account for only a small portion of all congenital and neonatal enterovirus infections. The pathologic findings in infants with milder infections, such as nonspecific febrile illness, have not been described.
The pathologic findings in fatal neonatal poliomyelitis are similar to those seen in disease of older children and adults. The major findings have involved the CNS, specifically the anterior horns of the spinal cord and the motor nuclei of the cranial nerves. Involvement is usually irregularly distributed and asymmetric. Microscopically, the anterior horn cells show neuronal destruction; gliosis; and perivascular, small, round cell infiltration. Myocarditis has also been observed, characterized by focal necrosis of muscle fibers and various degrees of cellular infiltration.
Records of neonatal illnesses associated with coxsackieviruses A are rare. Gold and coworkers, in a study of sudden unexpected death in infants, recovered coxsackievirus A4 from the brains of three children. Histologic abnormalities were not identified in the brains or spinal cords of these patients. Baker and Phillips reported the death of twins in association with coxsackievirus A3 intrauterine infections; the first twin was stillborn, and the second twin died when 2 days old of viral pneumonia.
Eisenhut and associates described a full-term neonate with coxsackievirus A9 infection with meningitis, myocarditis, and disseminated intravascular coagulation who died on the seventh day of life.
Of the enteroviruses, coxsackieviruses B have been most frequently associated with severe and catastrophic neonatal disease. The most common findings in these cases have been myocarditis or meningoencephalitis, or both. Involvement of the adrenals, pancreas, liver, and lungs has occurred.
The meninges are congested, edematous, and occasionally mildly infiltrated with inflammatory cells. щ
? References .
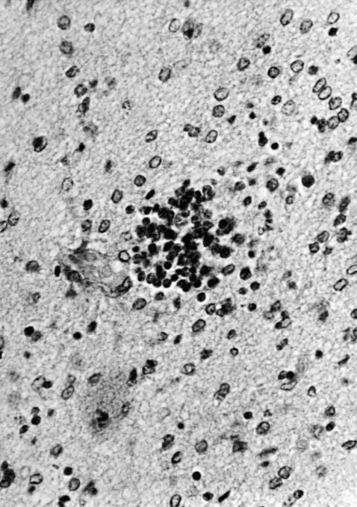
Lesions in the brain and spinal cord are focal rather than diffuse but frequently involve many different areas. The lesions consist of areas of eosinophilic degeneration of cortical cells, clusters of mononuclear and glial cells ( Fig. 25-5 ), and perivascular cuffing. On occasion, areas of liquefaction necrosis unassociated with inflammation are seen.
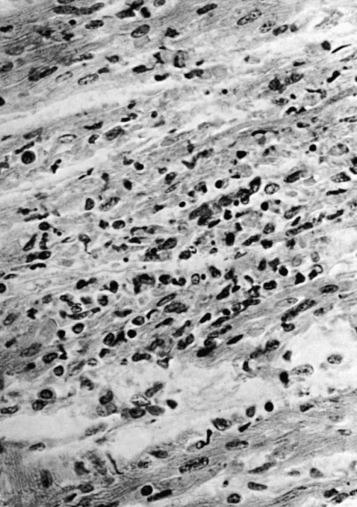
Grossly, the heart is usually enlarged, with dilation of the chambers and flabby musculature. ¶
¶ References .
Microscopically, the pericardium frequently contains some inflammatory cells; and thickening, edema, and focal infiltrations of inflammatory cells may be found in the endocardium. The myocardium ( Fig. 25-6 ) is congested and contains infiltrations of inflammatory cells (i.e., lymphocytes, mononuclear cells, reticulum cells, histiocytes, plasma cells, and polymorphonuclear and eosinophil leukocytes). Involvement of the myocardium is often patchy and focal but occasionally is diffuse. The muscle shows loss of striation, edema, and eosinophilic degeneration. Muscle necrosis without extensive cellular infiltration is common.
The lungs commonly have areas of mild focal pneumonitis with peribronchiolar mononuclear cellular infiltrations. Massive pulmonary hemorrhage has been observed. The liver is frequently engorged and occasionally contains isolated foci of liver cell necrosis and mononuclear cell infiltrations. A neonate with a coxsackievirus B1 infection developed a sepsis-like illness on the fourth day of life with severe hepatitis and subsequently developed progressive liver calcifications. In the pancreas, infiltration of mononuclear cells, lymphocytes, and plasma cells has been observed, and occasional focal degeneration of the islet cells occurs. Congestion has been observed in the adrenal glands, with mild-to-severe cortical necrosis and infiltration of inflammatory cells.
In an earlier period, although frequently responsible for neonatal illnesses, echoviruses were rarely associated with fatal infections. During the past 40 years, however, there have been many reports of fatal illnesses in newborns from echovirus type 11. #
# References .
In virtually all cases, the major pathologic finding was massive hepatic necrosis; other findings included hemorrhagic necrosis of the adrenal glands, hemorrhage in other organs, myocardial necrosis, and acute tubular necrosis of the kidneys. Wang and colleagues studied four neonates (three with echovirus 11 and one with echovirus 5 infections) with fulminant hepatic failure and observed two histopathologic patterns associated with minimal inflammation but extensive hemorrhagic necrosis. One pattern indicated ongoing endothelial injury with endotheliitis and fibrinoid necrosis. The second pattern, which was seen in the two neonates who initially survived, was that of venoocclusive disease. Virus has not been identified in hepatocytes. Extensive myositis of the strap muscles of the neck occurred in one case. Massive hepatic necrosis has also occurred in infections with echoviruses 3, 5, 6, 7, 9, 14, 19, 20, and 21. ∗a
∗a References .
Wreghitt and associates described a neonate with a fatal echovirus 7 infection. This infant was found to have massive disseminated intravascular coagulation, with bleeding in the adrenal glands, renal medulla, liver, and cerebellum.
At autopsy, one infant with echovirus 6 infection was found to have cloudy and thickened leptomeninges, liver necrosis, adrenal and renal hemorrhage, and mild interstitial pneumonitis. One infant with echovirus 9 infection had an enlarged and congested liver with marked central necrosis, and another with this virus had interstitial pneumonitis without liver involvement. Three infants with echovirus 11 infections had renal and adrenal hemorrhage and small-vessel thrombi in the renal medulla and in the medulla and the inner cortex of the adrenal glands. In these patients, the livers were normal. Two infants, one with echovirus 6 and the other with echovirus 31 infection, had only extensive pneumonias. Willems and colleagues described an infant with echovirus 11 infection who had pneumonia, persistent pulmonary hypertension, and purpura fulminans.
Neonatal parechovirus type 3 infections often involve the CNS, and extensive white matter injury has been repeatedly noted.
In this section, we present clinical manifestations by the specific viral agents and also by serotypes of the specific viruses. However, in recent years, a large number of enteroviral and parechoviral infections are now diagnosed by PCR assay, and serotype information is not always available. Because of this, much of the specific information in this section was determined more than 2 decades ago. In recent years, phenotypic presentations have been altered by recombination between different enteroviruses, and therefore clinical characteristics of specific enteroviral types today may be different from the findings identified 4 and 5 decades ago.
Poliomyelitis is associated with an increased incidence of abortion. Horn reported 43 abortions in 325 pregnancies complicated by maternal poliomyelitis. Abortion was directly related to the severity of the maternal illness, including the degree of fever during the acute phase of illness. However, abortion also was associated with mild, nonparalytic poliomyelitis. Schaeffer and colleagues studied the placenta and abortus 12 days after the onset of illness in a mother. Poliovirus type 1 was isolated from the placenta and the fetal tissues.
Other investigators have reported an increased incidence of abortions in cases of maternal poliomyelitis. Siegel and Greenberg noticed that fetal death occurred in 14 (46.7%) of 30 instances of maternal poliomyelitis during the first trimester. Kaye and colleagues reviewed the literature in 1953 and found 19 abortions in 101 cases of poliomyelitis in pregnancy. In a small study in Evanston Hospital in Illinois, the abortion rate associated with maternal poliomyelitis was little different from the expected rate. In a study of 310 pregnant women who received trivalent OPV, there was no increase in abortions above the expected rate. In a later study in Finland that involved about 9000 pregnant women immunized with OPV, there was no evidence of an increase in stillbirths.
Although in the late 1950s and early 1960s there were extensive outbreaks of illness caused by coxsackievirus A16, there was no evidence of adverse outcomes of pregnancy related to this virus. Because infections with other coxsackieviruses rarely involve large segments of the population, rate studies have not been performed.
Frisk and Diderholm found that 33% of women with abortions had IgM antibody to coxsackieviruses B, whereas only 8% of controls had similar antibody. In a second, larger study, the same research group confirmed their original findings.
There is no available evidence suggesting that echovirus infections during pregnancy are a cause of spontaneous abortion. Landsman and associates studied 2631 pregnancies during an epidemic of echovirus 9 and could find no difference in antibody to echovirus 9 between mothers who aborted and those who delivered term infants. A similar study in Finland revealed no increase in the abortion rate among mothers infected in early pregnancy with echovirus 9.
Ljungan virus (a parechovirus) is endemic in some rodent populations, and it can cause fetal death in these animals. It has been suggested that this virus may be the cause of some intrauterine deaths in humans. In one study, both PCR assay and immunohistochemical staining identified Ljungan virus in specimens from five human intrauterine fetal deaths.
In the study by Horn of 325 pregnancies, 9 infants died in utero. In each instance, the mother was critically ill with poliomyelitis. Horn also observed that 45 infants weighed less than 6 pounds, and 17 of these had a birth weight of less than 5 pounds. These low-birth-weight infants were born predominantly to mothers who had had poliomyelitis early in pregnancy. A similar finding was reported by Aycock. In New York City, Siegel and Greenberg also documented an increase in prematurity after maternal poliomyelitis infection. This was specifically related to maternal paralytic poliomyelitis. There has been no observation of stillbirth or prematurity in relation to vaccine administration.
Bates reported a fetus of 8 months of gestational age who was stillborn and had calcific pancarditis and hydrops fetalis at autopsy. Fluorescent antibody study revealed coxsackievirus B3 antigen in the myocardium. Burch and colleagues described three stillborn infants who had fluorescent antibody evidence of coxsackievirus B myocarditis, one each with coxsackieviruses B2, B3, or B4. They also reported a premature boy who had histologic and immunofluorescent evidence of cardiac infection with coxsackieviruses B2 through B4; he lived only 24 hours. A macerated stillborn girl was delivered 2 weeks after the occurrence of aseptic meningitis caused by coxsackievirus A9 in a 27-year-old woman. Virus was recovered from the placenta but not from the infant. Coxsackievirus B6 has been recovered from the brain, liver, and placenta of a stillborn infant. A baby of 26 weeks of gestation with nonimmune hydrops fetalis with an intrauterine infection with coxsackievirus B3 was reported by Ouellet and coworkers.
Freedman reported the occurrence of a full-term, fresh stillbirth in a woman infected with echovirus 11. Because the infant had no pathologic or virologic evidence of infection, he attributed the event to a secondary consequence of maternal infection from fever and dehydration rather than primary transplacental infection. Echovirus 27 has been associated with intrauterine death on two occasions.
In an extensive study of neonatal enteroviral infections in Milwaukee in 1979, Piraino and associates found that 12 of 19 stillbirths occurred from July through October, coincident with a major outbreak of enterovirus disease. Echovirus 11 was the main agent isolated during this period. A 1300-g fetus, stillborn after 26 weeks of gestation, had hydrocephalus, fibrotic peritonitis, and hepatosplenomegaly and was found to have an enterovirus 71 infection by PCR assay and immunohistochemical study.
The congenital malformation rate associated with poliovirus infection, as determined in the National Institutes of Health (NIH)-sponsored Collaborative Perinatal Research Project of 45,000 pregnancies, was 4.1%. Although isolated instances of congenital malformation and maternal poliomyelitis have been reported, there is little statistical evidence demonstrating that polioviruses are teratogens. In their review of the literature, Kaye and colleagues identified six anomalies in 101 infants born to mothers with poliomyelitis during pregnancy. In the reviews of Horn, Bates, and Siegel and Greenberg, there was no evidence of maternal poliovirus infection–induced anomalies.
The possibility of congenital anomalies associated with attenuated OPV has also been studied. Pearson and coworkers studied the fetal malformation rate in a community in which a large vaccine field trial had been carried out; although it is probable that pregnant women became infected with vaccine virus by secondary spread, there was no community increase in fetal malformations. Prem and associates studied the infants of 69 women who received attenuated vaccine before 20 weeks of gestation and found that none had anomalies. In contrast, the rate of congenital defects in Blackburn, England, increased coincident with mass vaccination with trivalent poliomyelitis vaccine. However, there is no evidence of cause and effect related to this observation. Connelly and colleagues commented on a child with a unique renal disease acquired in utero. The child’s mother had received OPV during the second month of pregnancy.
In February 1985, a mass vaccination program with live OPV was carried out in Finland. Although pregnant women received vaccine, there was no evidence that vaccine virus had a harmful effect on developing fetuses.
In a large prospective study, Brown, Evans and Brown, Brown and Evans, and Brown and Karunas made a serologic search for selected maternal enteroviral infections in association with congenital malformations. In one study, serum samples from 22,935 women had been collected. From this group, serum samples from 630 mothers of infants with anomalies and from 1164 mothers of children without defects were carefully studied. Specifically, serologic evidence was sought for infection during the first trimester and last 6 months of pregnancy with coxsackieviruses B1 through B5 and A9 and with echoviruses 6 and 9. In this study, infants were examined for 113 specific abnormalities; these anomalies were grouped into 12 categories for analysis. The investigators demonstrated a positive correlation between maternal infection and infant anomaly with coxsackieviruses B2 through B4 and A9. The overall first-trimester infection rate with coxsackievirus B4 was significantly higher in patients with anomalies than that in control subjects. Maternal coxsackievirus B2 infection throughout pregnancy, coxsackievirus B4 infections during the first trimester of pregnancy, and infection with at least one of the five coxsackieviruses B during pregnancy were all associated with urogenital anomalies. Coxsackievirus A9 infection was associated with digestive anomalies, and coxsackieviruses B3 and B4 were associated with cardiovascular defects. When coxsackieviruses B were analyzed as a group (B1-B5), there was an overall association with congenital heart disease; the likelihood of cardiovascular anomalies was increased when maternal infection with two or more coxsackieviruses B occurred. In this study, the mothers had been instructed to keep illness diary sheets. There was no correlation between reported maternal clinical illnesses and serologic evidence of infection with the selected enteroviruses. This suggests that many infections that may have been causally related to the anomalies were asymptomatic. A disturbing finding in this study was the lack of seasonal occurrence of the births of children with specific defects. Because enteroviral transmission is most common in the summer and fall, the birth rate of children with malformations should have been greatest in the spring and summer if coxsackieviruses were a major cause of malformation.
In the NIH-sponsored Collaborative Perinatal Research Project, Elizan and coworkers were unable to find any relationships between maternal infections with coxsackieviruses B and congenital CNS malformations. Scattered case reports in the literature describe congenital anomalies associated with maternal coxsackievirus infections. Makower and colleagues reported a child with congenital malformations who was born at 32 weeks of gestation and from whom a coxsackievirus A4 strain was recovered from the meconium. The child’s mother had been well throughout pregnancy, except for a febrile illness during the first month. The relationship of the viral infection to the congenital malformations or to the prematurity is uncertain.
Gauntt and associates studied the ventricular fluids from 28 newborn infants with severe congenital anatomic defects of the CNS. In four infants (two with hydranencephaly, one with an occipital meningocele, and one with aqueductal stenosis), neutralizing antibody to one or more coxsackievirus B types was found in the fluid. In one case, IgM-neutralizing antibody to coxsackievirus B6 was found. The investigators concluded that their data suggested the possibility of an association between congenital infections with coxsackieviruses B and severe CNS defects.
In the large prospective study of Brown and Karunas, the possible association of maternal infections with echoviruses 6 and 9 and congenital malformations was examined. Maternal infection with these selected echoviruses apparently was not associated with any anomaly. In three other studies, no association was found between maternal echovirus 9 infection and congenital malformation.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here