Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Despite improvements in surgical techniques, biomechanical constructs, and implants, a proportion of patients still experience the sequelae (chronic shoulder pain and dysfunction) of a nonhealed rotator cuff. Tendon healing typically comprises four components: progenitor cells, growth factors, scaffold, and vascular supply. Unfortunately, tendons have a poor ability to regenerate owing in part to their poor vascular supply. The rotator cuff tendon has limited intrinsic ability to reform all zones of the tendon-to-bone insertion site, as demonstrated by a number of animal studies investigating healing at the tendon-to-bone interface. The zonal insertion site, or enthesis, progresses from tendon to unmineralized fibrocartilage, mineralized fibrocartilage, and bone. Instead, fibrovascular scar tissue rich in type III collagen is produced at the site of healing that is biomechanically weaker than the native structure. It is believed that this weaker product renders repairs prone to subsequent failure.
Scarring results in impaired motion, loss of joint function, and poor quality of life. Improving rotator cuff repair requires augmentation of the repair through progenitor cells, growth factors, or scaffolds to ideally limit scar formation and improve tendon-to-bone healing. Various biologic strategies have been employed to improve tendon regeneration and limit scar tissue formation at the repair site; however, no ideal augmentation strategy exists. Rotator cuff repairs have been augmented using various types of biologics: platelet-rich plasma (PRP), growth factors, bone marrow and stem cells, growth factors, and scaffolds. This chapter will give an overview of the various existing types of biologics and explain their function and use in rotator cuff surgery.
PRP is a collection of autologous blood with a physiologically greater concentration of platelets. Platelets are originally derived from megakaryocytes, lack a nucleus, and circulate for approximately 7 days, serving hemostatic and coagulation functions. Platelets have various secretory vesicles (e.g., α-granules, dense granules) containing nearly 1500 different protein factors, including growth factors, peptide hormones, and chemoattractants. Activation of platelets results in exocytosis and degranulation of the secretory vesicles with an initial burst release of growth factors (GF) followed by production and sustained release. Growth factors in PRP are known to augment the healing process by promoting angiogenesis, thus allowing for an influx of blood supply and nutrients to the repair site. This influx of nutrients stimulates cellular repair and regeneration, and the efflux clears out cellular debris.
PRP has been investigated for its application in bone, cartilage, and ligament regeneration and provides supraphysiologic concentrations of platelets, which in turn serve as a storehouse of GF. Some of these growth factors include transforming growth factor-β1 (TGF-β1), platelet-derived growth factor (PDGF), vascular endothelial growth factor (VEGF), hepatocyte growth factor (HGF), insulin-like growth factor 1 (IGF-1), fibroblast growth factor (FGF), and epidermal growth factor. The release of these growth factors has been reported to promote bone and soft tissue healing.
Reports of PRP date back to the 1980s with Ferrari et al. using PRP for intraoperative blood salvage during cardiothoracic surgery. Marx et al. described a number of applications of PRP in oral maxillofacial surgeries. PRP is now being used in a number of different fields to biologically augment tendon repairs, ligament regeneration, wound healing, and sports medicine. PRP contains an abundance of growth factors, which act to stimulate tissue regeneration, cell proliferation, and angiogenesis.
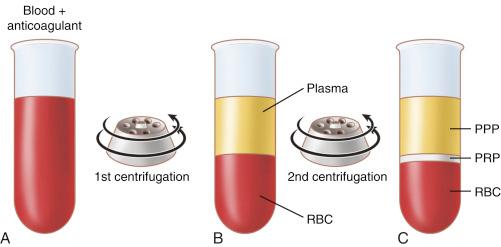
PRP can be generated via a number of different commercially available systems using anticoagulated blood and typically contain about three to five times as many growth factors as baseline values. There is a dose-dependent mitogenic effect of PRP and a platelet concentration between 200 × 10 3 platelets/μL and 1000 × 10 3 platelets/μL is considered therapeutic, whereas higher concentrations do not provide further biological advantage. PRP can be generated with centrifugation of whole blood following citrate addition, which prevents activation of the clotting cascade by binding ionized calcium. Centrifugation allows for the removal of red and white blood cells, as well as platelet-poor plasma (PPP). The clotting cascade is then initiated using various commercially available products, resulting in platelet activation, exocytosis, and degranulation of the secretory granules, releasing a milieu of growth factors.
There are numerous commercially available devices used to produce PRP ( Table 24.1 ). These devices vary in their method of separation (plasma-based or buffy coat systems) ( Fig. 24.1 ), as well as one-step or two-step separation, the centrifugation time, the centrifugation speed, and other variants in processing, which result in different concentrations of PRP. Furthermore, there is a high variance of white blood cells between the different PRP products, which has been widely discussed. White blood cells are considered to enhance an inflammatory process, and this may have a negative effect on tissue healing. Because of this characteristic, some investigators have considered not using PRP products with a higher concentration of white blood cells. However, there are studies that show a positive effect of white blood cells owing to antibacterial and immunological resistance and a higher release of growth factors. Therefore the concentration of white blood cells should be tailored specifically to the PRP application.
| Plasma-Based Systems | Buffy Coat System |
|---|---|
| ACP (Arthrex Inc.) | Accelerate (Exactech) |
| Cascade (MTF Sports Medicine) | Angel System (Arthrex Inc.) |
| Endoret (BTI Biotechnology Institute) | Arteriocyte (Magellan) |
| PlasmaPrep (STI Separation Technology Inc.) | GPS III (Biomet) |
| Harvest PRP (Terumo BCT) | |
| PEAK (Mitek Sports Medicine) |
PRP is progressively being used to supplement rotator cuff repairs with the purpose of improving tendon-to-bone healing. Numerous studies have demonstrated a positive effect with the use of PRP on tendon healing. Randelli et al. conducted a prospective, randomized, controlled, double-blind study with 53 patients undergoing arthroscopic repair of a complete rotator cuff tear, with one group augmented with autologous PRP and thrombin using a buffy coat system. The results demonstrated improved early healing, better functional recovery after rotator cuff repair, and improved postoperative pain (lower visual analogue scale [VAS] score) in the PRP group. Significant differences in clinical outcomes were noted at the 3-month follow-up in the PRP group. Barber et al. assessed the effects of platelet-rich plasma fibrin matrix (PRPFM) on tendon healing in a comparative study and demonstrated lower retear rates on the basis of magnetic resonance imaging (MRI) with the use of PRPFM. Jo CH et al. conducted a randomized clinical trial with 74 patients undergoing arthroscopic repair of medium to large rotator cuff tears and demonstrated decreased retear rates and increased cross-sectional area of the supraspinatus in the PRP group (using a plasma-based system); however, the speed of healing was not improved. In another randomized controlled trial, Jo CH et al. showed significantly lower retear rates as assessed on the basis of MRI or computed tomography (CT) at 9 months (primary outcome) and increased cross-sectional area of the supraspinatus in the PRP-augmented group (20%) (using a plasma-based system) compared with the conventional treatment group (55.6%). No significant differences between the groups were observed in terms of pain, range of motion, strength, satisfaction, and functional scores.
Despite various studies highlighting the beneficial effects of PRP, many studies demonstrate no significant benefit of PRP for tendon healing. In a level I randomized controlled trial by Castricini et al., 88 patients were investigated to assess the efficacy of PRPFM augmentation during rotator cuff repair for small and medium-sized rotator cuff tears using a plasma-based system. No significant differences were noted in total Constant score or MRI tendon score when comparing the two groups with and without the PRPFM. In another randomized controlled trial, by Rodeo et al., investigating the effect of PRPFM (using a plasma-based system) on tendon healing, no demonstrable effect on tendon healing, tendon vascularity, muscle strength, or clinical scores (American Shoulder and Elbow Surgeons Standardized Shoulder Assessment [ASES] and L’Insalata Shoulder Questionnaire scores) was noted. Weber et al. found no significant improvement in perioperative morbidity, clinical outcomes, VAS scores, narcotic use, recovery of motion, Simple Shoulder Test and ASES scores, or structural integrity in a 1-year follow-up of patients undergoing PRPFM augmentation in rotator cuff surgery. Bergeson et al. reported a statistically significant higher retear rate in the PRPFM group versus control subjects and no significant improvement in functional scores postoperatively in the PRPFM group. Vavken et al. conducted a meta-analysis to analyze the cost-effectiveness of PRP and concluded that even though PRP may promote healing of small and medium-sized tears to reduce retear rates, it is not cost-effective.
Good-quality studies exist as evidence for and against the use of PRP to augment rotator cuff repair. Controversy over the use of PRP is ongoing owing to the conflicting data. There is a lack of consistency with the use of PRP; however, this may be explained by the fact that there are many different forms of PRP used, different techniques for application, and wide patient variability.
Using animal models, it is understood that rotator cuff healing occurs in three phases. First, in the inflammatory phase, cytokine-mediated signaling directs macrophages to the site of injury via IGF-1, PDGF, and TGF-β. Macrophages then secrete TGF-β1, commencing the repair phase, which is characterized by fibroblast proliferation and deposition of type III collagen. Finally, the remodeling phase takes place over years and is marked by contraction of the fibrovascular scar tissue mediated by matrix metalloproteinases.
With this understanding in mind, researchers have continuously searched for ways to manipulate the healing process to counteract the results of scar tissue formation. Efforts have focused on strengthening the repair with stronger sutures and other surgical techniques, but failure rates remain high. This has prompted a closer look at the biologics of rotator cuff repair. Specifically, there is great interest in the use of biologic agents that limit scar tissue formation and promote normal tendon-to-bone composition.
Growth factors play important roles in cell chemotaxis, cell proliferation, extracellular matrix (ECM) synthesis, and cell differentiation. Studies have identified an upregulation of specific growth factors in an animal model of rotator cuff injury. These growth factors include basic fibroblast growth factor (bFGF), IGF-1, bone morphogenetic protein 12 (BMP-12), BMP-13, BMP-14, cartilage oligomeric matrix protein, connective tissue growth factor, PDGF-B, and TGF-β1. It is believed that manipulating expression or delivering these growth factors exogenously over the course of healing can promote normal rotator cuff composition.
The goal of using growth factors in the augmentation of rotator cuff repair is to recreate the native tendon-to-bone insertion site that has similar functionality and strength to the original structure. However, challenges remain in developing such a therapy. These include identifying which growth factor or growth factors produce the desired effects, determining the optimal concentration and timing for introducing factors, and last, the method of delivery ( Table 24.2 ).
| Growth Factor | Most Representative Function |
|---|---|
| BMP | Mediates bone formation. Promotes tendon and cartilage formation. |
| bFGF | Mitogenic for fibroblasts, osteoblasts, and chondroblasts. |
| TGF-β | Increases fibroblast activity and synthesis of extracellular matrix. |
| PDGF | Mitogenic and chemotactic properties as well as increase cell proliferation and matrix remodeling. |
| VEGF | Induces angiogenesis and capillary permeability. |
| IGF-1 | Anabolic function with increased DNA, collagen and glycosaminoglycan production. |
| HGF | Antifibrotic effects and has been shown to reduce inflammatory-induced organ damage. |
| EGF | A mitogen associated with mesenchymal stem cell and fibroblast proliferation. |
BMPs are a subset of the larger TGF-β family. The name comes from its role in mediating bone formation. In addition to bone, BMPs have received significant attention for their role in tendon and cartilage formation. BMP-12, BMP-13, and BMP-14 have been shown to promote tendon formation when injected ectopically in rats. Additional studies support the involvement of BMP-12 and BMP-13 in embryonic development at sites of tendon formation and insertion.
Many studies have looked at the effect of adding BMPs in animal models. Administration of BMP-12, BMP-13, and BMP-14 in various animal models was associated with increased tensile strength in the repaired tissue. Additional benefits in tendon strength were seen when recombinant adenoviruses expressing BMP-13 and BMP-14 were applied to rat Achilles tendon. Although many of these studies have focused on the effects of BMPs on healing tendons throughout the body, several studies have looked specifically at the effect of these proteins at the site of rotator cuff repair. First, in a sheep model, researchers demonstrated that administering recombinant human BMP-12 (rhBMP-12) and rhBMP-13 leads to the formation of neotendon and ligament formation in rats and improved healing of tendon injury. In another study examining a sheep rotator cuff repair model, administration of rhBMP-12 in an absorbable type I collagen sponge was 2.7 times stronger than in untreated controls. Similarly, delivery of rhBMP-12 in a hyaluronan sponge increased repaired tendon strength by a factor of 2.1. This not only exhibits the potential for BMP use in rotator cuff repair but also demonstrates the impact of growth factor delivery systems. This study also examined histological effects at the repaired tendon insertion and noted increased glycosaminoglycan and reestablishment of collagen fiber continuity, further suggesting improved healing.
Although most of the current research supports the use of BMP as a strong candidate for augmentation of rotator cuff repair, a lack of complete understanding remains. Adenovirus-mediated gene transfer of human BMP-13 in mesenchymal stem cells (MSCs) in a rat model of rotator cuff healing showed no difference in cartilage formation, collagen fiber organization, or biomechanical strength at the repair site compared with MSCs alone. Further research is needed to gain a better understanding of the role of BMPs and the complex signaling pathways in which they participate during tendon repair.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here