Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Over the past 15 years, cell therapies have attracted intense interest in their use as a potential treatment in a multitude of neurologic disorders including stroke. A large number of preclinical studies have provided important information on the mechanisms, efficacy, and potential clinical application of cell therapies to enhance recovery after stroke.
Cell therapies can be obtained from a variety of sources ranging from embryonic, birth associated, and adult tissues.
Increasing evidence points toward paracrine and immunomodulatory mechanisms of action for many different types of cell therapies rather than tissue replacement and restoration of severed connections. The principal mediators of benefit may be a range of biologic factors secreted by cell therapies including microparticles. Systemic delivery of cell therapies may even lead to biologic changes within peripheral organs that promote anti-inflammatory and pro-regenerative responses after stroke.
Multiple clinical trials have shown safety of various types of cell therapies in patients with acute, subacute, and chronic stroke. These trials have involved different delivery routes including intravenous, intra-arterial, and intracerebral injections. Some randomized, placebo-controlled clinical trials suggest potential treatment effects, which have led to phase 3 trials.
Further work in this emerging field should focus on better defining mechanisms of action, conducting more clinical trials in selected patients for acute, subacute, and chronic stroke, and applying clinically relevant biomarkers to monitor treatment effects and track labeled cells in patients.
Cell therapy refers to cellular material with biologic activities that cause a desired effect either in vitro or in vivo. For over 20 years, an expanding number and variety of cell types, prepared for exogenous administration, have been discovered with therapeutic activity demonstrated in animal models of neurologic disorders. , Cell therapies as a potential treatment for neurologic disease began in the 1990s as a transplantation source for certain neurodegenerative disorders. The first clinical trials were conducted to graft fetal tissues in patients with Parkinson disease. Some of the transplanted patients showed sustained improvement over time and no longer required dopaminergic medications. The results were preliminary but served as a proof of principle that grafting cellular tissue might effectively treat a neurologic disorder. Since then, the field of cell therapy evolved in a completely different direction when it was found that the bone marrow and umbilical cord contain various different types of cells that reduce injury and promote recovery in a range of animal models of stroke, traumatic brain injury, spinal cord injury, multiple sclerosis, and neurodegenerative disorders. Some of these cell therapies possess the properties of bona fide stem cells while others are heterogeneous collections of progenitor, immature, and/or mature cell types. The mechanisms underlying how these types of cell therapies enhance stroke recovery are completely different than cell transplantation and need to be understood within the context of their intended use.
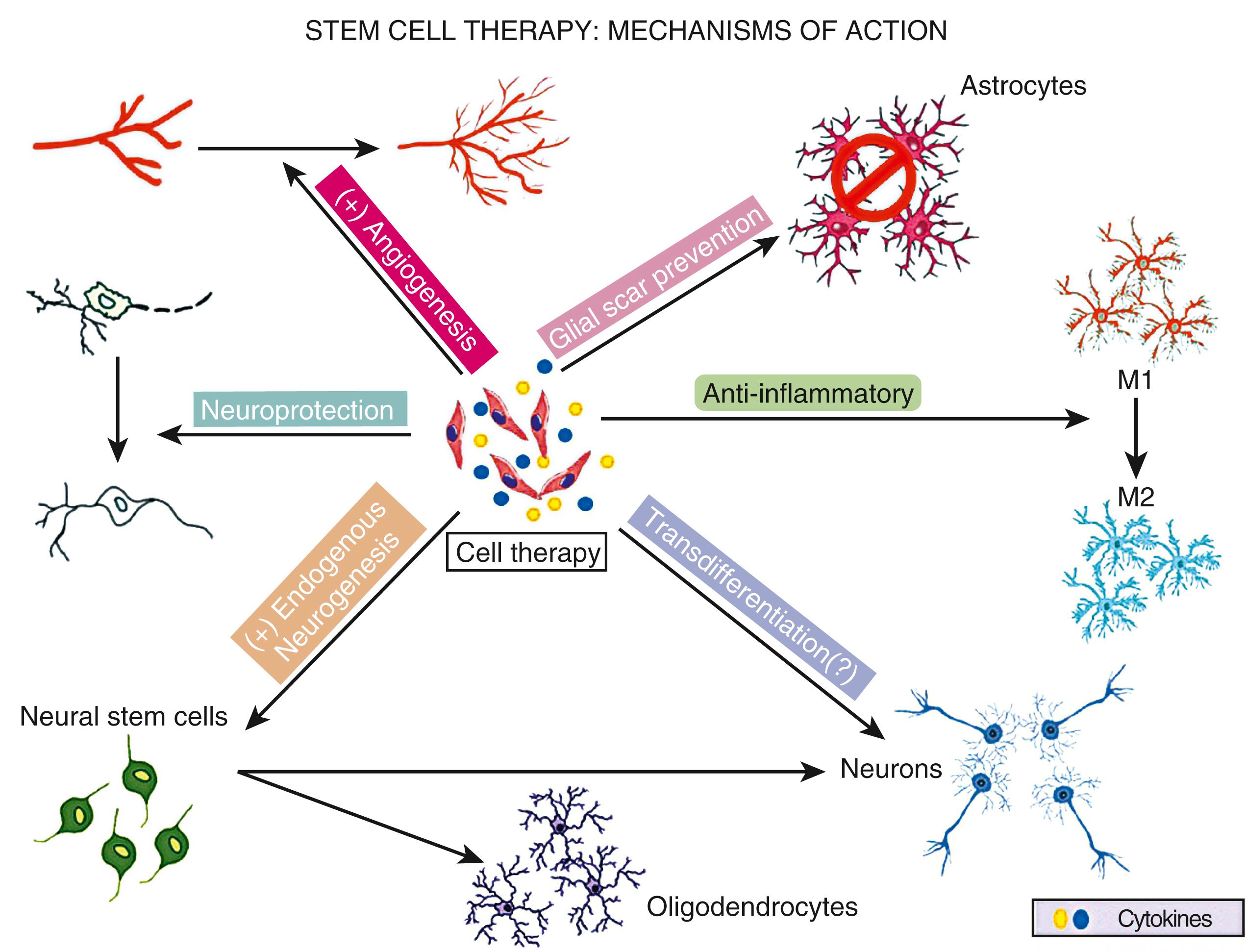
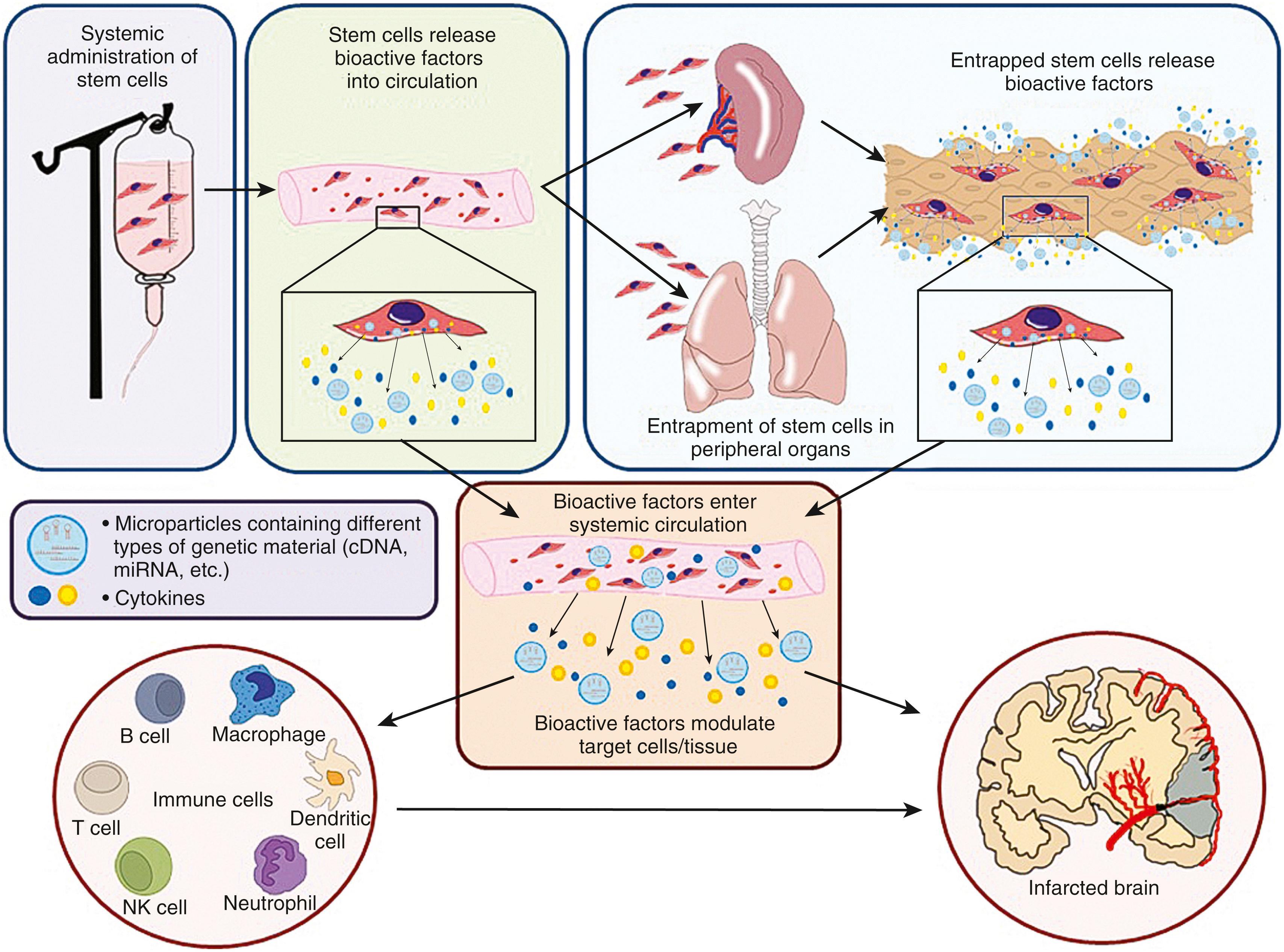
Rather than serving as a source of cell replacement, almost all types of cell therapies at the present time for clinical application are under development for stroke because they release biologic factors that modulate other endogenous host cells within the peripheral organs and/or the brain. Many types of cell therapies release growth factors and cytokines involved in brain repair, immunomodulation, and cell survival ( Fig. 62.1 ). , An emerging paradigm to explain how systemically delivered cell therapies enhance stroke recovery is based upon numerous preclinical studies showing modulation of immune cells in the circulation, lungs, and spleen. These changes reprogram peripheral immune cells, alter immune cell trafficking into the brain, and lead to the release of anti-inflammatory and pro-regenerative factors, ultimately changing the microenvironment within the brain and restoring homeostasis ( Fig. 62.2 ). Some types of cell therapies release biologically active microparticles, such as exosomes that by themselves are much smaller in size than cells, can circulate through the body and directly cross the blood-brain barrier (BBB) and enter into the brain to promote endogenous repair (see Fig. 62.2 ). , Therefore, there is a strong biologic rationale to apply certain types of cell therapies with systemic administration. The timing of administration of cell therapies depends upon the intended targets; there is extensive evidence that the acute to subacute stages of ischemic stroke may be an important window to consider for clinical applications. In the days to weeks after stroke, there is a brisk inflammatory response involving multiple interconnected processes both within the brain and in the periphery. During this same time period, there is a cascade of molecular signaling events leading to angiogenesis, neurogenesis, and axonal sprouting. Furthermore, neuroplasticity occurs at the molecular and cellular levels within the peri-infarct zone and areas remote from the infarct. Certain types of cell therapies modulate the inflammatory response and/or may facilitate and amplify many of the repair mechanisms operating during this time period. The temporal window for cell therapies during this subacute to chronic period of stroke is not well-defined and likely depends on the intended targets, cell type, and delivery route ( Fig. 62.3 ).
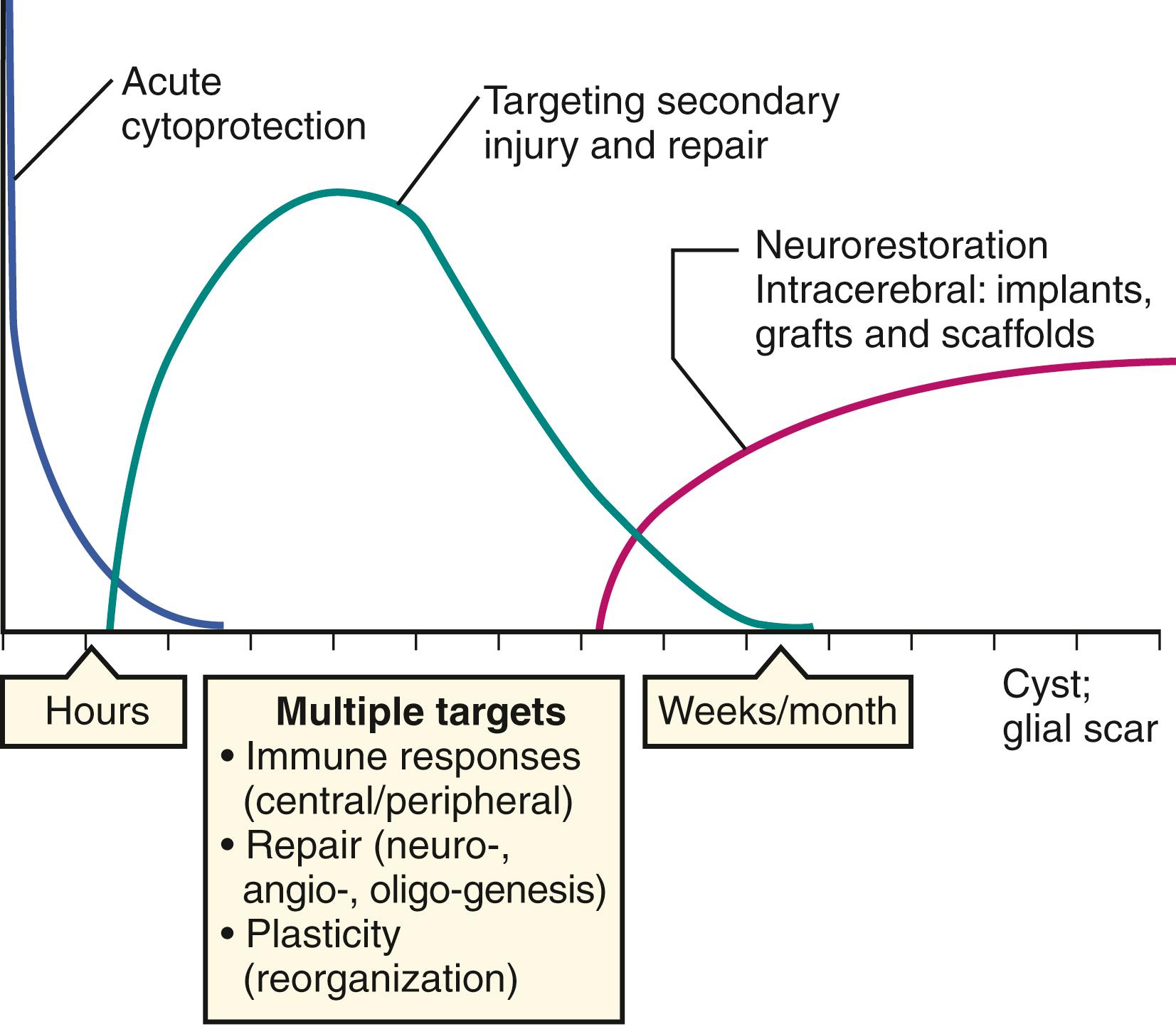
In the chronic period of stroke, months after symptom onset, the potential therapeutic applications of cell therapies are also being studied in several different ways (see Fig. 62.3 ). The route of delivery has principally involved a stereotactic intracranial administration, with the intent of placing therapeutic cells around the infarct. In the 1990s, there were preliminary clinical trials studying intracranial delivery of neural cells in patients with chronic stroke, , studies that were designed in the wake of fetal transplantation approaches for patients with Parkinson disease. The goal of these pilot trials was to graft neural cells in the infarct cavity, but current intracranial clinical studies have been designed with a different intention: to implant cells to stimulate local endogenous repair pathways and provide local trophic and extracellular matrix support. The current industry-sponsored cell therapy platforms for chronic stroke principally involve direct injections of (1) modified marrow stromal cells (sponsored by San Bio) and (2) fetal-derived neural fetal cells (ReNeuron and Neuralstem). Other investigator-initiated studies have also been studying direct intracranial injections of bone marrow stromal cells in Japan and elsewhere.
It is important to point out from the beginning of this chapter that the concept of grafting cells to restore lost neural circuitry is still in an early stage of preclinical development for any neurologic disorder. There are several biologic and logical challenges and barriers to consider for stroke that are well covered in other reviews, but there are some promising preclinical studies.
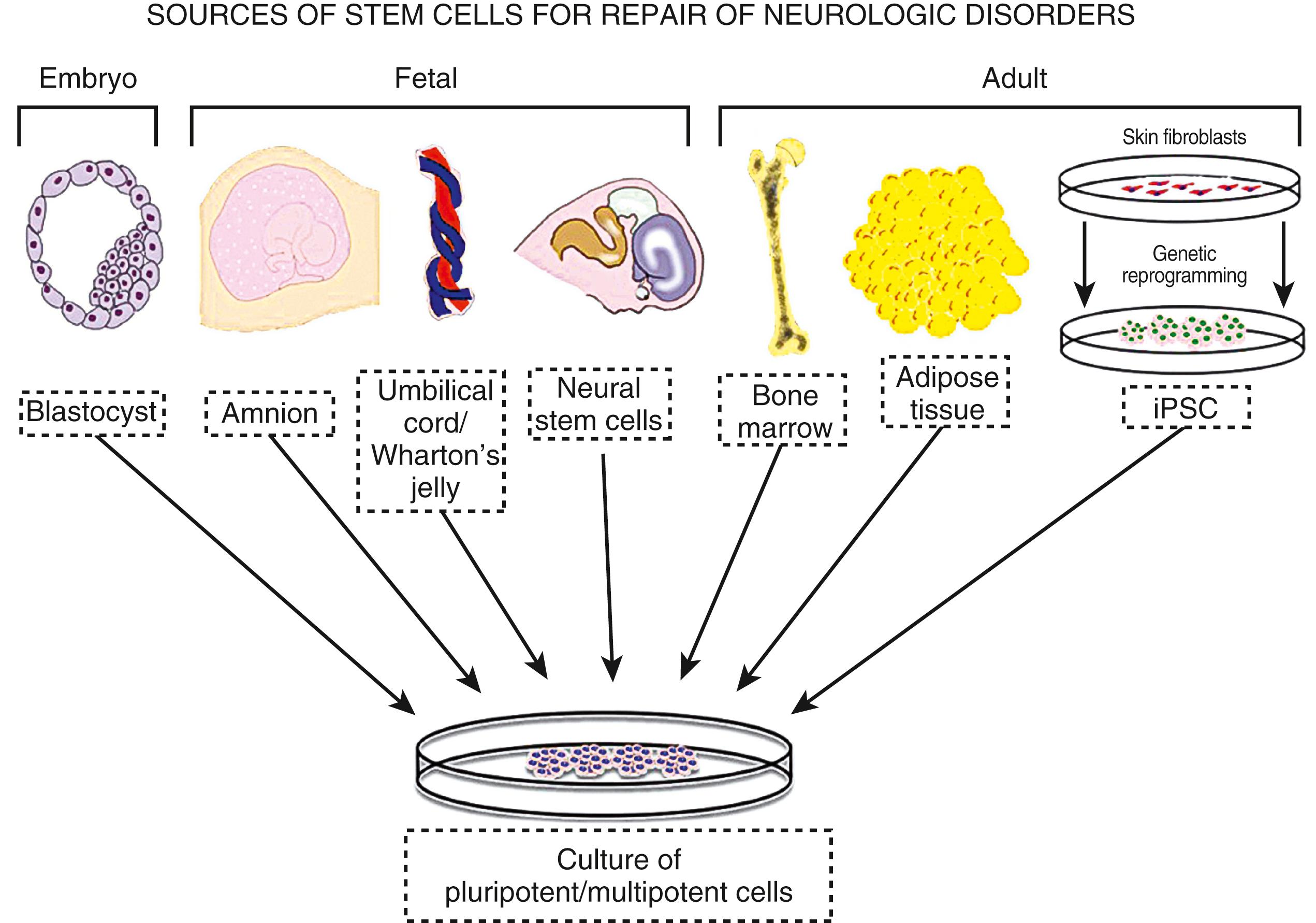
Among the various types of cell therapies, there are different kinds of cells: embryonic, fetal, birth-related, and adult cell types. All are under development as potential new treatments for stroke ( Fig. 62.4 ). A growing body of extensive animal data suggest that cell therapies derived from a range of tissues improve neurologic outcome in rodent models of stroke. Among the most studied, the investigation of adult-derived cell therapies has surged during the past 15 years, when it was discovered that the bone marrow harbored mesenchymal stromal cells, hematopoietic stem cells (HSCs), and other cell types that exert therapeutic effects in rodent stroke models, even when administered by intravenous delivery. Subsequently, a range of either purified cell types or mixed cell types from bone marrow, umbilical cord, and adipose and other tissues have been shown to improve neurologic outcome in rodents and other animals with stroke.
In addition to a wide variety of cell types, there are several delivery routes under consideration for cell delivery. The intravenous route is a preferred approach because of the ease of administration; it is the most effective means if the intent is to circulate cells throughout the body. For example, the intent for some cell therapy platforms in the acute to subacute stage of stroke is for cells to target the immune response after stroke. Umbilical cord cells, for example, bone-marrow-derived multipotent adult progenitor cells (MAPCs), HSCs, and even neural stem cells (NSCs) may exert their main therapeutic effects in models of stroke and traumatic brain injury by targeting the spleen. If the spleen and other immune-related organs are the main targets of certain cell therapies, an IV delivery route may be optimal. Furthermore, over 10 years of research have consistently shown that IV administration of MSCs improves neurologic outcome after stroke , and other acute neurologic disorders, while it remains contentious whether these cells enter the CNS in any significant numbers. Others have even shown that umbilical cells do not need to enter the brain to exert therapeutic effects. Both MSCs and umbilical cord cells may enhance recovery because of the factors that they release into the circulation, suggesting that their products are the critical mediators of their effects and not the cells themselves (see Fig. 62.2 ). All of these studies support the IV delivery route as a preferred approach for several types of cell therapy platforms.
The endovascular route is also being explored by several investigators because intra-arterial (IA) delivery (1) selectively targets therapeutic cells to an area of injury within the brain, (2) delivers a more concentrated number of cells at a lower dose to the brain, and (3) bypasses the entrapment of cells in peripheral organs. Overall, IA could lead to better control of cell delivery and cover a greater surface area within the brain at a lower dose compared to any other delivery route. However, for small cell types, IA delivery will likely still lead to cell migration into the systemic circulation and other organs once they pass through the CNS. Our studies indicate that for smaller-sized cells, IA does not lead to a greater effect on various different biologic endpoints compared with IV. There are also risks that need to be addressed with the IA route. IA delivery of larger-sized cells can lead to microvascular plugging and focal reductions in cerebral blood flow, leading to ischemic injury. This risk depends on the cell size, number of cells injected, adhesiveness of the cells, target arteries involved, and infusion rate. Various imaging modalities for preclinical studies are used to track the fate of injected cells, including magnetic resonance imaging (MRI), single-photon emission computed tomography (SPECT), and bioluminescence imaging (BLI). Intra-arterial delivery will likely be optimized with the use of imaging and tracking of injected cells to assess localization and viability of the injected cells. In addition, bioengineering or sorting specific cells with increased surface expression of adhesion molecules could improve the efficacy of intra-arterial delivery of transplanted cells.
In contrast to systemic delivery routes, stereotactic injection ensures placement of cells in a focal area and is a preferred route for such cell types where migration outside of the CNS may be unwanted. There are various opinions as to whether intracranial delivery is a preferred approach and is superior to systemic delivery. Decisions on which approaches to use require an understanding of intended effects, with planned trajectories and placement of cells, the cell types involved, a demonstration that intracranial delivery leads to a clear superior effect compared with systemic delivery, and clear safety data that the cells do not cause tumors or cause neurologic worsening. There are other routes of delivery under investigation, including intrathecal delivery and intranasal delivery.
Recognizing the tremendous potential of cell therapies to become a new future treatment for stroke, investigators from academia have convened with industry leaders together with members of regulatory agencies in a meeting called the STEPS (Stem Cells as an Emerging Paradigm for Stroke) conference. Having met four times, the group’s principal goals are to discuss how to successfully translate cell therapies from animal studies to clinical trials. The meetings follow the format of the STAIR conferences and set forth a series of guidelines for anyone interested in developing cell therapies for stroke. These conferences have stressed the importance of defining dose responses, therapeutic time window, optimal delivery route, biocompatibility of delivery devices, cell therapy development in the context of rehabilitation, and interactions with comorbidities and medications. Of special concern unique to cell therapies is ensuring that the cell product is fully characterized with immunophenotyping and other molecular and biologic approaches. The mechanisms of action should be understood within the context of the therapeutic intent. Is the cell product being developed as a cell replacement/transplantation approach, or a paracrine/modulator of the endogenous repair and immune responses? If the cells are allogeneic, will immunosuppressive agents be needed in a clinical trial or will the cells themselves modulate the immune system such that suppressive agents are not needed or would even be contraindicated?
In this section, we focus on specific cell therapies under development for stroke.
The bone marrow contains a number of different types of stem cells including HSCs, marrow stromal cells, MAPS, and endothelial progenitor cells (EPCs).
Mononuclear cells (MNCs) are a mixture of different types of cells and contain most of the different stem cells within this component of the marrow, but principally contain a number of immature and mature cell types of different myeloid, lymphoid, and erythroid lineages. MNCs were first tested in conditions such as ischemic heart disease over 10 years ago. Many trials suggest that MNCs can improve outcomes in patients with heart disease, while other studies have not confirmed a beneficial effect. MNCs are an attractive cell therapy because they can be rapidly isolated from patients after a bone-marrow aspiration, do not require culture, and therefore permit autologous applications for patients with acute to subacute disorders. For stroke, many different laboratories around the world, including our own, have found that bone-marrow-derived MNCs improve neurologic outcome, reduce inflammatory processes, and upregulate various repair mechanisms such as neurogenesis in rodent models of stroke. In a sheep model of stroke, MNCs reduce lesion size, lymphocytic infiltration, and axonal degeneration. Based on these studies involving both small and large animal models, we initiated and completed a phase 2a trial testing autologous bone marrow MNCs in patients with acute ischemic stroke in which we found that MNCs are safe to administer to patients within 24–72 hours after an ischemic stroke. MNC treatment was associated with neurologic improvement compared to a historical cohort, and with improvement in white-matter integrity of the corticospinal tract. There have been several other small clinical trials testing MNCs in patients with subacute and chronic stroke , and a randomized trial for patients beyond 7 days after stroke that found no evidence for improvement in neurologic outcome on the Barthel Index compared with a control group.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here