Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Explain energy metabolism in general and how the challenges to provide adequate energy to all cells change during the digestive phase versus the fasting phase.
List the primary hormones involved in the regulation of energy metabolic homeostasis, and describe their site of synthesis, regulation of production, and receptor signaling pathways.
Diagram the hormonal regulation of specific enzymatic pathways in the hepatocyte, skeletal muscle fiber, and adipocyte during the digestive phase.
Diagram the hormonal regulation of specific enzymatic pathways in the hepatocyte, skeletal muscle fiber, and adipocyte during the fasting phase.
Explain the role of adipose tissue as an endocrine organ.
Explain imbalances in energy metabolism and their consequences in type 1 and type 2 diabetes mellitus.
Cells continually perform work to grow, proliferate, and migrate; to maintain their structural integrity and internal environment; to respond to stimuli; and to perform their differentiated functions such as contraction, secretion, phagocytosis and propagation of an action potential. For example, while reading the previous sentence, your heart contracted and relaxed about 16 times, and about 3.5 million red blood cells entered your blood from bone marrow. Indeed, the resting metabolic rate (as when sitting and reading) constitutes about 60% to 70% of total potential energy expenditure.
Cells derive their energy to perform this nonstop work primarily from the universal energy carrier, adenosine triphosphate (ATP) . The enzymatic hydrolysis of the terminal one or two phosphate groups releases a significant amount of energy that is coupled to and drives many energetically unfavorable reactions. However, ATP cannot be stored. Rather, cells need a continually renewable supply of ATP, which is achieved by continually oxidizing carbon-based fuels ( Figs. 3.1A and B ). The primary, monomeric fuels are monosaccharides (primarily glucose) , free fatty acids (FFAs ; also called nonesterified fatty acids), amino acids (AAs), and ketone bodies . Certain cells can store some of these fuels as polymers and access them as needed (e.g., skeletal muscle stores glucose as glycogen to be used during exercise). However, most cell types continually import monomeric fuels that are delivered to the extracellular microenvironment from the circulation and then enter cells via specific transporters.
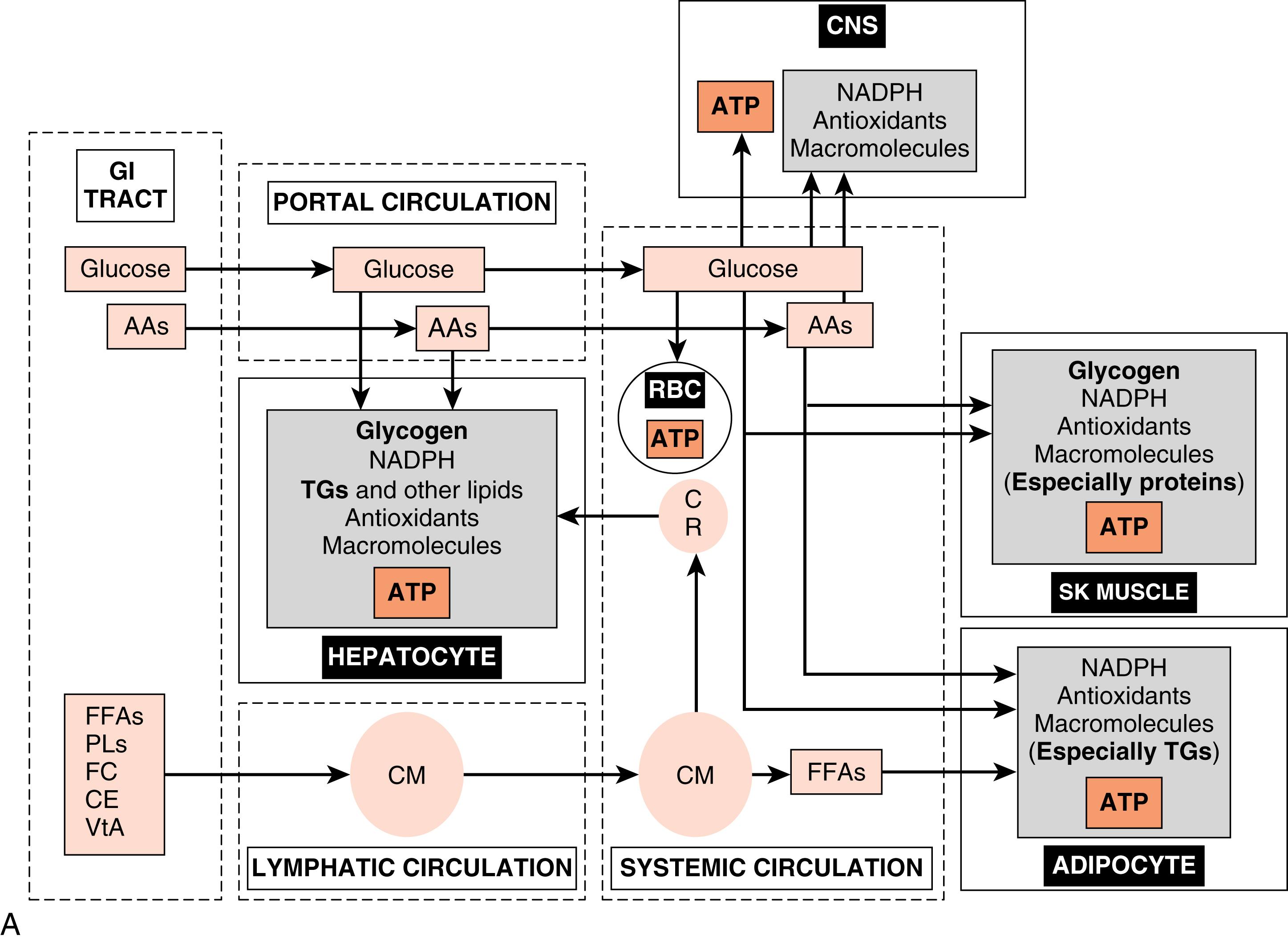
Because cells depend on the continuous delivery of monomeric fuels from the circulation, the body as a whole must (1) maintain adequate circulation to all cells and (2) maintain adequate levels of primary fuels and O 2 within the circulation at all times. Inability to do so results in reversible cell injury and, ultimately, irreversible cell injury and cell death. Conversely, while inadequate levels of fuels can lead to cell dysfunction and death, excessive levels of fuels, both extracellularly and intracellularly, can also lead to serious injury (see later). Thus the regulation of whole body energy metabolism serves to maintain adequate levels of intracellular ATP in all cell types at all times, while keeping the concentrations of intracellular and circulating fuels below critical thresholds. The amount and type of circulating fuels available to cells change during the day, as well as during exercise, fasting, or starvation. Consequently, whole body metabolism needs to be dynamic and flexible. This is achieved through regulation of metabolism by endocrine, paracrine , and neuronal signaling , and by intracellular components that act as sensors of specific nutrients, metabolites, ions, or the adenosine monophosphate (AMP)/ATP ratio.
For a specific fuel to be utilized by a specific cell type, that cell must express (1 ) cell membrane–associated transporters and, in some cases, intracellular organelle–associated transporters , that can import and export a specific fuel; and (2) the enzymes and cofactors that comprise specific metabolic pathways for a given fuel. Key components of these are regulated by hormones, metabolites, and intracellular energy sensors during the digestive versus fasting phases.
The cell types that play major roles in the coordination of fuel utilization and are major targets of hormonal and neuronal regulation are (1) the hepatocyte , (2) skeletal muscle fiber , and (3) the adipocyte (see Fig. 3.1A and B ). After the introduction of insulin and glucagon synthesis and regulation, this chapter will discuss the metabolic function and its regulation for each of these cell types in the context of the digestive phase and the fasting phase .
The digestive phase (or fed state) refers to the period of about 2 to 3 hours after the ingestion of a meal. Circulating fuels typically rise to levels that exceed the metabolic needs of the body. Because human metabolism has evolved to be very thrifty, excessive fuels are not wasted, but rather partitioned into various storage depots. The storage form of glucose is glycogen , the storage form of FFAs is triglyceride (TG) , and the storage form of AAs is protein , especially skeletal muscle proteins (see Fig. 3.1A ).
A major challenge associated with the digestive phase is to prevent prolonged excessive circulating and intracellular levels of nutrients . Insulin is the primary digestive phase hormone that promotes the movement of fuels out of the circulation and into cells, and their subsequent oxidation for energy or polymerization for storage or, in the case of AAs, for the synthesis of functional proteins. Insulin also promotes anabolic pathways that consume carbons from glucose, FFAs, and AAs, as well as consuming ATP, to build macromolecules to maintain cellular integrity and viability, or to promote cell growth and proliferation.
During the interdigestive phase between meals, the fasting phase during sleep and daytime fasting, and during prolonged physical work or exercise, the levels of circulating fuels begin to decline. In a normal, nonprotracted fasting phase (i.e., 8 to 12 hours), the brain is absolutely dependent on blood glucose for normal function. Thus a major challenge associated with the fasting phase is the maintenance of blood glucose levels above a critical threshold (60 to 70 mg/dL). This is required to avoid autonomic responses (e.g., nausea, heart racing), neuroglycopenic symptoms (e.g., confusion, lethargy), and even coma and death, due to increasingly severe degrees of hypoglycemia. Blood glucose levels are maintained by two processes (see Fig. 3.1B ):
Hepatic glucose production. During the initial period of a fast, the liver breaks down stored glycogen via glycogenolysis to glucose-6-phosphate (G6P), converts G6P to glucose by the liver-specific enzyme, glucose-6-phosphatase, and exports glucose through the bidirectional GLUT2 transporter. As a fasting phase progresses, hepatic glucose production switches from primarily glycogenolysis to primarily gluconeogenesis, or the production of glucose from 3-carbon metabolites, including lactate, pyruvate, glycerol, and several “glucogenic” AAs that are released from proteolysis.
Glucose sparing. Most cell types can utilize fuels other than glucose, thereby sparing glucose for use by the central nervous system (CNS). First, low levels of insulin during the fasting phase minimize the flow of glucose into skeletal muscle and adipocytes. TGs within adipocytes are mobilized so that FFAs are released into the circulation as FFAs, and preferentially used by skeletal and cardiac muscle, adipocytes, and other cell types for ATP production. Also, during the fasting phase, the liver converts FFAs and some “ketogenic” AAs into ketone bodies, and exports these fuels into the circulation for use by extrahepatic tissues. During a prolonged fast (i.e., 1 to 2 weeks), the CNS itself switches to the utilization of ketone bodies. Finally, skeletal muscle stores glycogen, which is mobilized especially during exercise/work for use within the muscle fiber.
The hormones glucagon and epinephrine , along with the sympathetic neurotransmitter, norepinephrine , represent the primary signals that induce the mobilization of energy stores and new synthesis of glucose and ketone bodies during the fasting phase, thereby preventing blood glucose from declining to dangerously low levels (see later). Several other hormones, including cortisol and growth hormone , also play important roles in the mobilization of energy stores, the control of circulating glucose and lipids, and the balance between protein synthesis and degradation; these are discussed in Chapters 5 and 7 .
The islets of Langerhans constitute the endocrine portion of the pancreas (also called the endocrine pancreas ; Fig. 3.2 ). About 1 million islets, making up about 1% to 2% of total pancreatic mass, are spread throughout the pancreas. The islets are composed of several cell types, each producing a different hormone. In islets situated in the body, tail, and anterior portion of the head of the pancreas (all of which have a common embryologic origin), the most abundant cell type is the β cell . The β cells make up about three-fourths of islet cells and produce the hormone insulin . The α cells comprise about 10% of these islets and secrete the hormone glucagon . The third major cell type of the islets within these regions is the Δ cells , which make up about 5% of the cells and produce the peptide somatostatin (gastric somatostatin was discussed in Chapter 2 , as an inhibitor of gastrin secretion). A fourth cell type, the F cell, represents about 80% of the cells in the islets situated within the posterior portion of the head of the pancreas (including the uncinate process) and secretes the peptide, pancreatic polypeptide .
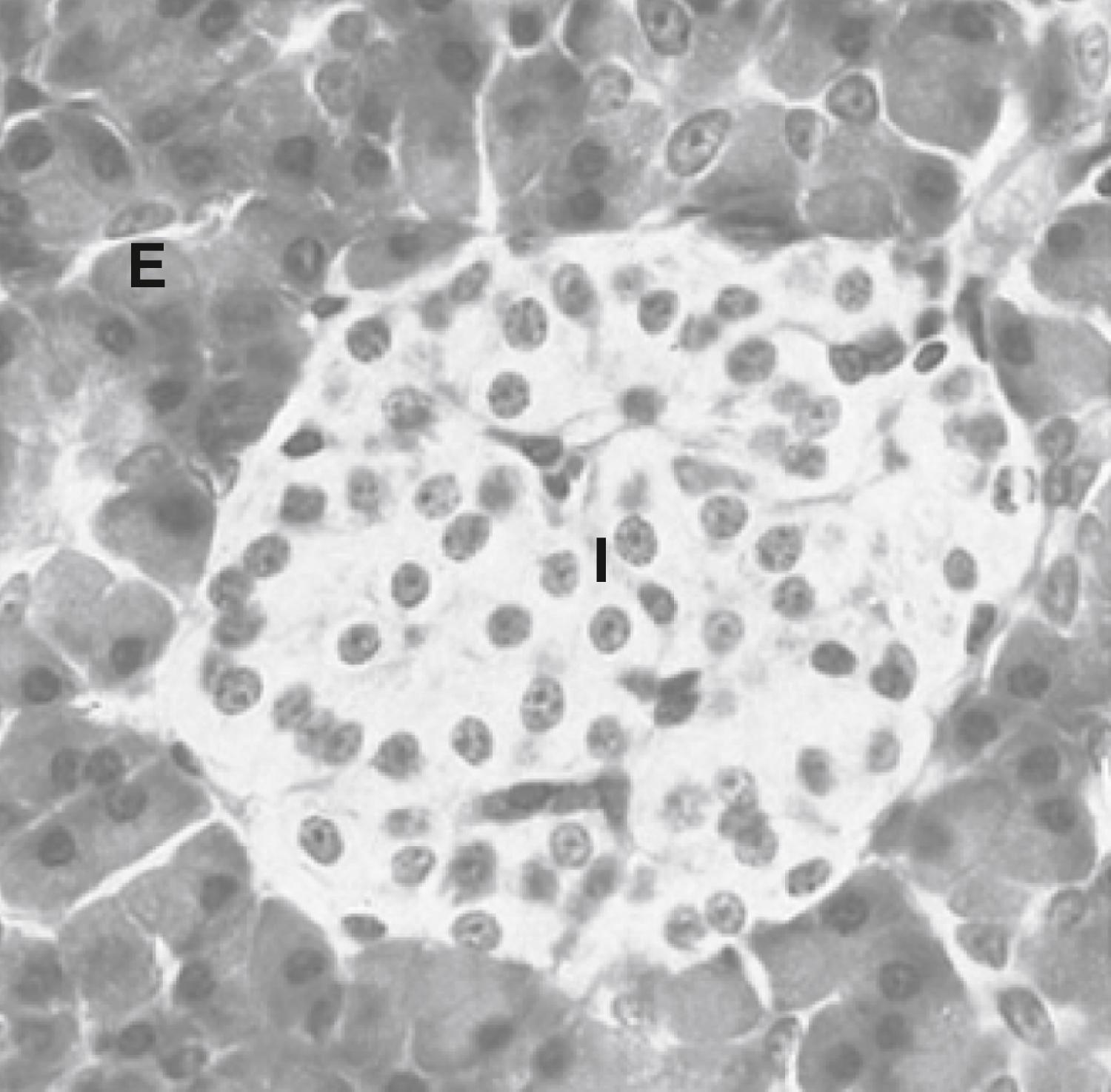
Because the physiologic function of pancreatic polypeptide in humans remains obscure, it is not further discussed here. Blood flow through the islets is somewhat autonomous from the blood flow to the surrounding exocrine tissue. Insulin secreted from the inner β cells reaches the outer α cells. Consequently, the first cells affected by circulating insulin are the α cells, in which insulin inhibits glucagon secretion.
Insulin is the primary anabolic hormone that is responsible for maintaining the upper limit of blood glucose and FFA levels. Insulin achieves this by the following mechanisms:
Increasing glucose uptake by skeletal muscle and adipocytes.
Increasing metabolism of glucose in liver, skeletal muscle, and adipocytes.
Increasing glycogen storage in liver and skeletal muscle.
Increasing the clearance of chylomicrons from the blood.
Increasing TG synthesis and storage in the liver and adipose tissue.
Suppressing glucose output by the liver.
Suppressing lipolysis of adipose TG stores.
Suppressing very-low-density lipoprotein (VLDL) production by the liver.
Insulin also promotes protein synthesis from AAs and inhibits protein degradation in peripheral tissues. Finally, insulin regulates metabolic homeostasis through effects on satiety.
Insulin is a protein hormone that belongs to a gene family that also includes insulin-like growth factors I and II (IGF-I, IGF-II), relaxin , and several insulin-like peptides. Organized, functional islets appear in the human pancreas at the beginning of the third trimester of gestation. Insulin gene expression and islet cell biogenesis are dependent on several transcription factors (e.g., hepatocyte nuclear factor-4 [HNF-4α], HNF-1α, or HNF-1β; pancreatic and intestinal homeobox-1 [PDX1]; neuroD1) (Clinical Box 3.1).
The insulin gene encodes preproinsulin . Preproinsulin is converted to proinsulin by microsomal enzymes as the peptide enters the endoplasmic reticulum. Proinsulin is packaged in the Golgi apparatus into membrane-bound secretory granules. Proinsulin contains the AA sequence of insulin plus the 31-amino acid C (connecting) peptide . The proteases that leave proinsulin, prohormone (or proprotein) convertase-2 and -3, are packaged with proinsulin within the secretory vesicle. The mature hormone consists of two chains, an α chain and a β chain, connected by two disulfide bridges ( Fig. 3.3 ). Insulin is stored in secretory vesicles in zinc-bound crystals. Because the entire contents of the granule are released, equimolar amounts of insulin and C peptide are secreted, as are small amounts of proinsulin. C peptide has no known biologic activity, and proinsulin has about 7% to 8% of the biologic activity of insulin.
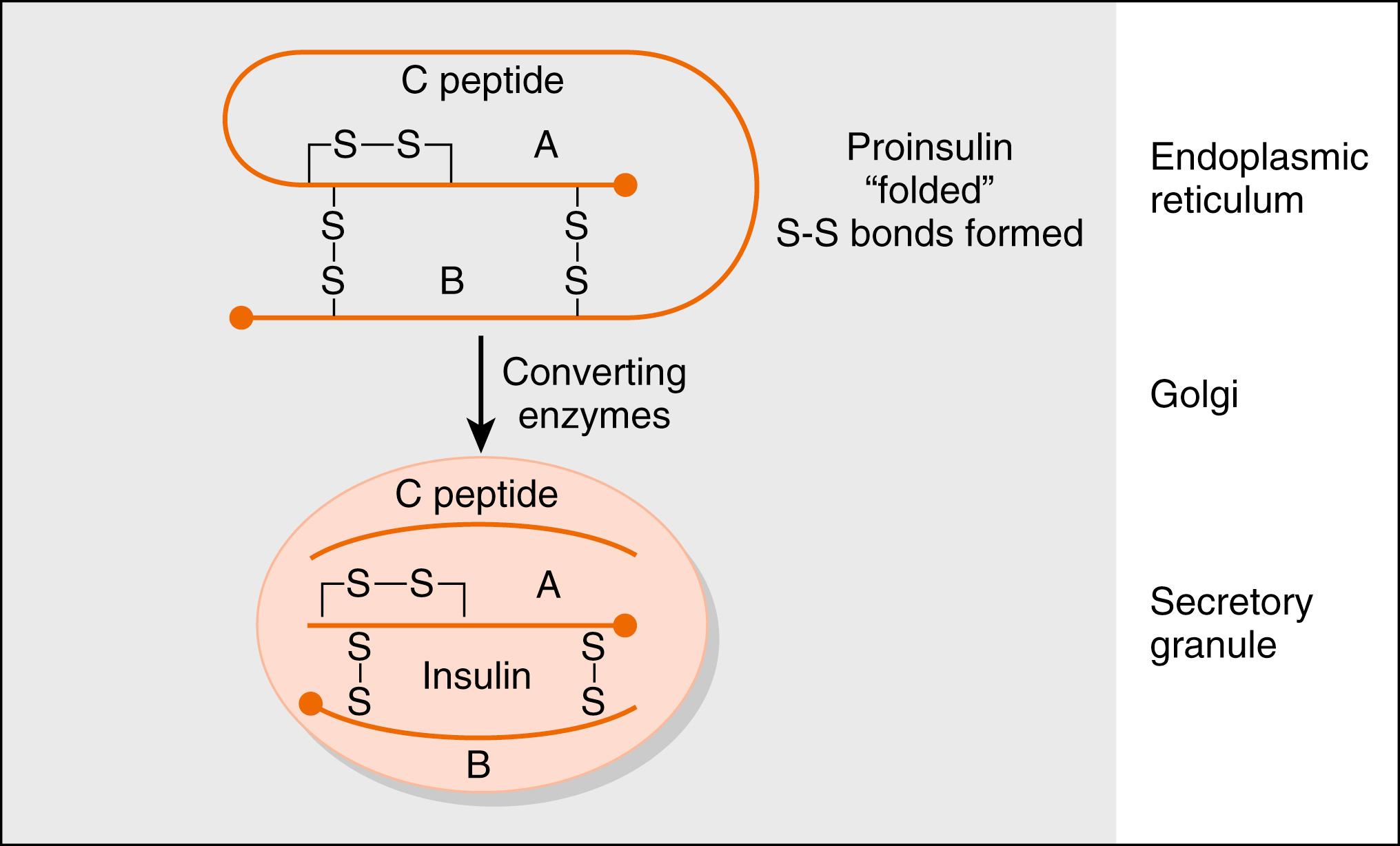
Measurements of C peptide in the blood are used to quantify endogenous insulin production in patients receiving exogenous insulin, which has been purified from C peptide. Insulin has about a 5-minute half-life and is cleared rapidly from the circulation by receptor-mediated endocytosis. It is degraded by lysosomal insulin degrading enzyme (IDE) in the liver, kidneys, and other tissues. Because insulin is secreted into the hepatic portal vein, almost one half of the insulin is degraded before leaving the liver. Recombinant human insulin and insulin analogs are now available, with different characteristics of onset and duration of action and peak activity.
Serum insulin levels normally begin to rise within 10 minutes after food ingestion and reach a peak in 30 to 45 minutes. The higher serum insulin level rapidly lowers blood glucose to baseline values. When insulin secretion is stimulated, insulin is released rapidly (within minutes), and this is called the early phase of insulin secretion. If the stimulus is maintained, insulin secretion falls within 10 minutes and then slowly rises over a period of about 1 hour. The second phase is referred to as the late phase of insulin release.
Glucose is the primary stimulus of insulin secretion ( Fig. 3.4 ). Glucose entry into β cells is facilitated by the GLUT2 transporter . Once glucose enters the β cell, it is phosphorylated to G6P by the low-affinity hexokinase, glucokinase (GK) . GK is referred to as the glucose sensor of the β cell because the rate of glucose entry is correlated to the rate of glucose phosphorylation, which, in turn, is directly related to insulin secretion. Heterozygous mutations in GK are one defect that leads to inadequate insulin release in patients with mature-onset diabetes of the young (MODY) . G6P is metabolized by β cells, increasing the intracellular ATP levels and closing an ATP-sensitive K + channel (see Fig. 3.4 ). This results in depolarization of the β-cell membrane, which opens voltage-gated Ca 2+ channels. Increased intracellular Ca 2+ levels activate exocytosis of secretory vesicles.
Heterozygous mutations in any one of the islet transcription factors, as well as glucokinase (see later), result in progressively inadequate production of insulin. This leads to MODY, which typically manifests in patients younger than 25 years of age.
TYPE gene mutated (requires insulin or oral hypoglycemic)
MODY 1 HNF-4α (yes)
MODY 2 Glucokinase (no)
MODY 3 HNF-1α (yes)
MODY 4 IPF-1 (yes)
MODY 5 HNF-1β (yes)
MODY 6 Neuro D1 (yes)
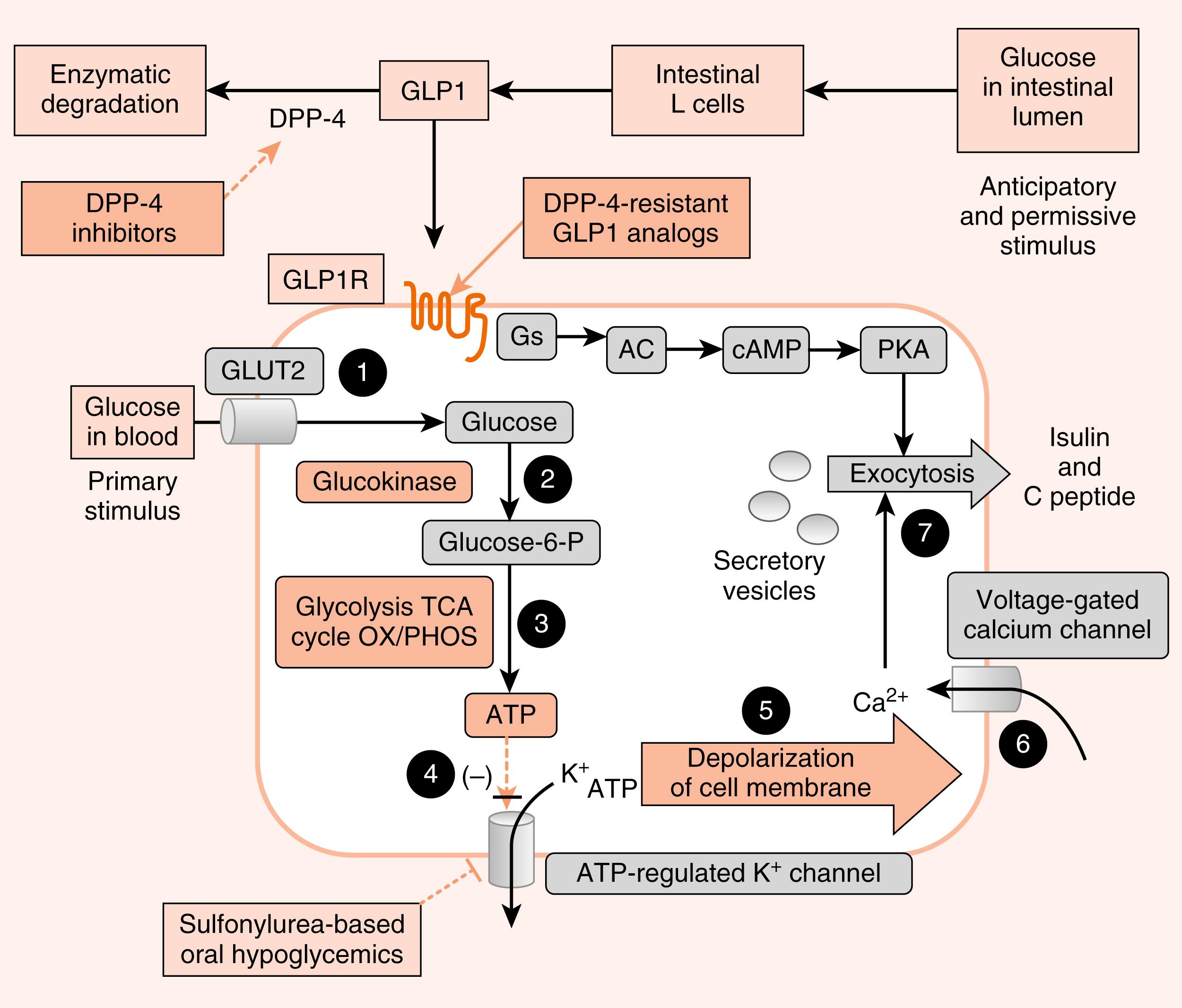
In addition to glucose, certain AAs (leucine) and vagal (parasympathetic) cholinergic innervation (i.e., in response to a meal) also stimulate insulin through increasing intracellular Ca 2+ levels. Long-chain FFAs also increase insulin secretion, although to a lesser extent than glucose and AAs.
As discussed in Chapter 2 , nutrient-dependent stimulation of insulin release is enhanced by the incretin hormones, glucagon-like peptide-1 (GLP-1) and gastric inhibitory peptide (GIP) and possibly other gastrointestinal (GI) hormones (e.g., cholecystokinin [CCK]). These act primarily by raising intracellular cyclic AMP (cAMP) within the β cell, which amplifies the intracellular effects of glucose on Ca 2+ (see Fig. 3.4 ). Intracellular cAMP acts both through phosphokinase A (PKA)-dependent and EPAC (exchange protein activated by cAMP)–dependent pathways (see Chapter 1 ) in β cells. Incretin hormones minimally increase insulin secretion in the absence of glucose.
Insulin secretion is inhibited by α 2 -adrenergic receptors , which are activated by epinephrine (from the adrenal medulla) and norepinephrine (from postganglionic sympathetic fibers). The α 2 -adrenergic receptors are coupled to a Gi-containing trimeric G-protein complex that inhibits adenylyl cyclase and decreases cAMP levels. Adrenergic inhibition of insulin serves to protect against hypoglycemia, especially during exercise. Although somatostatin from D cells inhibits both insulin and glucagon, its physiologic role in pancreatic islet function is unclear. Nevertheless, somatostatinomas are malignant tumors of the islets that produce high levels of somatostatin, which can lead to mild glucose intolerance.
The ATP-sensitive K + channel is a protein complex that contains an ATP-binding subunit called SUR1 . This subunit is also activated by sulfonylurea and meglitinide drugs , which are used as ingested drugs ( oral hypoglycemics ) to treat hyperglycemia in patients with partially impaired β-cell function (see Fig. 3.4 ). As these drugs act within the direct pathway for glucose-stimulated insulin secretion (GSIS) , they are associated with hypoglycemic episodes as a side effect. Rare mutations in the ATP-sensitive K + channel that keep it in the open conformation, thereby blocking glucose-induced insulin secretion, result in early-onset diabetes.
Analogs of GLP-1 (so-called “glutide” drugs ) are also used to enhance GSIS (see Fig. 3.4 ).
GLP-1 is rapidly degraded by the enzyme dipeptidyl dipeptidase-4 (DPP-4) . Inhibitors of DPP-4 (so-called “gliptin” drugs ) are also effective at enhancing GSIS. Both glutides and gliptins confer a significantly lower risk of hypoglycemia, as they only act in the presence of elevated glucose.
The insulin receptor is a member of the receptor tyrosine kinase (RTK) family (see Chapter 1 ), which includes receptors for several other growth factors, such as IGFs, platelet-derived growth factor (PDGF), and epidermal growth factor (EGF). The insulin receptor is expressed on the cell membrane as a homodimer composed of α/β monomers ( Fig. 3.5 ). The α/β monomer is synthesized as one protein, which is then proteolytically cleaved, with the two fragments connected by a disulfide bond. The two α/β monomers are also held together by a disulfide bond between the α subunits. The α subunits are external to the cell membrane and contain the hormone-binding site. The β subunits span the membrane and contain tyrosine kinase on the cytosolic surface.
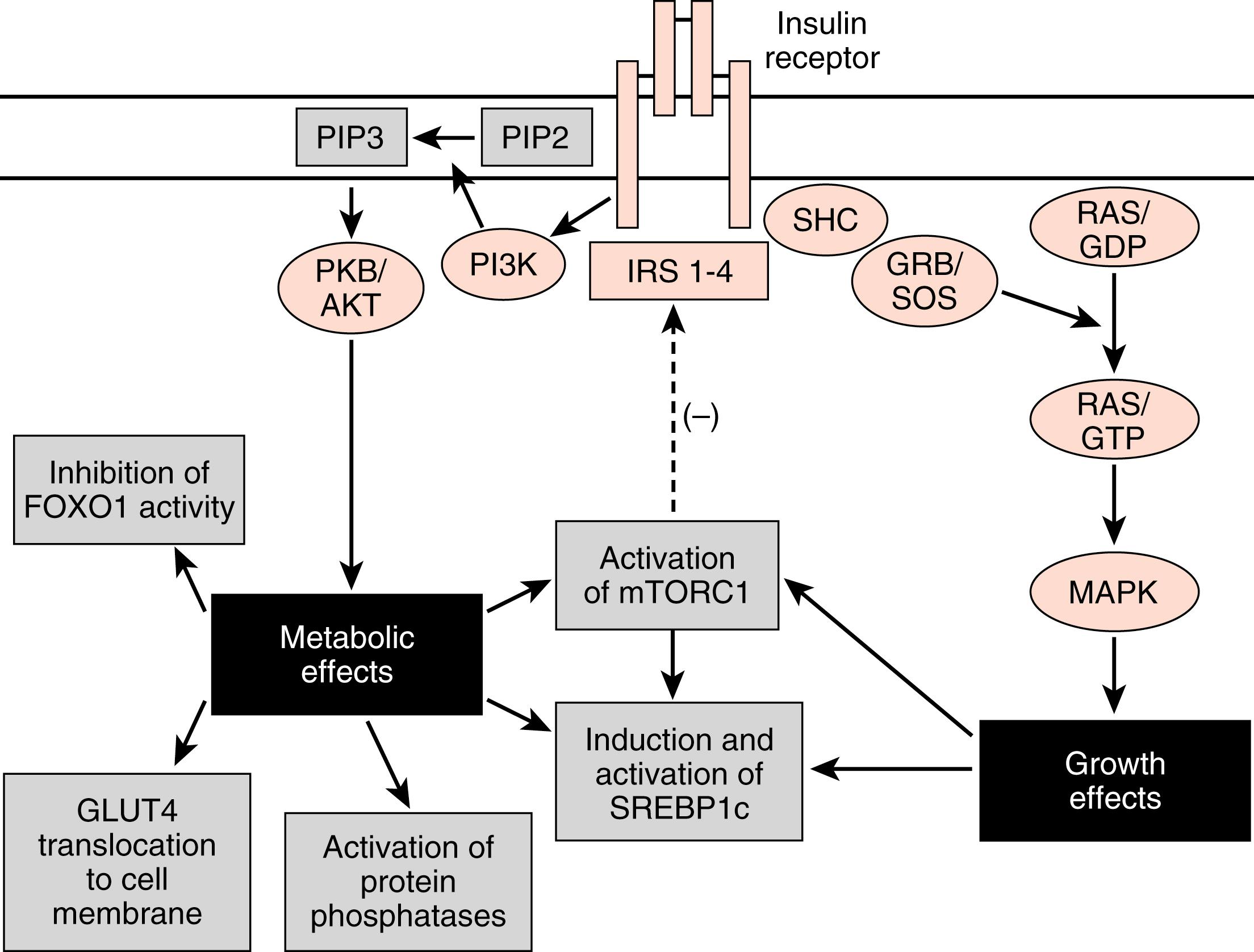
Insulin binding to the insulin receptor induces the β subunits to cross-phosphorylate each other on three tyrosine residues. These phosphotyrosine residues recruit two major classes of adaptor proteins: insulin-receptor substrate isoforms 1 to 4 (IRS1-4) and Shc protein (see Fig. 3.5 ). The IRS proteins are phosphorylated by the tyrosine kinase activity of the insulin receptor. The phosphotyrosine residues on IRS recruit PI3 kinase to the membrane, where it is phosphorylated and activated by the insulin receptor. PI3 kinase converts phosphoinositide-4,5-bisphosphate (PIP2) to phosphoinositide-3,4,5-triphosphate (PIP3). PIP3 recruits to the membrane and leads to the activation of a pleiotropic protein kinase, called Akt (see Fig. 3.5 ).
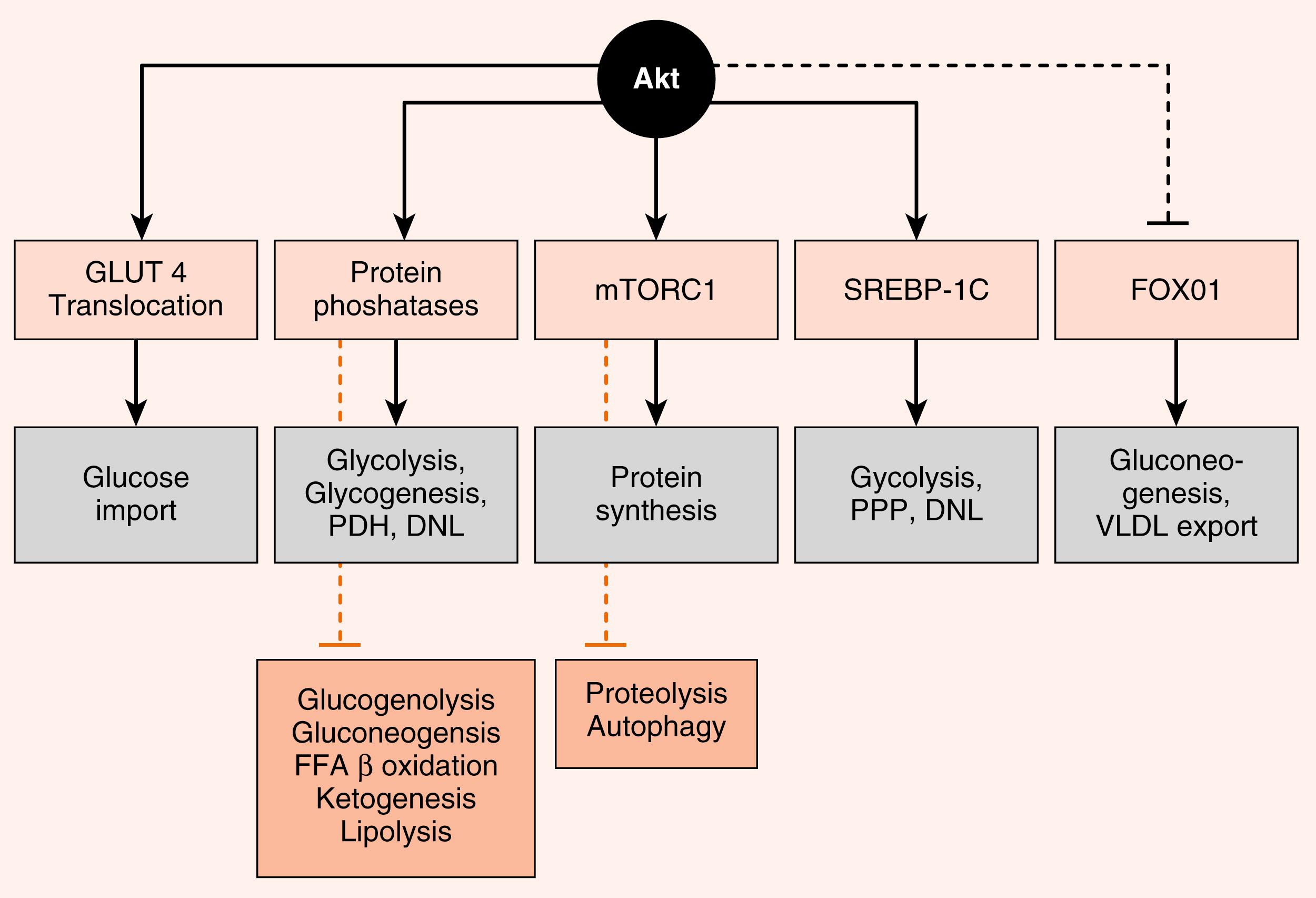
Akt regulates numerous enzymes and transcription factors that mediate the metabolic actions of insulin. Akt acts in five general ways that largely account for the metabolic effects of insulin ( Fig. 3.6 ):
Phosphorylation of exocytosis components that induce the insertion of GLUT4 glucose transporters into the cell membranes of muscle and adipose tissue (see later). This action requires combined IRS/PI3K-dependent signaling and an additional adaptor protein-dependent pathway that activates a small G-protein pathway (not shown).
Activation of protein phosphatases that, in turn, regulate metabolic enzymes through dephosphorylation. For example, dephosphorylation of liver pyruvate kinase activates the enzyme, whereas phosphorylation by cAMP/PKA signaling inactivates it.
Activation of mammalian target of rapamycin complex-1 (mTORC1) (see later). mTORC1 is a kinase complex that promotes ribosomal RNA synthesis, ribosome assembly, and protein synthesis. mTORC1 also increases sterol-regulatory element–binding protein-1c (SREBP1c) activity.
Direct induction of synthesis and activation of the lipogenic transcription factor, the SREBP1c. SREBP1c coordinately stimulates the expression of enzymes involved in glycolysis, lipogenesis, and the pentose phosphate pathway.
Phosphorylation and inactivation of the hepatic transcription factor, FOXO1 .
FOXO1 stimulates the expression of some gluconeogenic enzymes, as well as VLDL-related apoprotein B100.
The Shc protein is linked to the mitogen-activated protein kinase (MAPK) pathway (see Fig. 3.5 ), which mediates the growth and mitogenic actions of insulin (in conjunction with the activation of mTORC1).
The termination of insulin receptor signaling is a topic of high interest because these mechanisms potentially play a role in insulin resistance . Insulin induces the downregulation of its own receptor by receptor-mediated endocytosis . Additionally, several serine and threonine protein kinases are indirectly activated by insulin and by other molecules (such as inflammatory cytokines) and subsequently inactivate the insulin receptor or IRS proteins. These include mTORC1, which negatively feeds back on IRS proteins (see Fig. 3.5 ). Another serine threonine kinase that reduces activity or levels of the insulin receptor and IRS proteins is the suppressor of cytokine signaling (SOCS) family of proteins. In the presence of inflammation , proinflammatory cytokines activate SOCS kinase, which negatively feeds back on both the cytokine receptors and the insulin receptor. Thus inflammation can lead to insulin resistance. Finally, intracellular accumulation of TGs and other lipids increase cytosolic levels of lipid intermediates such as diacylglycerol (DAG) and ceramide . These intermediates lead to the activation of serine/threonine kinases such as protein kinase C (PKC) isoforms that phosphorylate and inhibit the insulin receptor and IRS proteins.
Glucagon is an important counterregulatory hormone that opposes insulin actions, and increases blood glucose levels through its effects on liver glucose output. Glucagon promotes the production of glucose through elevated hepatic glycogenolysis and gluconeogenesis and through decreased glycolysis and glycogen synthesis. Glucagon inhibits hepatic FFA synthesis ( de novo lipogenesis ) from glucose. Glucagon also maintains blood glucose indirectly through stimulation of ketogenesis , which provides an alternative energy source that leads to glucose sparing in many tissues.
As discussed in Chapter 2 , glucagon is a member of the secretin gene family . Preproglucagon is proteolytically cleaved in the pancreatic islet α cell in a cell-specific manner to produce the 29-AA glucagon (see Fig. 2.9 ). Glucagon is highly conserved among mammals.
Like insulin, glucagon circulates in an unbound form and has a short half-life (about 6 minutes). The predominant site of glucagon degradation is the liver, which degrades as much as 80% of the circulating glucagon in one pass. Because glucagon (either from the pancreas or the gut) enters the hepatic portal vein and is carried to the liver before reaching the systemic circulation, a large portion of the hormone never reaches the systemic circulation. The liver is the primary target organ of glucagon, with lesser effects on adipose tissue. As discussed later, glucagon opposes the actions of insulin. Thus several factors that stimulate insulin inhibit glucagon. Indeed, it is the insulin-to-glucagon ratio that determines the net flow of hepatic metabolic pathways. A major stimulus for glucagon secretion is a drop in blood glucose, which is primarily an indirect effect via the removal of inhibition of glucagon secretion by insulin ( Fig. 3.7 ). Circulating catecholamines, which inhibit insulin secretion through α 2 -adrenergic receptors, stimulate glucagon secretion through β 2 -adrenergic receptors (see Fig. 3.7 ). Serum AAs also stimulate glucagon secretion. This means that a protein meal will increase postprandial levels of glucagon along with insulin, thereby protecting against hypoglycemia. In contrast, a carbohydrate-only meal stimulates insulin secretion and inhibits glucagon secretion.
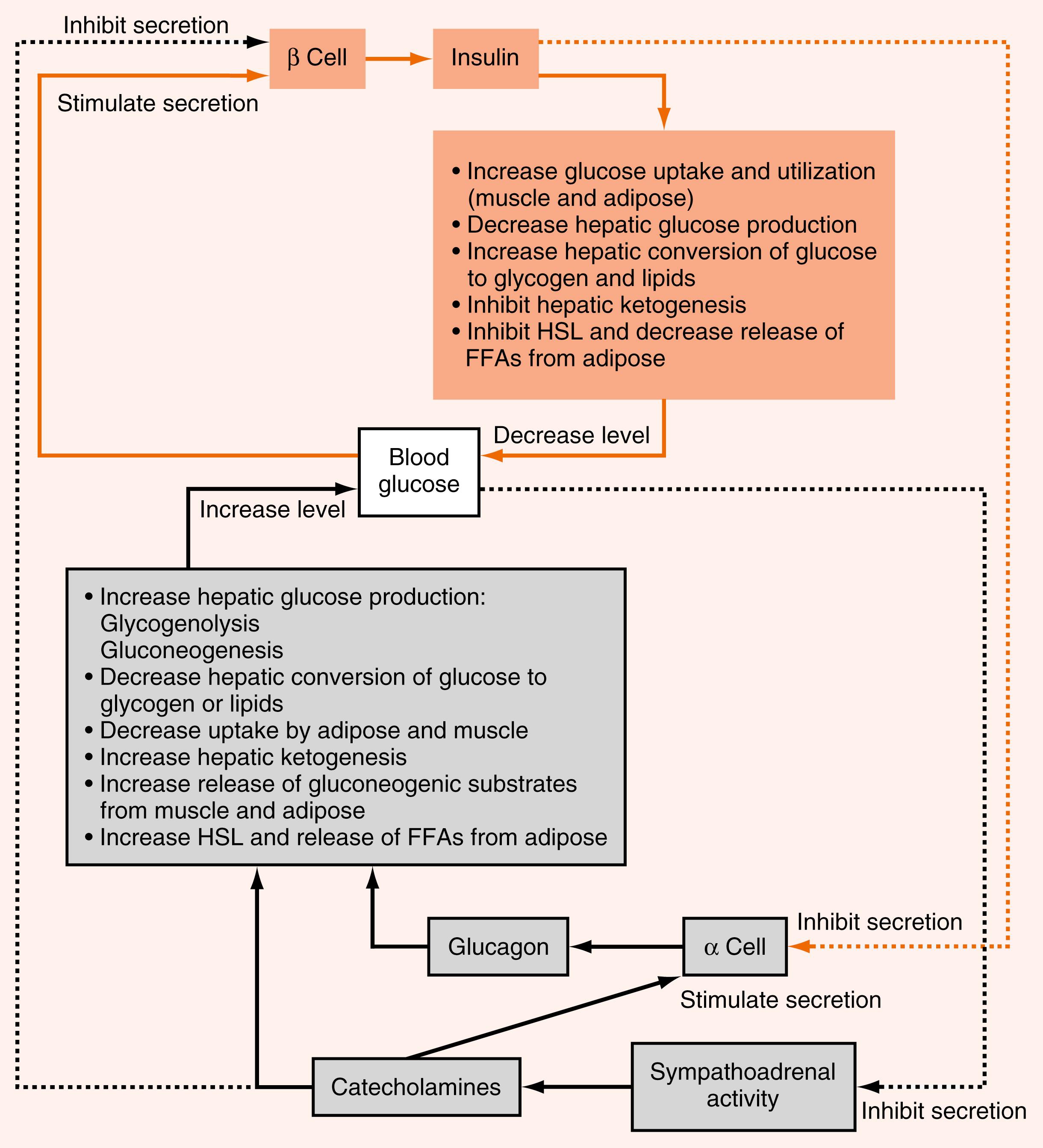
The glucagon receptor is a 7-transmembrane receptor primarily linked to Gs-containing heterotrimeric G-protein complex (see Chapter 1 ). Consequently, glucagon increases intracellular cAMP levels in the liver. The increase leads to activation of protein kinase A (PKA). Glucagon stimulated PKA opposes the effects of insulin/Akt-activated protein phosphatases at several steps within hepatic and adipocyte metabolic pathways. The glucagon receptor is expressed by hepatocytes and adipocytes, but not by skeletal muscle.
The other major counterregulatory factors are the catecholamines , epinephrine and norepinephrine . Epinephrine, and to a much lesser extent norepinephrine, are secreted by the adrenal medulla (see Chapter 7 ), whereas only norepinephrine is released from postganglionic sympathetic nerve endings. The direct metabolic actions of catecholamines are mediated primarily by β 2 - and β 3 -adrenergic receptors located on muscle, adipose, and liver. Like the glucagon receptor, β-adrenergic receptors are linked to a Gs signaling pathway that increases intracellular cAMP. Epinephrine also promotes glycogenolysis and gluconeogenesis through the α 1 -adrenergic receptor , which is coupled to the Gq/IP3/DAG signaling pathway.
Catecholamines are released from sympathetic nerve endings and the adrenal medulla in response to decreased glucose concentrations, stress or alarm, and exercise. Decreased glucose levels (i.e., hypoglycemia) are primarily sensed by hypothalamic neurons, which initiate a sympathetic response to release catecholamines.
Catecholamines circulate in the blood as free hormones, and both circulating and tissue catecholamines are rapidly enzymatically inactivated (see Chapter 7 ).
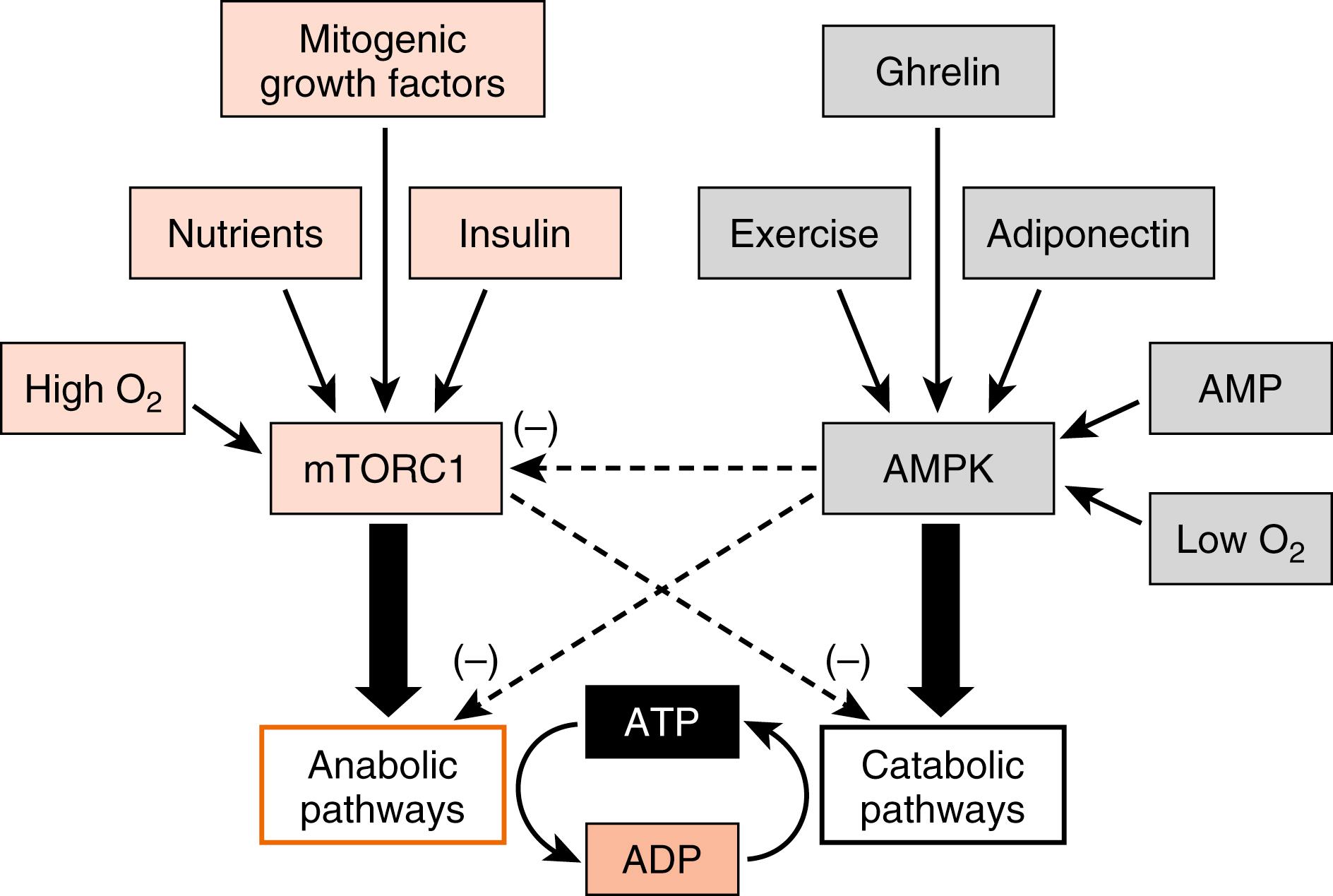
Importantly, all cells must balance energy needs associated with their differentiated function with other uses of carbon, especially the synthesis of macromolecules (lipids, nucleic acids, proteins) and the assembly of organelles that is associated with maintenance of cellular viability. There are several master regulatory nutrient and energy sensors that monitor intracellular nutrient levels and the relative amount of ATP to balance anabolic pathways with catabolic ones.
Two of these regulatory factors are mTORC1 and AMP-activated kinase (AMPK ; also a multimeric complex). In the presence of insulin and high levels of intracellular nutrients (AAs, glucose) that signal an abundance of carbons, mTORC1 promotes energy-consuming anabolic pathways, such as ribosomal RNA production and ribosome assembly, protein synthesis, and lipogenesis ( Fig. 3.8 ). Also, activation of mTORC1 inhibits the breakdown of intracellular macromolecules and organelles for energy and survival, a process termed autophagy . In contrast, a drain on energy supply associated with an elevation of AMP levels, or an indication of high energy use such as elevated intramuscular Ca 2+ levels and or reduced O 2 , activates AMPK (see Fig. 3.8 ). Hormones associated with a fasting state (ghrelin, adiponectin; discussed later) also activate AMPK. Active AMPK inhibits anabolic pathways (in part by inhibiting mTORC1 activity) and promotes energy-generating catabolic pathways, including glycolysis and β-oxidation of FFAs (see later). Although other important energy-sensing factors exist in cells, mTORC1 and AMPK have emerged as two centrally important factors involved in cellular energy balance, and the interaction of these two complexes with hormonal signaling is discussed later.
The hormonal regulation of the major metabolic pathways, with emphasis on key regulated enzymes and transporters, during the digestive phase compared with the fasting phase is presented in this section.
During the digestive phase, circulating nutrient levels increase. Following are some basic facts concerning the metabolism of glucose, FFAs and AAs during the digestive phase, with emphasis on hepatocytes, skeletal muscle and adipocytes.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here