Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Because of the increased use of central venous catheters and implantable pacemakers and defibrillators, upper extremity deep venous thrombosis (UEDVT) has become more frequent. Central vein stenosis and thrombosis, usually from neointimal hyperplasia, are commonly seen in dialysis-dependent patients or in patients who have had placement of an indwelling central venous catheter. Traditional risk factors also include thrombophilia and cancer. It is estimated that approximately 10% of all cases of deep venous thrombosis (DVT) occur in the upper extremity veins. Even though these cases are less likely to result in pulmonary embolism and postthrombotic syndrome than in lower extremity DVT, they are associated with a risk of pulmonary embolism (5.6%), venous gangrene, and disabling arm or neck swelling requiring proper recognition, diagnosis, and treatment.
UEDVT can be classified as primary, when it occurs without an inciting cause, and secondary, when it is related to an underlying catheter or previous catheter. Primary UEDVT, also known as effort thrombosis, usually occurs in healthy young individuals (third decade of life) who present with sudden, severe swelling of the upper extremity that may be associated with cyanosis and arm paresthesia. The condition is usually seen in patients after a repetitive activity and is known by the eponym Paget-Schroetter syndrome after a British and a German physician who simultaneously reported it. These patients usually have a compressive phenomenon at the thoracic outlet. It is traditionally seen at the level of the clavicular head and first rib. The scalenus anterior muscle and tendon, along with the bony structures, may compress the subclavian vein at this level, particularly at some stressed positions of the arm. The process can also be seen with the cervical ribs ( Figs. 24.1 and 24.2 ). The combination of the compression of the costoclavicular portion of the axillosubclavian vein and repetitive stress leads to intimal damage and subsequent fibrotic reactions of the underlying vein. This intimal damage along with the diminished flow from the compression then promotes thrombosis. Patients with effort thrombosis must be identified because if this condition is not treated, it can progress, and patients may experience chronic disability (25%–74%).
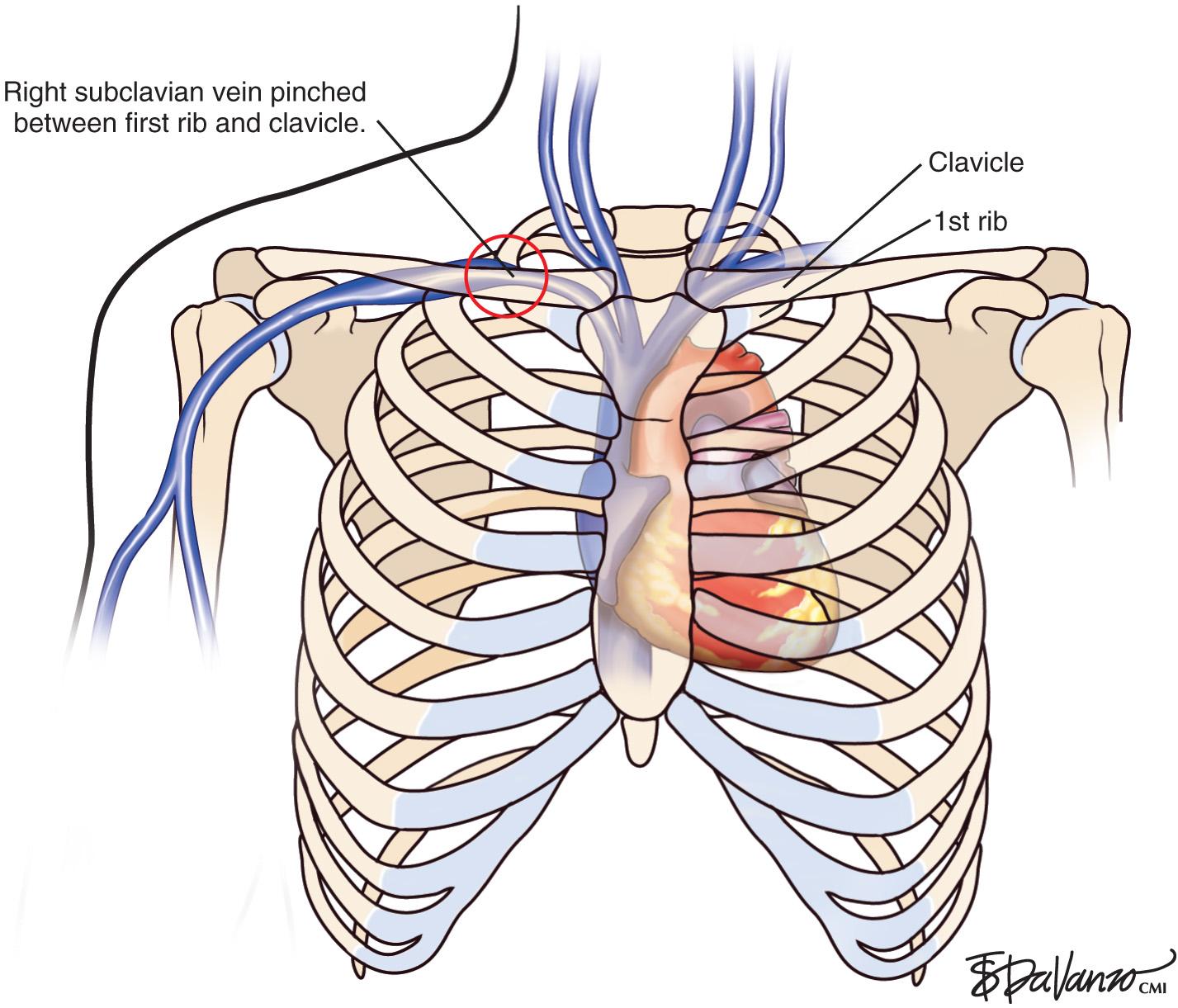
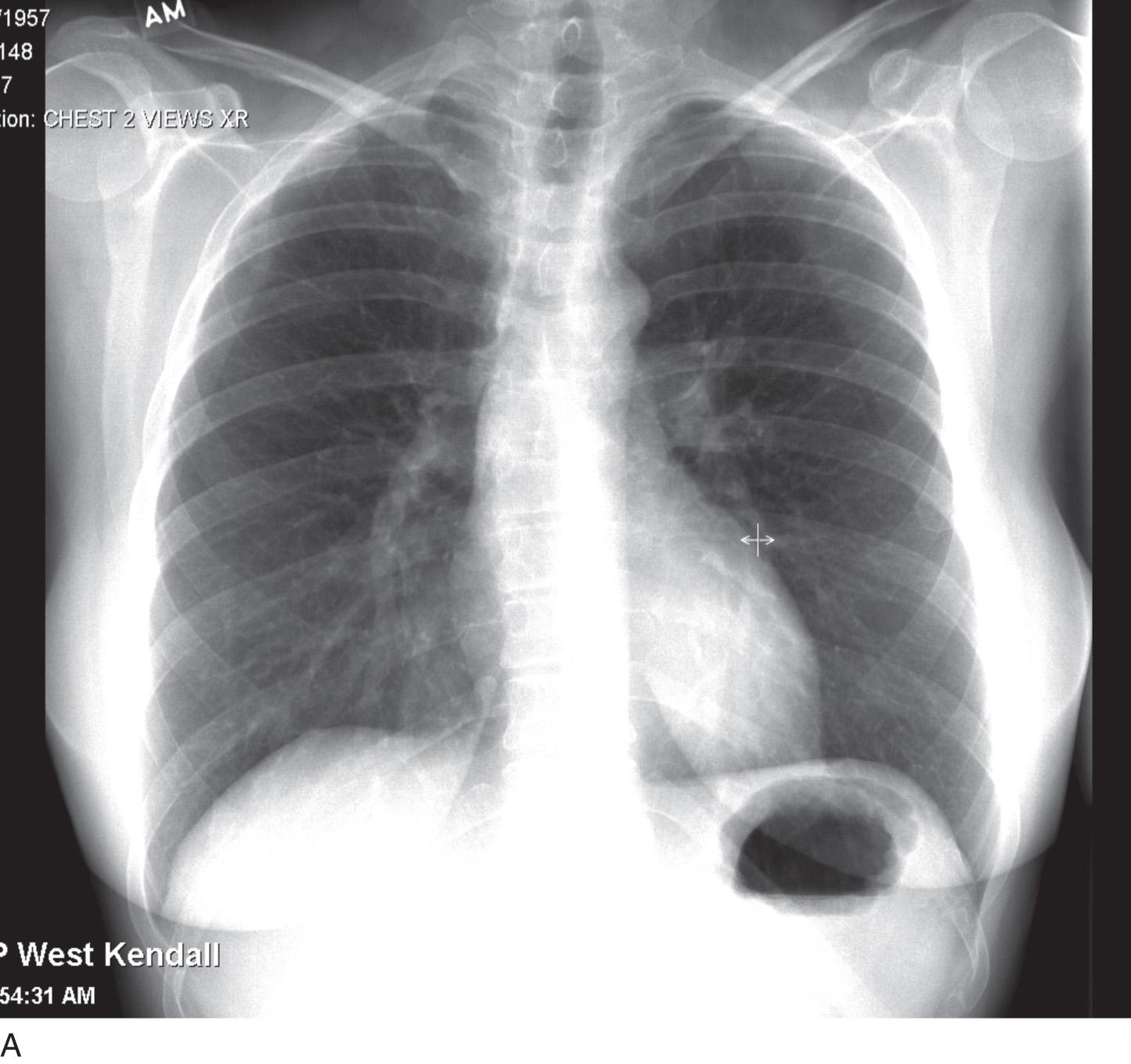
As opposed to patients with secondary UEDVT, in these patients there is an extrinsic venous compression responsible for the thrombosis. The goal of treatment is to restore venous patency and relieve the compression. When effort thrombosis is suspected in a patient, a catheter-directed chemical thrombolysis (if the patient is a candidate) is performed followed by a limited angioplasty, if needed, to restore flow. The results of the angioplasty are variable depending on the underlying injury to the vein. The hope is that the vein can be gently dilated to attempt to maintain patency; the patient can then undergo anticoagulation until decompression surgery can be performed. It is important to diagnose the compression during the thrombolysis procedures and understand that the long-term patency of angioplasty will be limited in the setting of external compression. Stents should never be placed in this compressive environment.
There is controversy about whether to operate immediately or several weeks after thrombolysis. Some surgeons will only operate in the setting of persistent symptoms. Typically, patients are treated with immediate surgery shortly after thrombolysis to relieve the compression. Several weeks after surgery, the status of the vein can be reassessed with ultrasound and angioplasty can be performed as needed to maintain or reestablish flow. There is also controversy whether it is worthwhile to resect the rib in patients who are asymptomatic with well-established collaterals; however, most institutions will proceed with first rib resection to attempt to reestablish patency of the axillosubclavian vein.
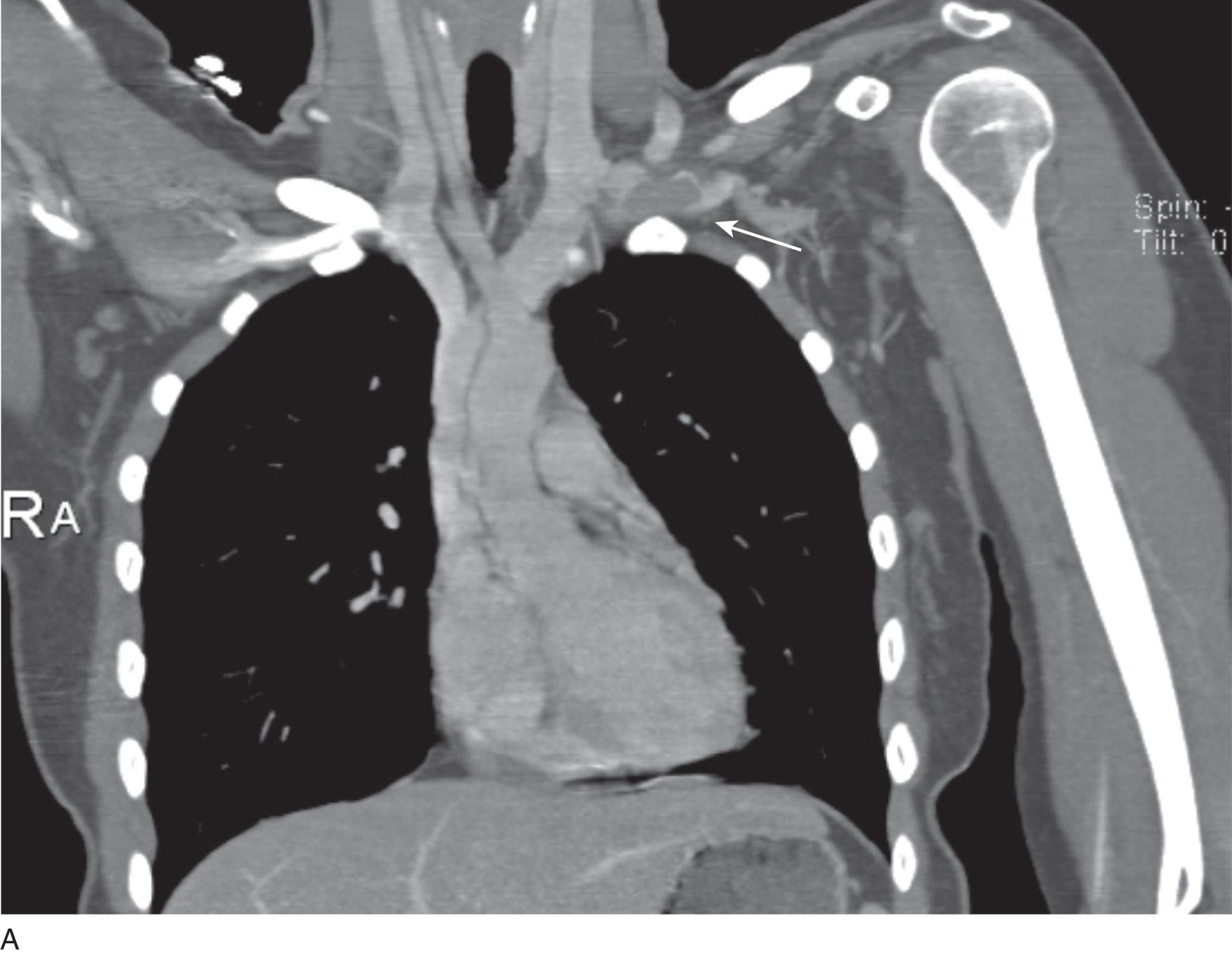
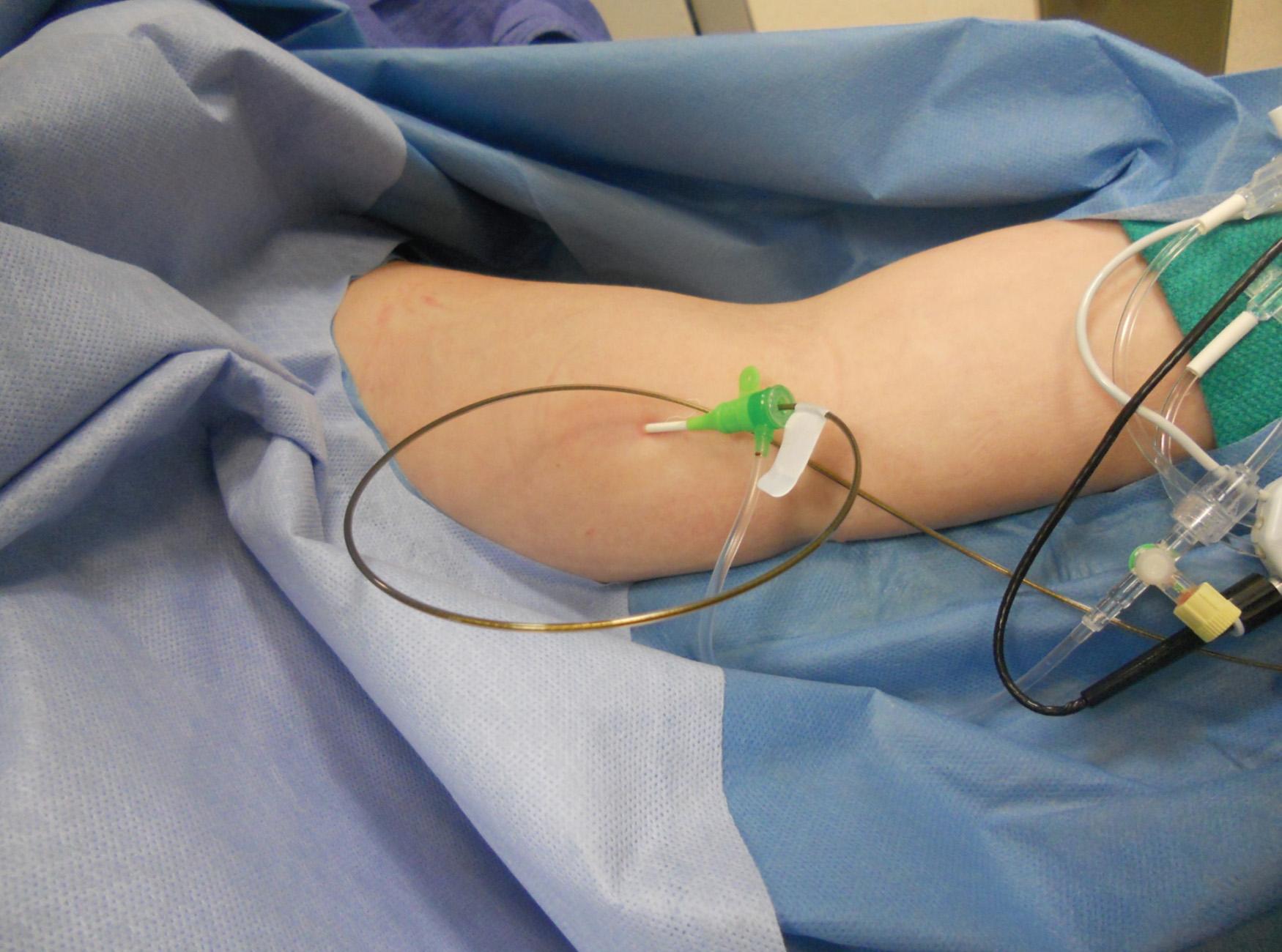
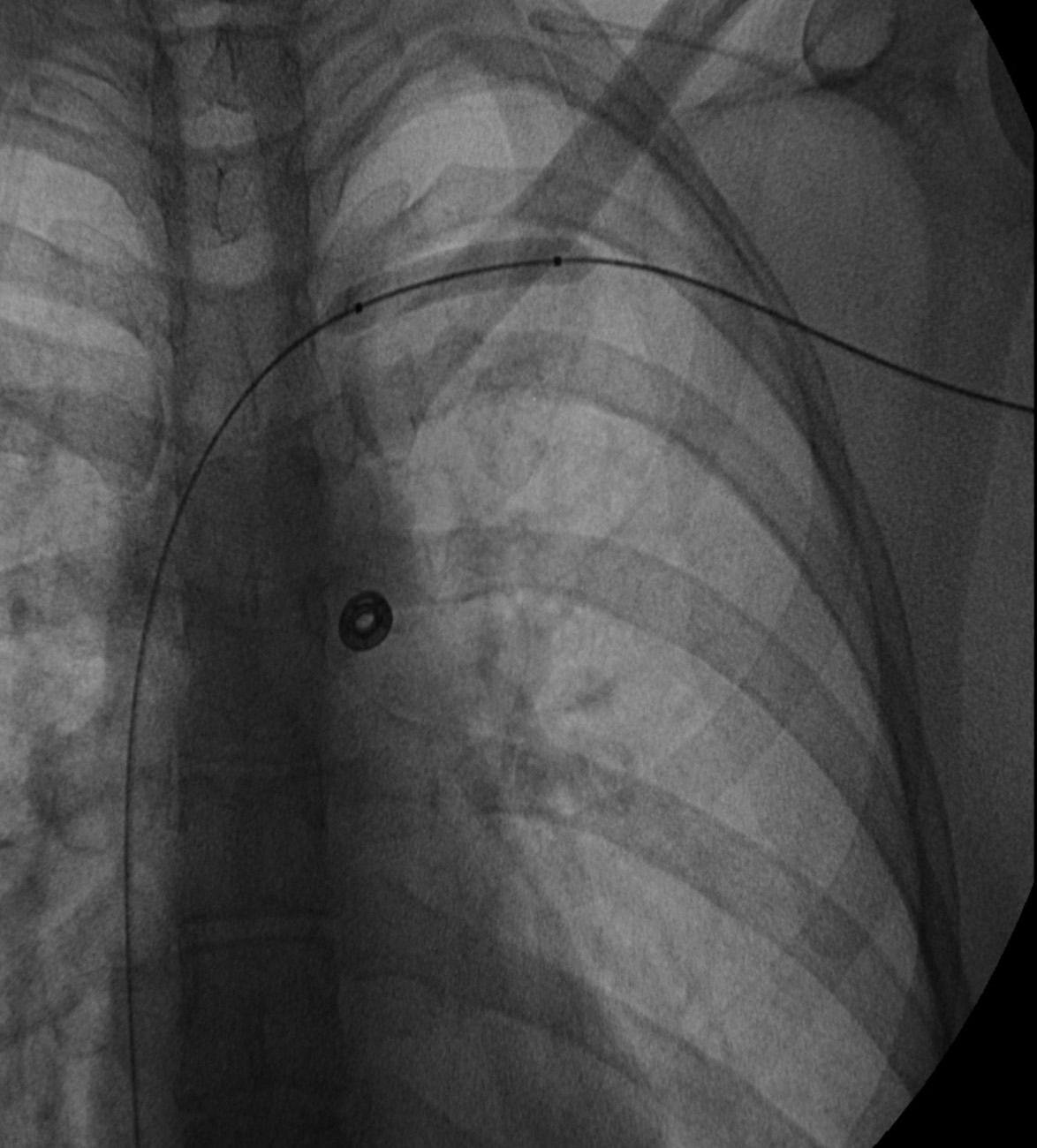
Before treatment, it is common for patients to have a venous duplex ultrasound demonstrating thrombosis. A computed tomography (CT) scan or magnetic resonance image highlighting the venous phase may be helpful in demonstrating the compression and excluding other sources of central occlusion ( Fig. 24.3 ). Ultrasound-guided vascular access is achieved traditionally into the basilic or brachial vein to obtain direct access into the axillosubclavian vein. It is important to establish that the vein being punctured is patent. A 4-Fr or 5-Fr ministick microcatheter system is used to perform an initial venogram and document the extent of thrombosis ( Fig. 24.4 ). Once the entry vein is found to be satisfactory, a 5-Fr or 6-Fr vascular sheath is placed. The axillosubclavian thrombosis is crossed with a soft tip guidewire (Bentson wire; Cook Medical, Bloomington, IN) and an angled catheter (Kumpe or multipurpose). The catheter is exchanged for a thrombolysis catheter that is placed across the area of thrombosis ( Fig. 24.5 ). The catheter infusion length is matched to the length of thrombosis, and the patient is treated overnight with thrombolytics. The choice and dose are usually dependent on the operator's choice, amount of thrombus, and patient risk factors. We usually coadminister peripheral low-dose heparin during the infusion, typically 300 units of heparin per hour ( Fig. 24.6 ). In the setting of an acute decompensation requiring rapid thrombectomy or a patient who may not be a candidate for chemical thrombolysis, mechanical thrombectomy may be performed.
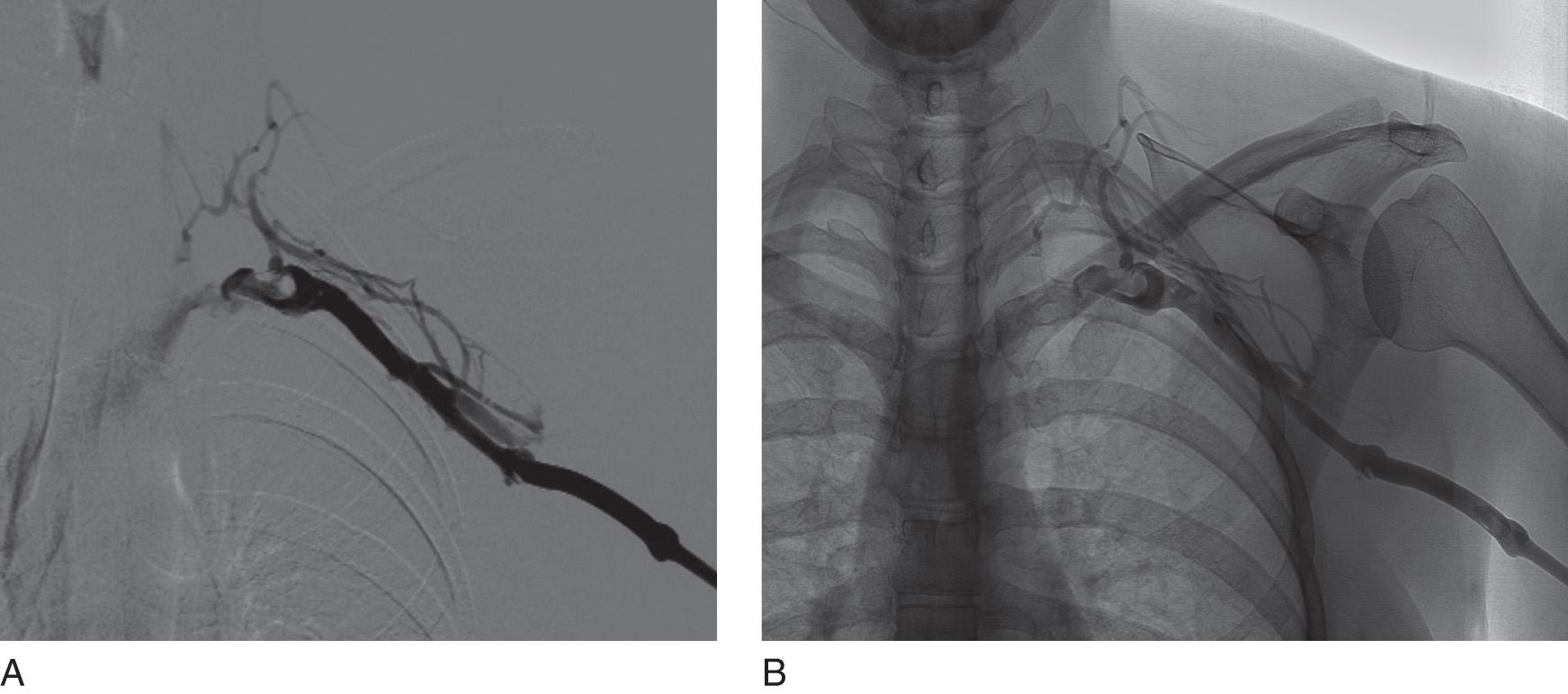
![Fig. 24.5, Frontal image demonstrating a wire across the occlusion and terminating in the inferior vena cava with a thrombolysis catheter (6-Fr EKOS ultrasound-aided infusion catheter [Bothell, WA]) positioned across the occlusion for overnight chemical thrombolysis. Fig. 24.5, Frontal image demonstrating a wire across the occlusion and terminating in the inferior vena cava with a thrombolysis catheter (6-Fr EKOS ultrasound-aided infusion catheter [Bothell, WA]) positioned across the occlusion for overnight chemical thrombolysis.](https://storage.googleapis.com/dl.dentistrykey.com/clinical/EndovenousManagementofCentralandUpperExtremityVeins/4_3s20B9780323511391000243.jpg)
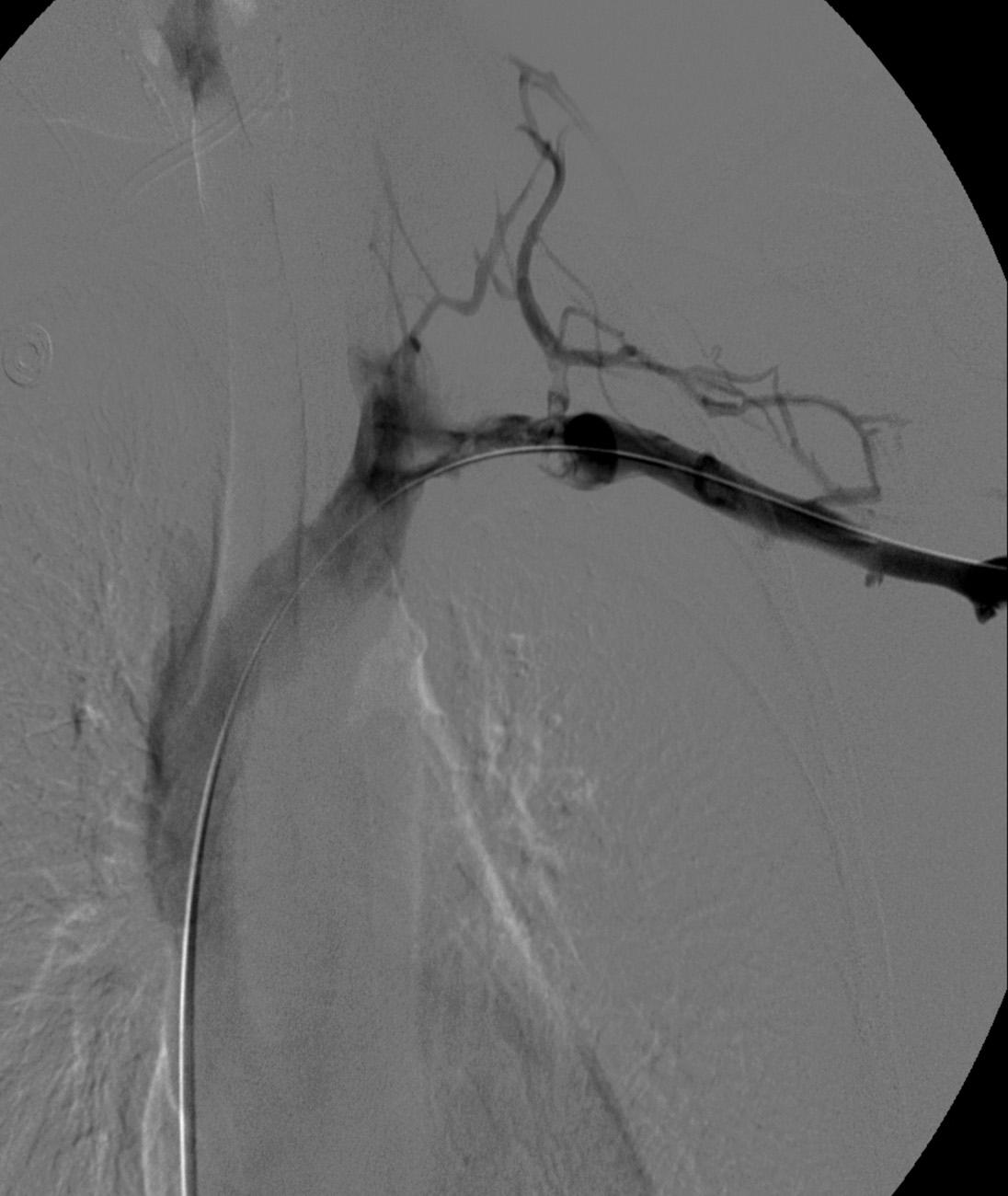
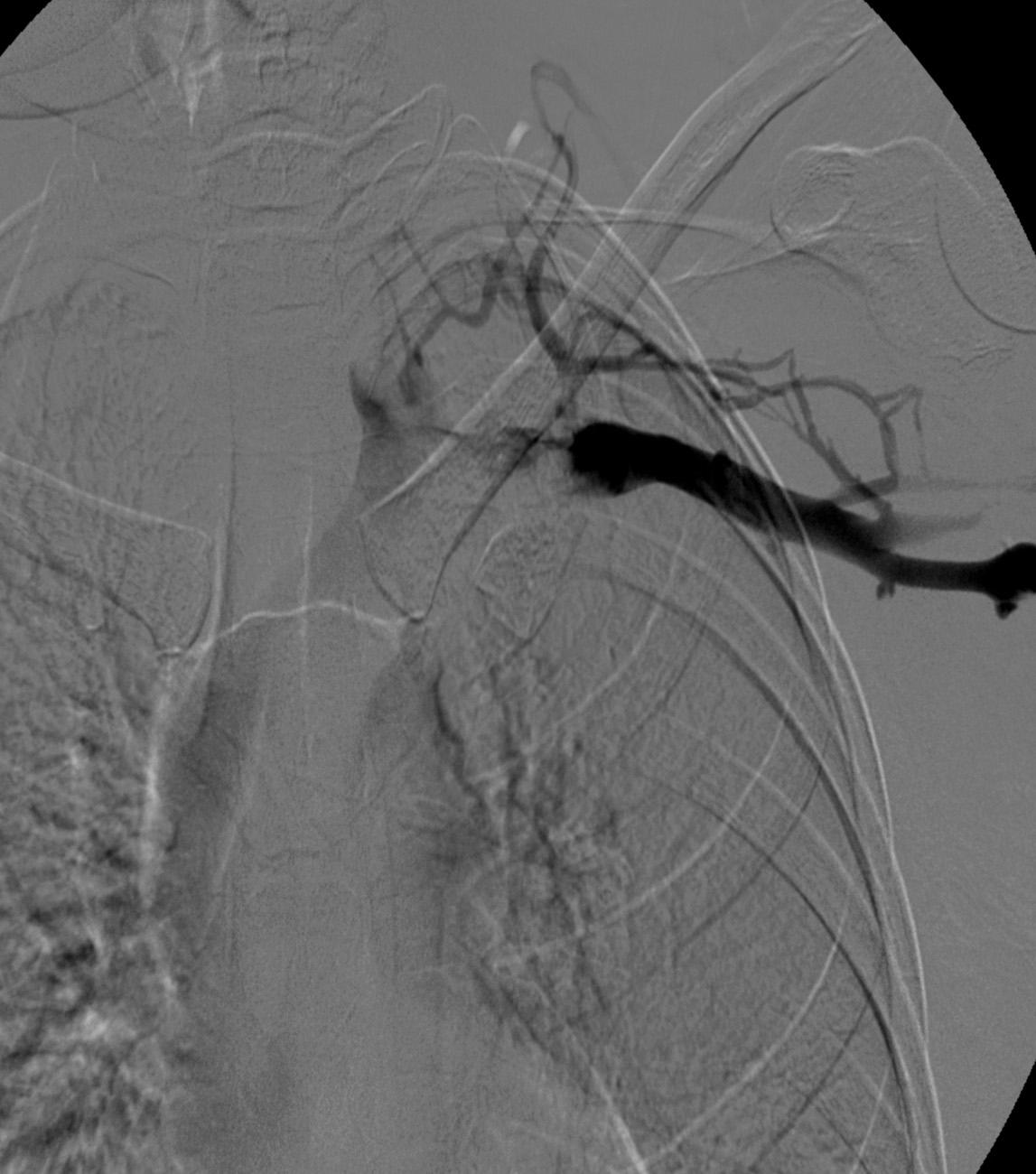
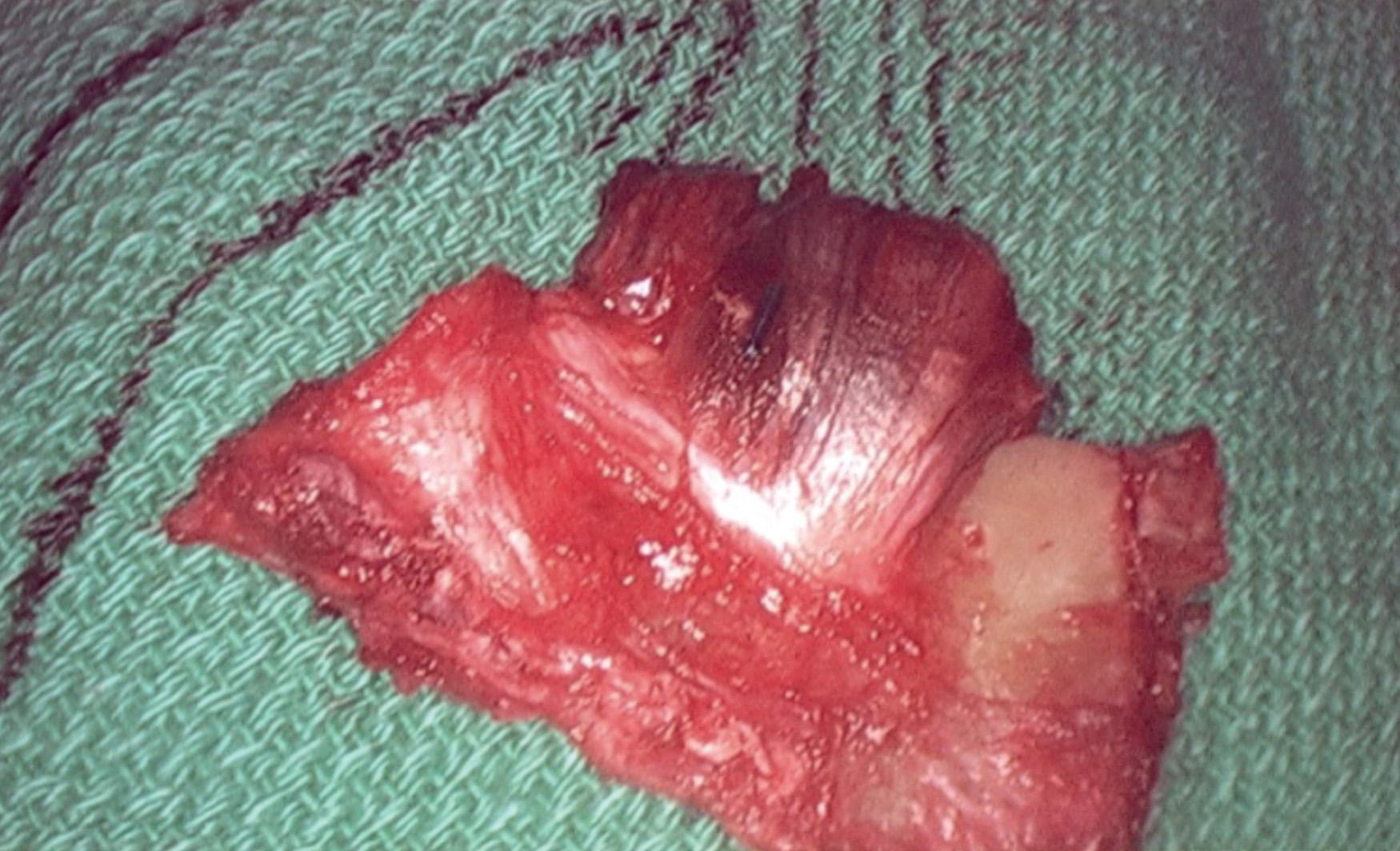
After overnight catheter directed thrombolysis, a venogram is performed to assess the patency of the axillosubclavian vein ( Fig. 24.7 ). Usually, overnight thrombolysis is sufficient to clear the associated thrombus, but in certain patients, a second day of thrombolysis may be necessary. As with any thrombolysis procedure, the patient is informed of the potential risk of bleeding and monitored carefully, as well as with fibrinogen levels. A venogram is then performed in a provocative position to assess the amount of compression and secure the diagnosis. We usually abduct and externally rotate the arm over the patient's head. The area of compression on the vein is usually still present after thrombolysis ( Figs. 24.8 and 24.9 ). Angioplasty can then be performed if there is a persistent high-grade stenosis ( Fig. 24.10 ). A low-pressure balloon is used, not solely to treat the venous obstruction but also to assess the amount of fibrosis and neointimal damage because a severely damaged vein may require a venous patch at the time of surgical decompression. The surgical decompression may be performed from a transaxillary or supraclavicular approach. A portion of the first rib along with the tendinous attachment of the transected anterior scalene muscle is usually removed ( Fig. 24.11 ).
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here