Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The first recorded bronchoscopic intervention is attributed to Gustav Killian, who in 1897 used an esophagoscope to remove a piece of pork bone from a patient's right mainstem bronchus. A few decades later, Chevalier Jackson, now known as the father of endoscopy, became instrumental in the advancement of the field of rigid endoscopy of both the airway and esophagus. Advancements in endoscopic technology have led to safer and more effective alternatives to surgery in nonoperative candidates. This chapter focuses on endoscopic bronchial and esophageal interventions, including photodynamic therapy, radiofrequency ablation, laser ablation, brachytherapy, and stenting. Evolving endoscopic procedures for benign esophageal diseases (achalasia, Zenker diverticulum, and gastroesophageal reflux) are also discussed.
The principle of photodynamic therapy (PDT) lies in the preferential accumulation and subsequent activation of a photosensitizing agent in target cells, that when activated by light of a specific spectrum, results in selective tissue destruction. Photosensitizers used in thoracic surgery include the most commonly used purified hematoporphyrin derivatives (porfimer sodium [Photofrin]), chlorines (temoporfin or m -tetrahydroxyphenyl chlorin [ m -THPC]), and 5-aminolevulinic acid (5-ALA). Each molecule is activated at an optimal wavelength of light absorption (630 nm for porfimer sodium and 5-ALA and 652 nm for m -THPC).
Although photosensitizers accumulate in all cells of the body, higher concentrations are retained within neoplastic cells and their interstitium within 1 to 4 days. Although the mechanism remains unclear, increased cellular proliferation, altered lymphatic drainage, and neovascularization are hypothesized to be responsible for the increased accumulation seen in neoplastic tissues. When laser light of the appropriate wavelength is delivered to neoplastic cells harboring the photosensitizer, it triggers a series of events culminating in cell destruction. The individual cells are bombarded with photons of a wavelength specific to the photosensitizer being used. The absorbed energy then acts as a catalyst in the formation of highly reactive oxygen species (e.g., superoxide anions, peroxide anions, singlet oxygen) whose primary cellular targets of photodamage include cellular membranes, amino acids, and nucleosides. It is important to remember that all cells will absorb some of the currently available photosensitizers; thus, surrounding normal tissue will be damaged to some degree and can limit the effectiveness of phototherapy.
The first step in PDT is the administration of the photosensitizer. Porfimer sodium (1.5 to 2.0 mg/kg) and m -THPC (0.075 mg/kg) are injected intravenously; 5-ALA (30-60 mg/kg) is given orally. Despite the benefit of being able to administer orally, increased tumor specificity and shorter duration of skin photosensitivity, 5-ALA has yet to gain widespread clinical acceptance. At the current oral dose, 5-ALA PDT accumulates preferentially in the mucosa and results in a more shallow tissue injury with necrosis only to a depth of 1 mm. Higher 5-ALA doses are avoided because of its increased side effects (e.g., malaise, nausea, vomiting, alopecia, transient elevation of liver enzymes). On the other hand, m -THPC is 100 times more photoactive than porfimer sodium, and it has a shorter elimination half-life; this results in deeper penetration and necrosis. As a result, m -THPC is typically reserved for use in malignant lesions that penetrate into submucosa or deeper.
The most commonly used photosensitizer is porfimer sodium, which has a depth of penetration of approximately 5 mm. It can be given in the outpatient setting, 1 to 4 days before endoscopic light application. It is most commonly used for endoluminal tumors of the airway and the esophagus, but other applications include anorectal, dermatologic, gastric, and hepatobiliary. Its short-term side effects include chest pain, odynophagia, and fever. Education of the patient is essential to minimize complications related to photosensitivity. Patients are instructed to avoid all sunlight and bright indoor light exposure for 30 days. Patients remain photosensitive for a period of 4 to 8 weeks, after which they can be gradually reexposed to sunlight.
Our preference has been to use conscious sedation and flexible endoscopy in most cases. A cylindrical diffuser fiber is used to deliver light therapy to the tumor. The sizes used are 1, 2.5, and 5 cm ( Fig. 6-1 ). Choice of fiber length is based on technical ability to expose the tumor maximally to the light and to minimize the surrounding normal tissue exposure. The diffuser fiber is placed alongside the tumor. If this is not possible because of occlusion of the lumen, the tumor can be impaled with the probe. Caution should be exercised if impalation of the tumor is attempted, because without viewing and defining the length of tumor obstruction and determining where and whether patent distal lumen begins it can be somewhat risky. Multiple light illumination cycles may be needed, depending on the length of the tumor relative to the length of the probe. The diffuser must be positioned to minimize light delivery to normal tissues. Endoscopy is repeated at 48 hours and sometimes a third time after an additional 48 hours. During the repeated endoscopic procedures, necrotic debris is removed by irrigation and suction. Usually, further light treatments are delivered to the tumor at the time of repeated endoscopy.
In many cases, PDT can be performed in the outpatient setting; however, caution should be exercised in treating nearly obstructing lesions of the esophagus or central airways, because perioperative edema and necrotic tissue can temporarily worsen obstruction, leading to dyspnea or, in the case of esophageal obstruction, initial worsening of the dysphagia.
Contraindications to PDT are few. Patients suffering from porphyria or those who are allergic to porphyrins cannot receive porfimer sodium. PDT is contraindicated in the presence of an esophagorespiratory fistula or in tumors eroding into a major blood vessel. PDT is not recommended for the treatment of obstruction caused by extraluminal compression, since the effectiveness is limited because of an inability to expose the tumor to the light activation. Caution should be used in treating patients with underlying renal or hepatic dysfunction.
Photodynamic therapy has been used in an investigational setting for the treatment of lung cancer since 1980. It is currently approved as a treatment option for centrally located early stage 0 (TisN0M0) and stage 1 ( T1N0M0 ) lung cancer. These small central tumors are rare, although some centers with active early screening programs, such as sputum cytology or light-induced fluorescence bronchoscopy, encounter these tumors more frequently. The results of PDT for these small lesions should be compared with the results of surgical resection. The central location of these tumors may necessitate complex airway reconstructions. PDT may be a suitable alternative to resection in patients who are not surgical candidates or as an adjunct in decreasing tumor burden prior to surgical resection. There are few central airway tumors that are discovered at a curable stage, because most of these early-stage, centrally located endobronchial lesions often go undetected by radiologic studies. In addition, only about 10% of all non–small cell lung cancer tumors occur in a central location. Superficially spreading tumors (<3 cm 2 ) and small endobronchial tumors (<1 cm 2 ) are reasonable targets for PDT. Initial complete response can be expected in 65% (<3 cm 2 ) to 85% (<1 cm 2 ) of superficial cancers and in 30% (>0.5 cm nodule) to 92% (<0.5 cm nodule) of nodular cancers. Long-term response rates (i.e., >1 year) vary between 30% and 80%. At 5 years of follow-up, complete response rates of up to 29% to 66% have been reported depending upon the exact size, location, and stage of the lesion.
In a study of 21 operative candidates treated initially with two courses of PDT, 52% (11 of 21) were free of tumor at 1 year. Curative PDT failed in the remaining 48% (10 of 21), and they were offered surgery; 8 consented. Of the 11 responders, 9 (9 of 21; 43%) did not require surgery (mean follow-up, 68 months), and 2 were operated on for a second primary lung cancer. It is noteworthy that of the patients who eventually underwent resection, 30% were found to have N1 disease, supporting the role of resection when possible. Our policy has been to reserve curative PDT for patients with significant comorbid factors or those who refuse pulmonary resection. In this group of patients, PDT is an excellent option, with an expected 1-year survival of up to 80%. In our initial experience of treating 10 superficial cancers, a complete response was seen in 70% at a follow-up of 30 months. In other prospective series, 80% to 93% of nonsurgical candidates were alive at 5 years of follow-up.
Resection should continue to be the main therapy for early-stage non–small cell lung cancer, because only a relatively small number of patients will have curable central tumors that are suitable for PDT with curative intent. A much larger group of patients with advanced, inoperable, or obstructive central lung cancers may benefit from palliative PDT. In this situation, the therapeutic goal should be symptomatic relief and preservation of quality of life and functional status. Patients most notably find improvement in obstructive symptoms, such as dyspnea or hemoptysis, yet the need for strict photosensitivity precautions should be considered in patients with limited survival.
A cohort of 175 patients with endobronchial non–small cell lung cancer treated with PDT was observed prospectively for a period of 14 years. Most patients had squamous cell carcinoma (89.3%), and treatment by conventional modalities, including chemotherapy, radiotherapy, laser ablation, and surgery, had failed in 73.1%. The mean number of treatments was 2.8 per patient. After multivariate analysis, stage was found to have the most significant effect on survival. Poor performance status (i.e., Karnofsky performance status < 50) negatively affected outcome in advanced-stage tumors (IIIa-IV). However, patients with a low Karnofsky performance status (<50) secondary to pulmonary symptoms still derived benefit from PDT. Prolonged survival (12 to 37 months) was observed in 66% of stage IIIa-IV patients with poor performance. The median survival for the entire group was 7 months. Of the 44 patients with stage IV disease, 21% survived 12 months or longer.
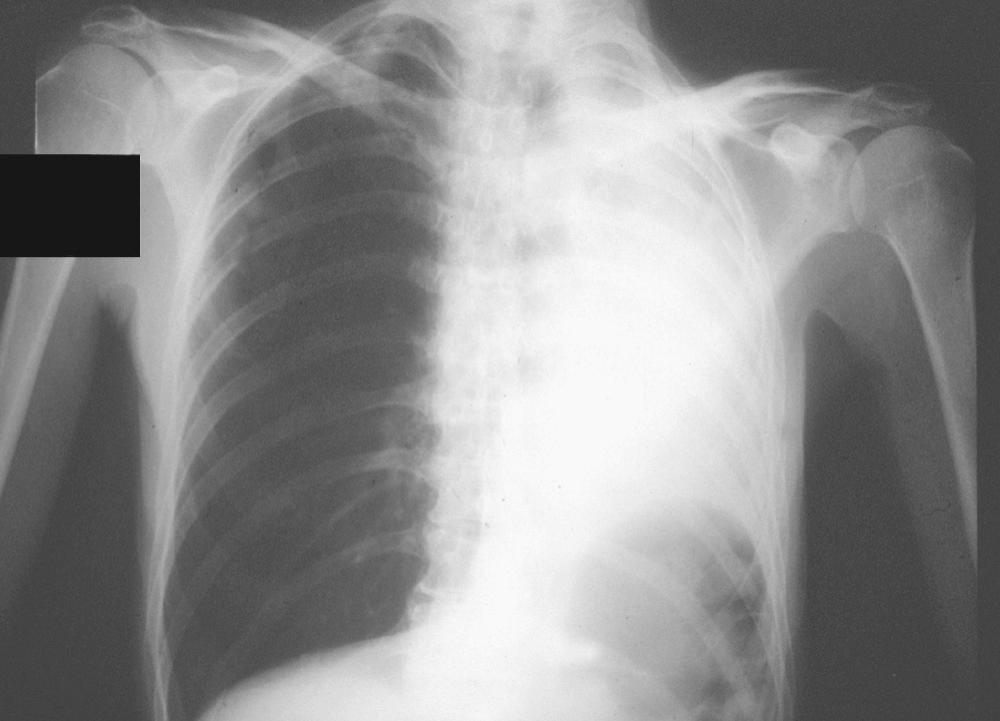
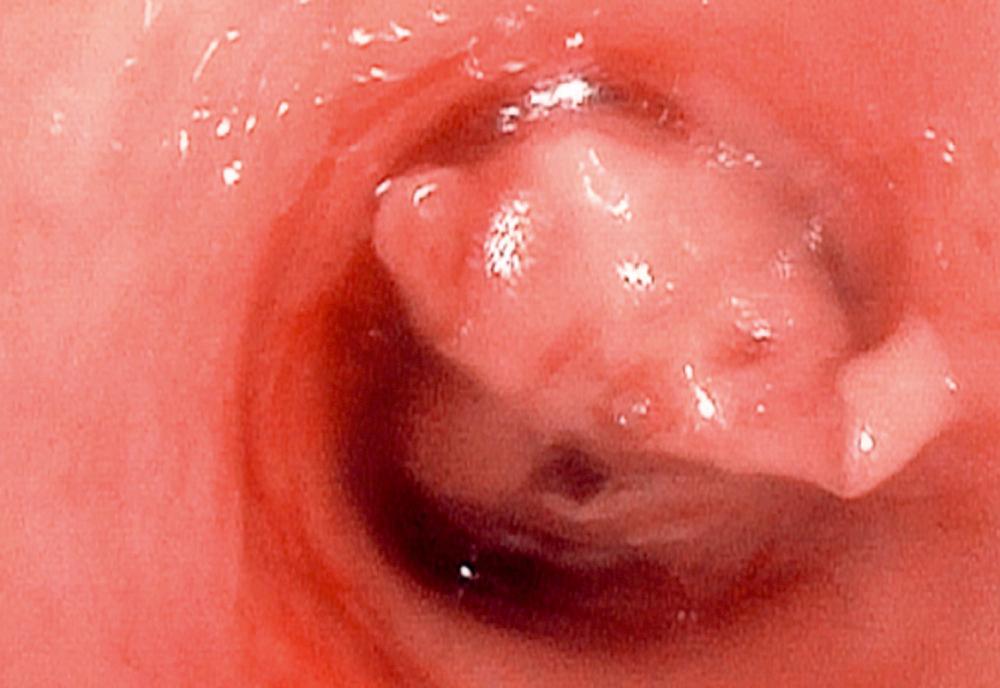
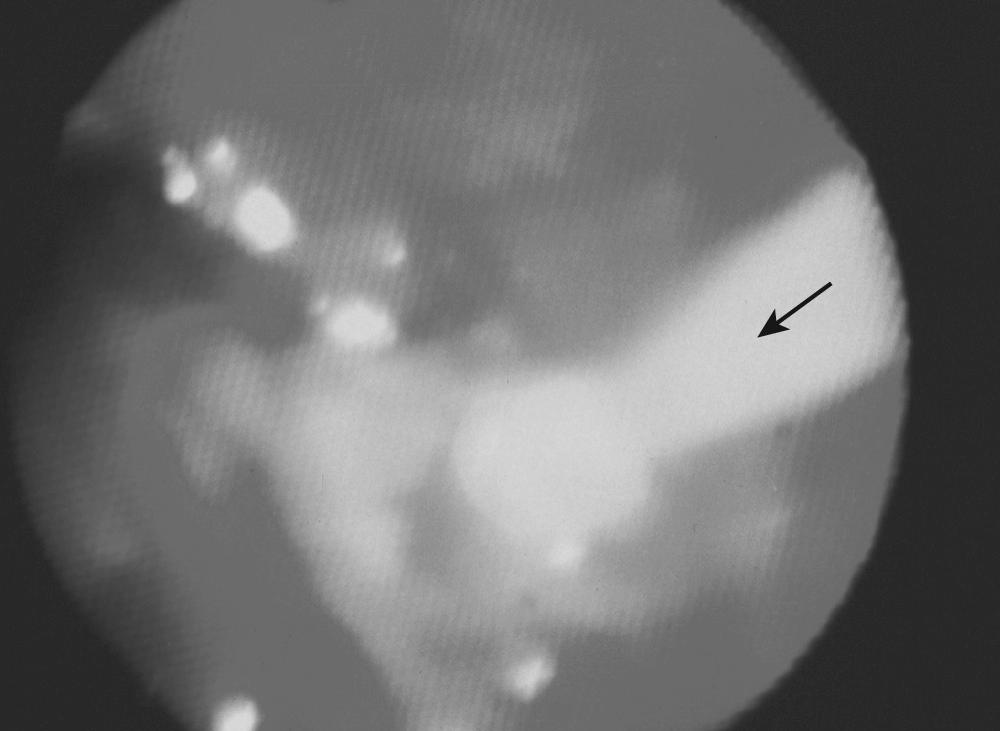
Our initial experience with palliative PDT involved 44 patients with lung cancer and symptoms related to obstruction and hemoptysis. Thirteen patients required a second treatment course at a mean of 2.3 months for recurrent symptoms. There were no fatalities, and 82% of treatments were performed without complications. Hemoptysis was effectively palliated in 90% of treatment courses. Obstruction was successfully palliated in 59% of treatments. Median survival was 2.3 months. PDT has also been shown to be effective in palliating hemoptysis and dyspnea in patients with endobronchial metastases from malignant neoplasms of nonpulmonary etiology. An example of a patient who was treated for a complete lobar obstruction is demonstrated in Figures 6-2 through 6-6 . In general, we have not used PDT for massive hemoptysis for practical reasons; those best suited have subacute but persistent hemoptysis of low volume and have visible endoluminal granular rumor with a bleeding surface area.
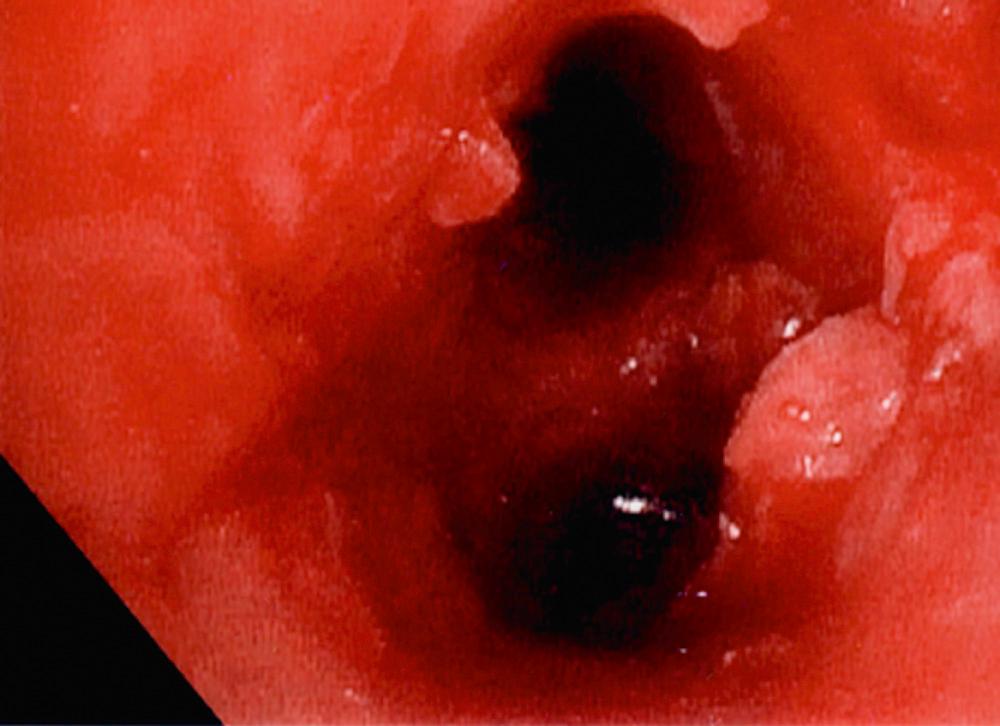
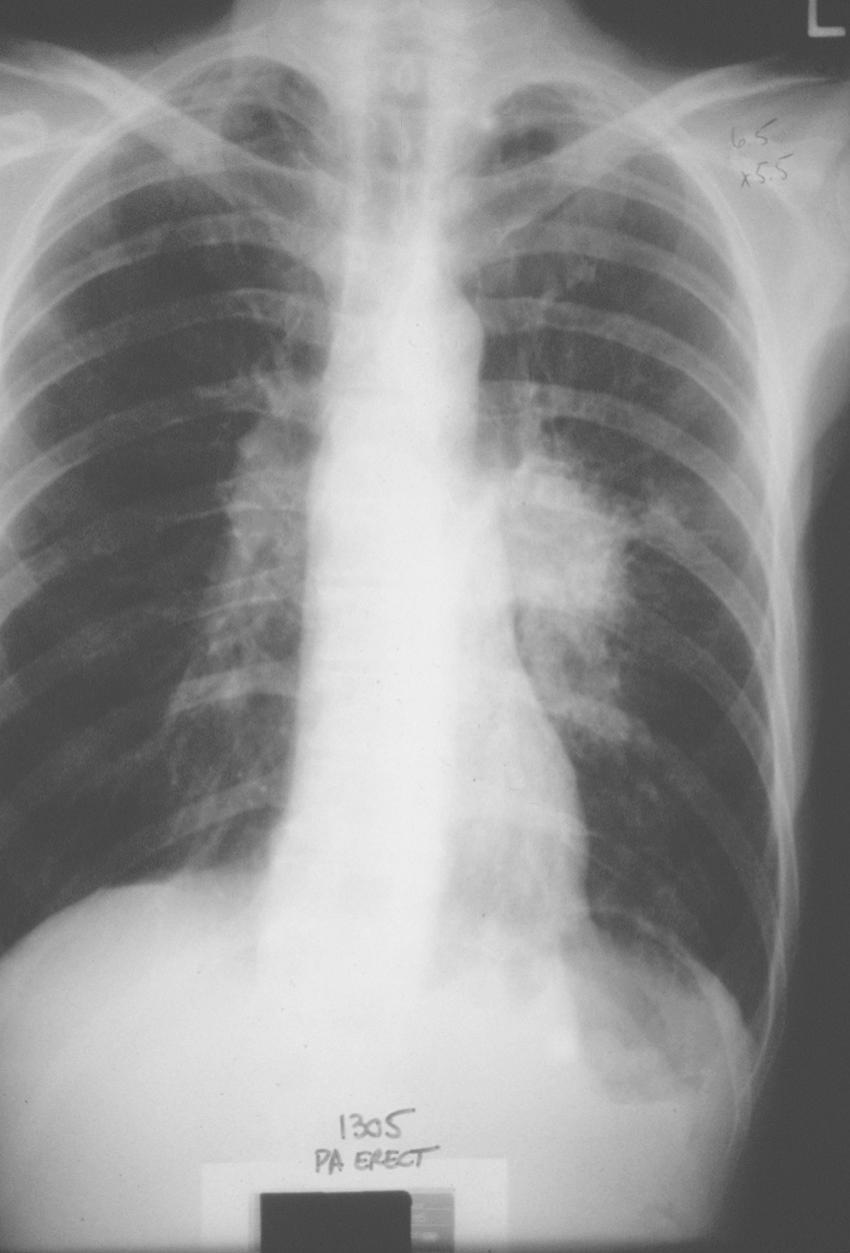
In a randomized prospective study, PDT was compared with standard laser techniques for inoperable non–small cell lung cancer. The bronchoscopic response rate (PDT, 38.5%; laser, 23.5%) and symptomatic improvement at 1 month were equivalent in each group. PDT provided significantly longer-lasting relief (50 days vs. 38 days) and improved median survival (265 days vs. 95 days) compared with laser resection. However, this finding may be explained in part by the lower proportion of advanced-stage tumors seen in the PDT group.
In general, PDT is well tolerated with minimal morbidity. All bright light and direct sunlight exposure must be avoided initially. Patients remain photosensitive for 4 to 8 weeks, after which they can be gradually reexposed to sunlight. Inadvertent direct sunlight exposure has been reported to cause first- and second-degree burns in up to 10% of patients. The restrictions imposed by photosensitivity and their potential impact on quality of life should be considered before treatment. Immediate postprocedure mortality for lung and esophageal PDT (30-day) ranges from 2.6% to 7.1%, but in large part is due to the comorbidities and advanced stage of many of the patients in these series.
The management of high-grade dysplasia associated with Barrett esophagus is controversial. In the recent past, most surgeons advocated esophagectomy because of the associated risks of occult malignant disease and lymph node metastases. On the other hand, concerns related to the morbidity and mortality of esophagectomy have led some clinicians to advocate alternative approaches, such as surveillance, endoscopic mucosal resection, or mucosal ablation. In our experience, we have reported good results and a mortality of approximately 1% with a minimally invasive esophageal resection for patients with Barrett esophagus and high-grade dysplasia. Currently available mucosal ablative methods include endoscopic mucosal resection, PDT, laser ablation, argon plasma coagulation, and radiofrequency ablation. All these techniques attempt to resect or eradicate Barrett epithelium with the hope that it will be replaced by squamous epithelium. Unfortunately, in some instances, islands of columnar epithelium may recur under a normal-appearing squamous epithelial layer leading to difficult detection. This problem is addressed by other endoscopic techniques, such as initial endoscopic mucosal resection or endoscopic submucosal dissection, whereby the esophageal mucosa is actually resected piecemeal. An in-depth discussion of endoscopic resection techniques is beyond the scope of this chapter, but it should be noted that this approach has become the standard of care in many centers for properly selected patients.
PDT is arguably the most established of these ablative techniques. In a large single-institution study, PDT was combined with the neodymium:yttrium-aluminum-garnet (Nd:YAG) laser to treat 73 patients with high-grade dysplasia and 14 patients with low-grade dysplasia. All patients were observed endoscopically for 12 months. Regression to low-grade dysplasia or no dysplasia was observed in 88% of high-grade dysplasia cases and in 93% of low-grade dysplasia cases. Complete eradication of Barrett epithelium occurred in only 9% of patients after PDT alone. In focusing on the PDT-treated area, a 49% eradication rate was reported. The addition of laser ablation to residual areas of Barrett epithelium increased the eradication rate to 87%. In a 5-year prospective randomized multicenter international trial, PDT with Photofrin plus omeprazole was compared with omeprazole only for the treatment of Barrett esophagus with high-grade dysplasia. Complete ablation of high-grade dysplasia was observed significantly more often in the Photofrin group compared with controls (77% vs. 39%). Progression to esophageal cancer was significantly decreased in the Photofrin group (13% vs. 28%). Treatment-related side-effects were more common in the Photofrin group (94% vs. 13%). Strictures developed in 36% of patients, with 98% resolving after dilation. Notably, the benefits on dysplasia resolution and cancer progression persisted at 5-year follow-up. At 5 years, the study demonstrated that the addition of PDT with Photofrin to proton pump inhibitor therapy resulted in a 38% increase in eradication of Barrett esophagus with high-grade dysplasia, a 14% decrease in the incidence of invasive carcinoma, and a longer time to progression to cancer.
PDT can also be used to treat early-stage esophageal tumors (T1-T2) in patients who are not candidates for surgical resection. In a group of patients that included mostly T1 tumors (12 of 13), regression to Barrett esophagus with low-grade dysplasia or without dysplasia was observed in 77% of patients (10 of 13). At our center, PDT with curative intent is reserved for patients with prohibitively high operative risk. All others undergo minimally invasive esophagectomy. We have reviewed our institutional experience, from 1997 to 2005, using PDT for patients with high-grade dysplasia or superficial esophageal cancer. A total of 50 patients, who either refused or were unfit for surgery, had PDT with curative intent. The majority of patients (70%) had high-grade dysplasia or small (≤T1) tumors. At a median follow-up of 28 months, 32% of patients were alive without recurrence, 30% were alive with recurrent disease, and 38% were deceased from cancer or other causes. The patients with recurrences were retreated with PDT. There was no procedure-related mortality, and the stricture rate was 42%, which were generally easily dilated. Although these results are clearly inferior to esophageal resection, endoscopic ablation appears to be a suitable treatment option for nonsurgical candidates. In another cohort of 28 patients with high-grade dysplasia treated with minimally invasive esophagectomy, 96% remained free of recurrence at 13 months. These results were corroborated by other investigators who have observed excellent long-term survival (100%) after esophagectomy for high-grade dysplasia.
In the treatment of Barrett esophagus with high-grade dysplasia, PDT is well tolerated with usually transient side effects, such as nausea, regurgitation, constipation, odynophagia, dysphagia, weight loss (average of 6 kg), and noncardiac chest pain. Other complications include stricture, tumor growth below the PDT-induced scarring, and Barrett esophagus and tumor recurrence. In our experience, approximately 30% of patients with high-grade dysplasia undergoing PDT will develop an esophageal stricture. Stricture is minimized by decreasing the intensity of light therapy compared with doses used for larger tumors.
Another endoscopic treatment modality for patients with Barrett esophagus is radiofrequency (RF) ablation. The components of the system include a sizing balloon (18 to 34 mm), a 3-cm balloon-based bipolar RF electrode, and a dedicated RF generator. The sizing balloon is used to ensure proper contact between the balloon electrode and the esophageal mucosa. The RF mucosal ablation is achieved by using a power of 300 W to deliver 10 to 12 J/cm 2 . The coagulum is then removed from the esophageal surface by irrigation and suction. The maximum ablation depth is 1 mm, which translates to a depth of injury that is superficial to the muscularis mucosa. The depth of injury is highly reproducible and has been confirmed in the human esophagus. In addition to the circumferential balloon electrode, the approval of an RF ablation device that can be attached to the end of a flexible esophagoscope can be used to ablate focal areas of esophageal mucosa. Encouraging results have been published showing complete eradication of Barrett mucosa and intramucosal carcinoma when RF ablation was used in combination with endoscopic mucosal resection.
With further analysis, it seems that patients with high-grade dysplasia without visible raised or nodular lesions are the optimal candidates for endoscopic management with RFA. The presence of visible lesions requires the addition of a mucosal resection technique to allow for adequate staging and treatment.
Visible lesions containing high-grade dysplasia or intramucosal carcinoma can be treated successfully with RFA, but only as an adjunct to a mucosal resectional technique such as endoscopic mucosal resection.
The Surveillance vs. Radiofrequency Ablation (SURF) trial was a randomized multicenter trial conducted in Europe that randomized patients with a confirmed histologic diagnosis of Barrett esophagus with low-grade dysplasia to treatment with either RFA or standard endoscopic surveillance (at 6, 12, 24, and 36 months). RFA was demonstrated to significantly reduce the risk of progression to high-grade dysplasia or esophageal adenocarcinoma compared with standard endoscopic surveillance (1.5% vs. 27%) while also reducing the risk of progression to esophageal adenocarcinoma (1.5% vs. 8.8%).
Stenting of the esophagus is often the most commonly used modality to palliate dysphagia ; however, PDT offers some advantages in certain clinical situations. In addition to relieving obstruction, PDT can often be used to control or prevent tumor bleeding. It can also prevent the globus sensation associated with stents in the proximal esophagus and the reflux symptoms observed with stents bridging the gastroesophageal junction. PDT is a potential alternative to stenting for endoluminal cancers in both of these locations.
PDT has been compared with laser ablation in a randomized trial. PDT was equivalent to laser ablation for malignant dysphagia and was associated with a lower rate of perforation (PDT, 1%; laser, 7%). We reviewed our experience with PDT in 215 patients suffering from obstructing esophageal cancer. The most common pathologic process was adenocarcinoma (83%). The distal esophagus was involved in 71% of cases. Dysphagia improved in 85% of patients, and bleeding was successfully controlled in 90%. Stenting was subsequently required in 24% of patients. As expected, median survival was poor at 4.9 months; however, effective palliation lasting 3 to 4 months was provided to many of these unfortunate patients. In another prospective series of 77 patients for whom conventional therapy had failed or who were deemed unfit for surgery, investigators reported a median survival of 6.3 months after PDT. The only significant predictor of mortality was clinical stage. Photosensitivity appears to be an acceptable tradeoff in these patients. In one study using satisfaction questionnaires, patients reported that the ability to swallow food was more important than the limitations of photosensitivity. In most cases, the potential benefits of PDT far outweigh the risk of complications.
In patients with obstructing aerodigestive tumors, PDT can result in adverse events. Esophageal strictures (4.8% to 5.2%), perforation (0% to 9.1%), and esophagorespiratory fistulas (0% to 5.2%) are among the most serious PDT-related complications. Esophageal perforation and fistula are probably best treated by esophageal stenting. Other factors contributing to the mortality rate and risk of esophageal perforation include the generally poor performance status of this particular group of patients with advanced tumors and the fact that some patients with obstructing tumors receive concurrent radiation therapy and chemotherapy.
Although several types of lasers including carbon dioxide, argon, potassium titanyl phosphate, and diode are available throughout medicine, the most commonly used endoscopic laser in thoracic surgery is the Nd:YAG laser. The Nd:YAG laser energy can be delivered through a small-caliber fiberoptic conduit, thus making it ideal for use with the flexible bronchoscope or esophagoscope. Adjustments in power level allow either photocoagulation (low power) or thermal necrosis (high power) of tissue. Depending on the energy level selected, the laser can penetrate tissue depths ranging from millimeters to several centimeters in depth. Airway and esophageal Nd:YAG laser procedures can be performed safely with sedation alone. During the past 25 years, experience with laser photoresection has led to the emergence of clinical safety guidelines and the characterization of lesions ideally suited to laser therapy. Communication among the surgeon, the anesthesiologist, and the rest of the operating team is essential to minimize risks to the patient and staff during laser procedures. The fractional inspired oxygen (Fi o 2 ) should be set to the lowest possible level required to maintain adequate oxygenation, and it should never exceed 40% because of the risk of airway fire. Experienced centers have published a set of simple rules to increase the safety of lasers in the airway ( Box 6-1 ). These rules, often referred to as the “Ten Commandments,” are also applicable to the esophagus. Other investigators have contributed an excellent mnemonic, “The Rule of Fours,” to remember favorable lesion characteristics, power and pulse settings, and techniques to optimize the results of airway lasering ( Box 6-2 ). Lesions of the central airways less than 4 cm in length, localized to one wall, and protruding through the lumen (e.g., exophytic, pedunculated) without complete obstruction are ideal for laser ablation.
Know the anatomy. The aortic arch, the pulmonary artery, and the esophagus are danger zones.
Have a well-prepared laser team that includes an anesthetist specialized in conscious sedation and two assistants equipped with emergency response procedures.
Any endoluminal growth is amenable to lasering, but purely external compression is a contraindication.
Use the rigid bronchoscope with two suction catheters for high-grade obstruction, especially if a malignant neoplasm is involved.
Monitor blood gases and cardiac performance. At the least sign of hypoxemia, interrupt treatment long enough to oxygenate the patient, if necessary under closed-circuit conditions.
Fire parallel to the wall of the airway. Never aim directly into it.
Coagulate at will but avoid using the laser at high-power settings; mechanical resection after laser coagulation is preferable to laser resection alone whenever possible.
Control hemorrhage. Even slow bleeding will lead to hypoxemia if it is left unattended.
Terminate each procedure with a thorough laser irradiation of the resected area and a tracheobronchial toilet to remove all secretions or debris.
Observe and monitor the patient in the recovery room for a reasonable time.
Length of lesion ≤ 4 cm
Duration of collapse < 4 weeks
Power: 40 watts
Pulse duration: 0.4 second
Endotracheal tube to lesion > 4 cm
Fiber tip to lesion: 4 mm
Flexible bronchoscope to tip: 4 mm
Fi o 2 < 40%
Number of pulses between cleaning < 40
Procedure time < 4 hours
Total number of treatments < 4
Life expectancy > 4 weeks
Laser team: 4 persons
Nd:YAG, Neodymium:yttrium-aluminum-garnet.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here