Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Endoscopic craniofacial resection (CFR) has emerged as an alternative to the traditional CFR originally credited to Ketcham in 1963.
The standard craniofacial approach for tumor resection combines a transfacial approach via a lateral rhinotomy or midface degloving to gain access for removal of the sinus component of the tumor with a transcranial approach to remove the skull base and intracranial portion of the tumor. The most common indications for CFR are tumors arising from the nasal cavity, frontal sinuses, or ethmoid sinuses extending to or through the anterior skull base.
The major reason for selecting endoscopic CFR is to avoid the morbidity associated with open CFR.
The rates of complication associated with open CFR as reported in the literature vary considerably.
In the largest study to date evaluating open CFRs, Ganly et al. reported an overall complication rate of 36.3% and a mortality rate of 4.7%.
The rate of CSF fistula after major CFR may be as high as 20%.
The rate of meningitis after CFR is reported in the literature to be 5% to 7.7%.
Despite the wide exposure afforded by the open CFR, the close proximity of the orbit, brain, and other critical neurovascular structures often prevents surgeons from taking large margins without causing significant morbidity. In fact, one multicenter study looking at outcomes of open CFRs reported a 30% incidence of positive margins.
It has been suggested that the magnified view provided by the endoscope can also make tumor mapping more precise, especially in deep areas such as the pterygopalatine fossa and sphenoid sinus, where visualization and illumination can be difficult in an open procedure. Furthermore, for some tumors, unilateral endoscopic resection is possible, thus preserving smell on the contralateral side.
A major criticism of endoscopic CFR is that the tumors are removed piecemeal. Traditional teaching is that tumors should be removed en bloc to ensure maximal survival. This principle is founded on the concept that surgical violation of the tumor predisposes to spread of the cancer through lymphatic or vascular channels, thereby increasing the risk of local, regional, and distant tumor spread. Furthermore, there is concern that tumor removal is more likely to be incomplete with a piecemeal as opposed to an en bloc resection. However, data from transoral laser microsurgery and transoral robotic surgery, as well as more recent data from endoscopic CFRs, show that this is not the case. The most important surgical variable affecting survival, regardless of the method used, is achieving complete tumor resection with negative margins.
A study comparing endoscopic versus open craniofacial resection found no significant differences in survival, metastatic rates, or complication rates between the two groups. The endoscopic group had shorter hospital stays and the added benefit of a better cosmetic outcome. However, comparison of oncologic outcomes between the groups was limited by discrepancy in histologic grade and clinical stage between the two retrospective, nonrandomized groups.
Surgery of the skull base is complex because of its proximity to the dura, brain, and orbit. Tumors will be adjacent to or involve one or more of these structures during endoscopic CFR.
Sphenoid sinus anatomy deserves special mention. The optic nerve and cavernous sinus are located along the lateral wall, whereas the sella turcica is located posteriorly and centrally.
Refer to the appropriate chapters in this text for more detailed anatomic descriptions of the sphenoid sinus, ethmoid roof, and frontal sinus.
Endoscopic CFR may be considered in the management of benign and malignant tumors of the paranasal sinuses involving the skull base.
This chapter focuses on the treatment of esthesioneuroblastoma, which is one of the more common tumors approached by this method. However, this technique can be applied to the majority of benign and malignant tumors involving the anterior skull base.
Preoperative images should be thoroughly analyzed; the findings will ultimately determine tumor resectability and critical anatomic constraints.
A multidisciplinary approach—including, when pertinent, specialists in neurosurgery, ophthalmology, medical oncology, and radiation oncology—is critical.
A definitive tissue biopsy is necessary preoperatively, especially when critical anatomic structures such as the orbit, dura, or neurovascular regions are involved.
Indications include any benign or malignant tumor of the nasal cavity and/or paranasal sinuses that involves the anterior skull base.
Contraindications include poor surgical candidacy because of comorbidities, involvement of skin or brain parenchyma, and bilateral orbital involvement.
Relative contraindications include extension into the cavernous sinus, orbit, and lacrimal system, as well as massive tumors with unfavorable histologic features. Another contraindication to the approach is the inability to reconstruct the skull base due to factors such as previous surgery, radiation exposure, or defect size.
Esthesioneuroblastoma, first described by Berger and Luc in 1924, arises from the olfactory epithelium and accounts for approximately 3% to 6% of malignancies of the nasal cavity and paranasal sinus.
Tumor staging is an important guide for prognosis and therapy. The most common staging systems are the Kadish staging system ( Table 25.1 ), the University of California at Los Angeles–Dulguerov and Calcaterra staging system ( Table 25.2 ), and the Hyams histopathologic grading system ( Table 25.3 ). In a 1993 study by Morita et al. examining prognostic factors, Hyams histopathologic grade was found to be the most significant prognostic factor, with a 5-year survival rate of 80% for 32 patients with low-grade tumors and 40% for 15 patients with high-grade tumors.
| Stage | Characteristics |
|---|---|
| A | Disease confined to nasal cavity |
| B | Disease in nasal cavity and one or more paranasal sinuses |
| C | Disease extending beyond the nasal cavity and paranasal sinuses |
| Stage | Characteristics |
|---|---|
| T1 | Tumor involving the nasal cavity and/or paranasal sinuses (excluding sphenoid), sparing the most superior ethmoid cells |
| T2 | Tumor involving the nasal cavity and/or paranasal sinuses (including the sphenoid) with extension to or erosion of the cribriform plate |
| T3 | Tumor extending into the orbit or protruding into the anterior cranial fossa, without dural invasion |
| T4 | Tumor involving the brain |
| N0 | No cervical lymph node metastasis |
| N1 | Any form of cervical lymph node metastasis |
| M0 | No metastases |
| M1 | Distant metastasis |
| Grade | Lobular Architecture Preservation | Mitotic Index | Nuclear Polymorphism | Fibrillary Matrix | Rosettes | Necrosis |
|---|---|---|---|---|---|---|
| I | + | None | None | Prominent | HW rosettes | None |
| II | + | Low | Moderate | Present | HW rosettes | None |
| III | +/− | Moderate | Prominent | Low | FW rosettes | Rare |
| IV | +/− | High | Marked | Absent | None | Frequent |
Before endoscopic CFR, the primary tumor site must be examined using magnetic resonance imaging (MRI) and computed tomography (CT). The scans should be analyzed closely for involvement of the eye, dura, brain, and neurovascular structures ( Fig. 25.1 ). As with any oncologic surgery, the goal of endoscopic CFR is to achieve clear margins. If this goal cannot be achieved, then other approaches should be used. For advanced-stage sinonasal malignancies, a workup for metastasis should include examination of the neck and lungs. Consideration can be given to performing positron emission tomography instead of neck and chest CT.
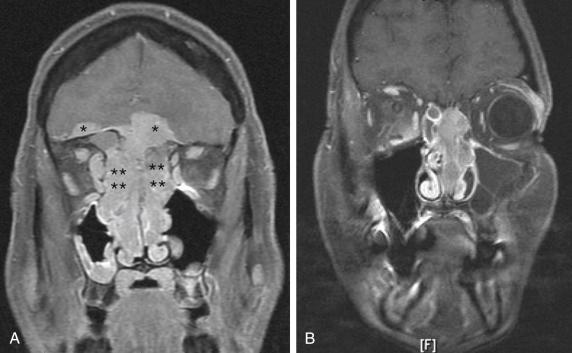
The extent of the tumor should be identified radiographically. Ideally, an additional layer of tissue should be included in the resection to obtain a margin; that is, if the tumor extends to the skull base, an additional dural margin should be taken once the involved skull base is resected.
Many of the instruments employed in endoscopic CFR are the same as those used in simple functional endoscopic sinus surgery. The following are the extra instruments specific to the endoscopic CFR procedure:
0-, 30-, and 70-degree endoscopes
Reverse endoscopes are preferred when working at the skull base. These endoscopes are designed to have the light cord directed upward, which frees up the area below the endoscope for the instruments.
Endoscopic bipolar forceps
Suction coagulator (suction Bovie)
Straight and angled endoscopic drills
Straight and angled microdébrider burs
Carotid Doppler ultrasound device
Image guidance system with possible MRI/CT fusion to better delineate tumor margins
Consideration should be given to placement of a lumbar drain at the onset of surgery.
A two-surgeon, four-handed, two-nostril technique is preferred for fine dissection at the skull base.
Endoscopic bipolar forceps and clip appliers are helpful for arterial ligation.
A complete set of skull base instruments is invaluable.
The size of the defect and available local reconstructive options should be continuously reevaluated as the tumor dissection proceeds.
A skilled pathologist is essential to assess the frozen-section margins.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here