Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Lesions considered here are characterized by müllerian differentiation on microscopic examination and reflect the metaplastic potential of the pelvic and lower abdominal mesothelium and the subjacent mesenchyme of females (‘secondary müllerian system’).
The müllerian potential of these tissues is consistent with their close embryonic relation to the müllerian ducts that arise by invagination of the coelomic epithelium. Displacement of coelomic epithelium and subcoelomic mesenchyme during embryonic development may account for identical lesions within pelvic and abdominal lymph nodes.
Other histogenetic mechanisms for some of these lesions exist. Indeed, most cases of peritoneal and ovarian endometriosis are likely a result of retrograde menstruation and implantation. Lymphatic and hematogenous spread likely account for endometriosis (and rare other lesions, such as benign metastasizing leiomyoma) in intra-abdominal lymph nodes and distant sites, respectively.
For a consideration of historical aspects of endometriosis, see A History of Endometriosis by Dr. Ronald Batt.
Endometriosis, defined as the presence of endometrial tissue outside the endometrium and myometrium, occurs in as many as 10–15% of women of reproductive age. The great majority of patients are in the reproductive age group, the disorder being less common in adolescents and postmenopausal women and exceptionally rare in prepubertal girls.
The typical symptoms are dysmenorrhea, lower abdominal, pelvic and back pain, dyspareunia, irregular bleeding, and infertility. Involvement of diverse common to rare sites ( Table 19.1 ) may be associated with localized clinical manifestations that may be catamenial.
| Common | Less common | Rare |
|---|---|---|
| Ovaries | Large bowel, small bowel, and appendix | Lungs, pleura |
| Uterosacral, round, and broad ligaments | Mucosa of cervix (see Chapter 4 ), vagina, and fallopian tubes (see Chapter 11 ) | Soft tissues, breast |
| Rectovaginal septum | Skin (scars, umbilicus, vulva, perineum, inguinal region) | Bone |
| Cul-de-sac | Ureter, bladder | Upper abdominal peritoneum |
| Serosa of uterus and tubes | Omentum, pelvic lymph nodes | Stomach, pancreas, liver |
| Serosa of other pelvic organs | Inguinal region | Kidney, urethra, prostate, paratesticular Sciatic nerve, subarachnoid space, brain |
Some cases are only discovered when an endometriosis-associated neoplasm is excised (uncommon) or it is an incidental microscopic finding in tissues (particularly the ovary) removed for other reasons (common).
Findings in less commonly involved sites include:
Intestinal endometriosis (>90% of which involve the rectosigmoid, the remainder the ileum, or rarely other sites) can mimic diverticulitis, appendicitis, Crohn's disease, chronic active colitis, irritable bowel syndrome, mucosal prolapse, or a neoplasm.
Ureteric endometriosis typically causes hydroureter, with hydronephrosis and/or pyelonephritis as additional complications.
Inguinal endometriosis may mimic a hernia, lymphadenopathy, or a neoplasm.
Abdominal wall endometriosis may present as a soft tissue tumor; almost all such cases occur within cesarean section scars facilitating the diagnosis (Wang et al.).
Pelvic examination may disclose tender nodules in the cul-de-sac and uterosacral ligaments; semi-fixed cystic ovaries; a fixed retroverted uterus; and an indurated rectovaginal septum.
Rare complications include ascites (sometimes with a right pleural effusion), hemoperitoneum, and infection (Simmons et al.) or rupture of an endometriotic cyst. Abdominal wall endometriosis associated with ventriculoperitoneal and lumboperitoneal shunts has caused compromise of the shunt.
Serum CA125 levels may be elevated and correlate with both the severity and the clinical course of the disease.
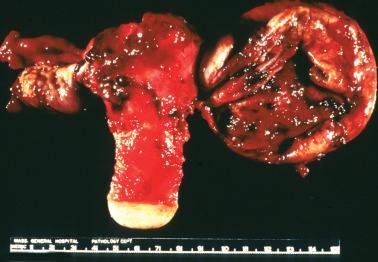
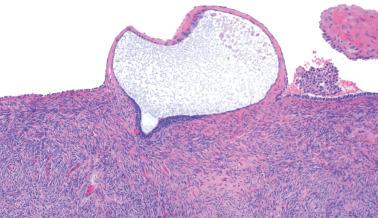
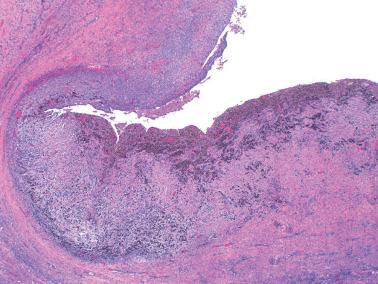
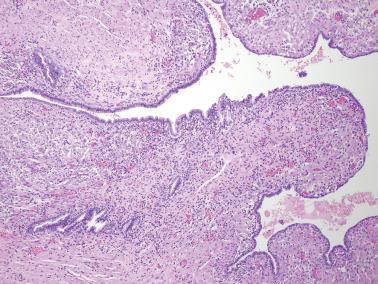
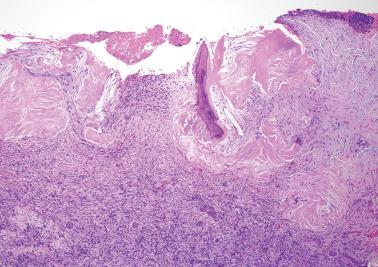
Endometriotic foci may appear as punctate, red, blue, brown or white spots, patches, or nodules with either a slightly raised or puckered surface; the lesions are frequently associated with dense fibrous adhesions.
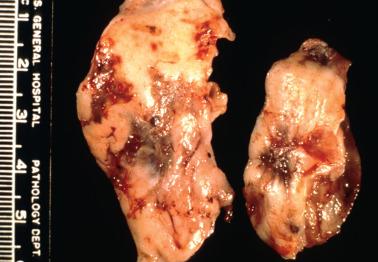
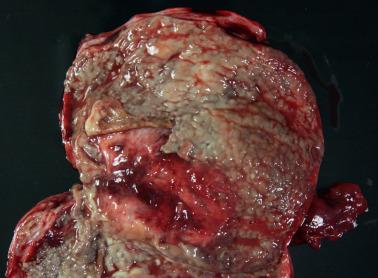
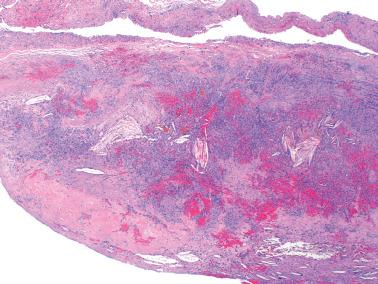
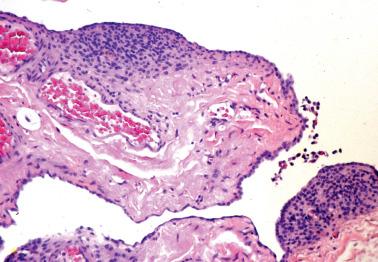
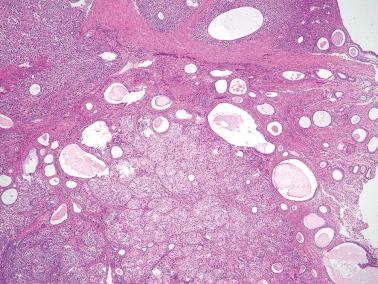
Endometriotic cysts, which most often involve the ovaries, usually have fibrotic walls, a smooth or shaggy, brown to yellow lining, and semifluid or inspissated, chocolate-colored cyst contents.
Fibrous adhesions may bind an endometriotic cyst to adjacent organs potentially mimicking an invasive ovarian cancer intraoperatively.
Mural nodules or intraluminal polypoid projections should be sampled for microscopic examination to exclude a neoplasm originating in the cyst.
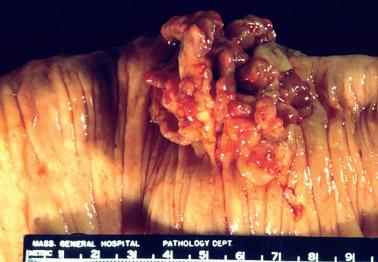
Intestinal endometriosis typically forms a solid, tumor-like mural mass that may impinge on the lumen or cause kinking of the involved segment; rarely there is an intraluminal mass that may mimic a neoplasm.
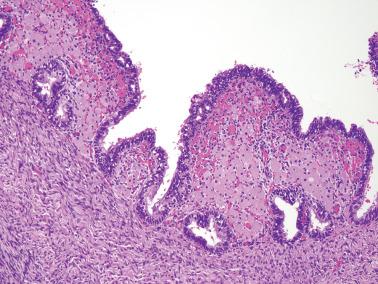
Polypoid endometriosis is a rare form of endometriosis characterized by polypoid, often multiple, mucosal or serosal masses that may mimic a neoplasm on clinical, intraoperative, and gross examination. Typical (nonpolypoid) endometriosis is often present in the same site or elsewhere.
Parker et al. found that the most common sites of polypoid endometriosis were, in descending order of frequency: colon, ovary (serosal or within an endometriotic cyst), uterine serosa, cervicovaginal mucosa, ureter, fallopian tube, omentum, bladder, paraurethral and paravaginal soft tissue, and retroperitoneum.
Some cases may be related to hyperestrinism and/or contain hyperplastic endometriotic tissue.
Rare cases of nonpolypoid endometriosis may form large solid and/or cystic pelvic masses that intraoperatively and macroscopically can simulate a neoplasm.
Both endometriotic epithelium and stroma are usually present, but a diagnosis of endometriosis is often possible when only one of these components is present.
The glands may be inactive or resemble those of eutopic proliferative or secretory endometrium.
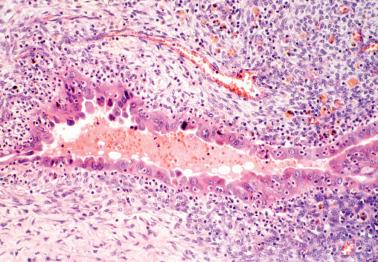
The stromal component is usually obvious and resembles typical endometrial stroma, including a network of arterioles.
Occasionally, however (particularly in a postmenopausal patient or following treatment), the endometriotic stromal cells are confined to inconspicuous cuffs around endometriotic glands or cysts and/or are obscured by histiocytes. They may be more spindled and fibroblastic than typical endometrial stromal cells, particularly around long-standing endometriotic cysts.
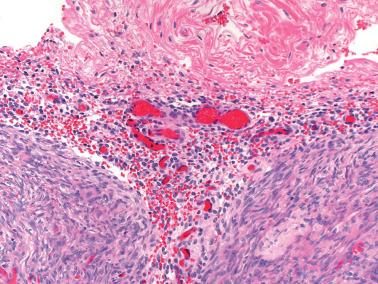
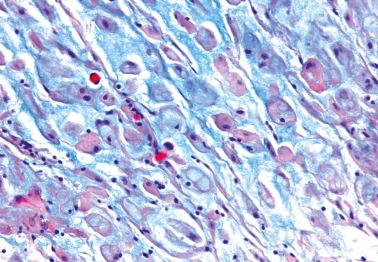
The presence of stromal arterioles, extravasated erythrocytes, and pigmented histiocytes in or around the lesion may be a clue to the diagnosis, as noted below, particularly when the endometriotic stroma is atrophic.
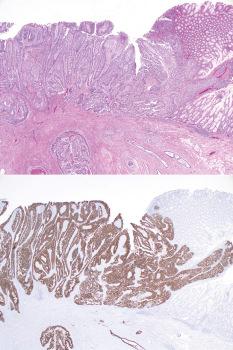
The typical CD10-positivity of endometriotic stromal cells can be diagnostically helpful, especially if the stromal cells are sparse or of uncertain nature, or when the glandular epithelium is sparse or absent.
Hemorrhage is common and often elicits an infiltrate of histiocytes (‘pseudoxanthoma cells’) that typically contain lipid and two types of brown granular pigment: ceroid (lipofuscin, hemofuscin) and hemosiderin. Occasionally similar pigment is present within the epithelial cells.
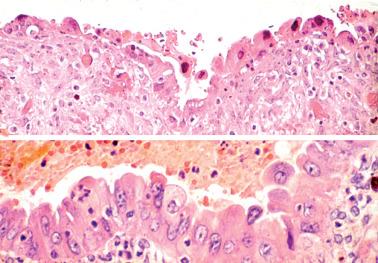
The epithelial lining of an endometriotic cyst may be attenuated, with a single layer of cuboidal epithelial cells that appear endometrioid. In cases with a nonspecific epithelial lining or in which the epithelium is completely denuded, a diagnosis of endometriosis is still tenable if foci of endometriotic stroma are present, sometimes requiring a careful search if extensively effaced by histiocytes.
The cyst lining may be totally replaced by granulation tissue, fibrous tissue, and pseudoxanthoma cells, an appearance that is strongly suggestive of endometriosis (‘presumptive endometriosis’), although rarely a similar appearance may be seen with other lesions.
The epithelial lining cells may be focally stratified and have abundant eosinophilic cytoplasm and large atypical hyperchromatic nuclei, sometimes with a hobnail appearance. This change is probably reactive in most cases, but occasionally merges with a neoplasm, suggesting a premalignant potential in some cases. Seidman reported 20 cases with this finding and no synchronous neoplasm: no endometriosis-associated tumor developed during a mean follow-up of ~9 years.
HNF1β, a marker typically present in clear cell carcinomas, may also be identified in reactive and atypical endometriotic epithelial cells.
Endometriosis involving smooth muscle (uterine ligaments, walls of hollow viscera) is typically associated with a proliferation of the smooth muscle that can be striking and result in an adenomyosis-like appearance.
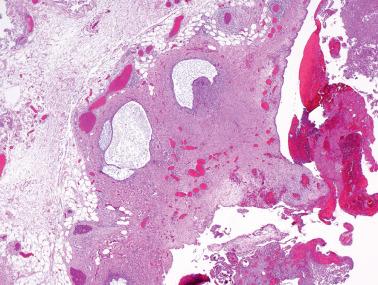
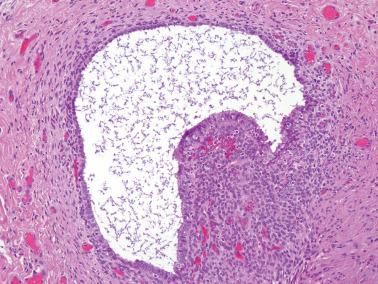
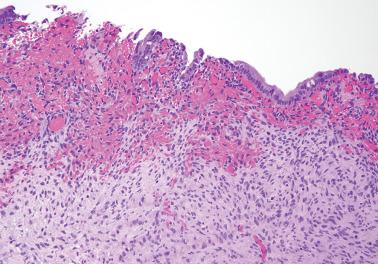
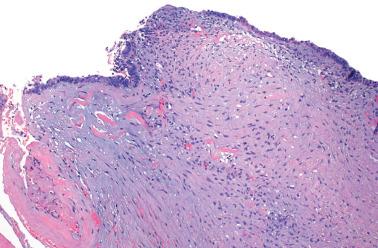
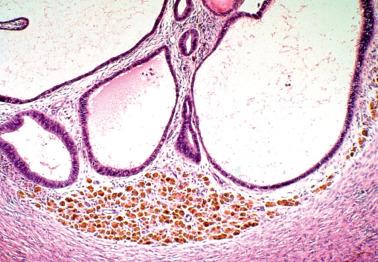
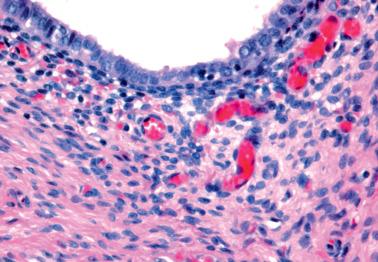
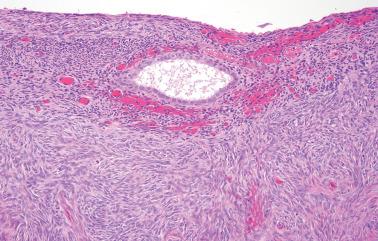
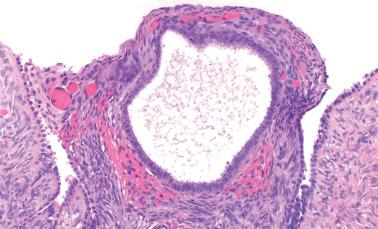
Endometriosis is often underdiagnosed by the pathologist, particularly in postmenopausal women, when the ovarian surface is involved, or when either the glandular or stromal component is absent or inconspicuous.
Small foci of endometriosis within the superficial ovarian cortex and on the ovarian surface (as plaques, small polypoid projections or cystic glands) are often underdiagnosed.
This underdiagnosis is often due to the endometriotic stroma being misinterpreted as ovarian stroma, the endometriotic glands being misinterpreted as epithelial inclusion glands or cysts, the occasional absence of glands (see next point), and a frequent absence of pigmented histiocytes.
The characteristic arterioles (often engorged with erythrocytes) within endometriotic stroma, the typical oval stromal cells (versus spindled ovarian stromal cells), and extravasated erythrocytes facilitate the diagnosis. CD10 staining, as noted above, can be helpful.
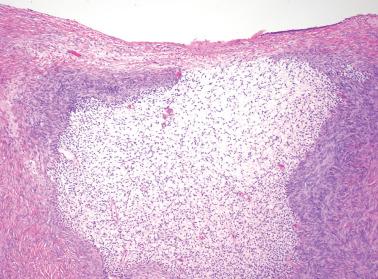
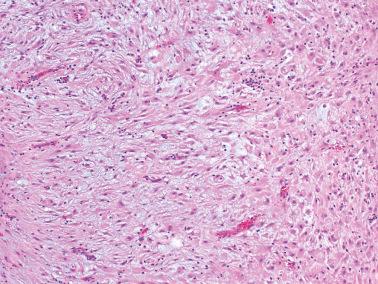
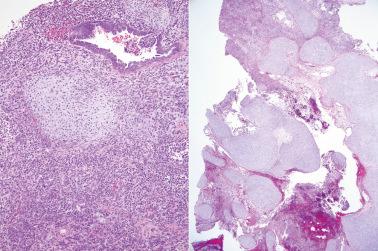
‘Stromal endometriosis’ (SE), which now refers to endometriotic foci composed only of endometriotic stroma, is frequently underdiagnosed. SE is most commonly encountered in peritoneal endometriosis.
Boyle and McCluggage found SE in 45% of laparoscopic biopsies in women with peritoneal endometriosis; in ~7% of cases it was the only form of endometriosis present.
SE typically occurs as serosal or subserosal nodules (‘micronodular SE’) or plaques; the stromal cells often have a whorled pattern. Decidualization of the stromal cells secondary to progestin treatment (see below) may obscure the diagnosis.
The presence of arterioles, extravasated erythrocytes, pigmented histiocytes, and staining for CD10 facilitate the diagnosis. In some cases deeper sections can disclose typical endometriosis (with glands and stroma).
The nodules of SE can be mistaken for lymphoid aggregates. CD10 staining can help confirm the stromal nature of the cells in such cases.
SE also occurs within the ovarian stroma and the superficial stroma of the uterine cervix (see Chapter 4 ); in both sites, it is usually as an incidental microscopic finding unassociated with pelvic endometriosis.
Stromal endometriosis may raise concern for endometrial stromal sarcoma or Kaposi’ s sarcoma (see Differential Diagnosis ).
Unusual hormonal changes:
Unopposed estrogen can result in precancerous hyperplasia similar to that seen in eutopic endometrium (see Atypical Endometriosis, below).
The progestational effects of pregnancy or progestin therapy typically result in gland atrophy (or occasionally the Arias-Stella reaction) and a fully developed or partial decidual reaction that can be subtle and lead to underdiagnosis. Cytoplasmic vacuoles in the decidual cells can create a signet-ring-like appearance, but the vacuoles contain acid rather than neutral mucin and the cells are cytokeratin negative. A stromal myxoid change may also occur (see below).
Inactive or atrophic changes in endometriosis are usual after the menopause and are also seen in premenopausal patients treated with oral contraceptives or danazol. The atrophic glands may retain subtle periglandular cuffs of stromal cells; CD10 positivity of the stromal cells in such cases facilitates the diagnosis.
Tamoxifen treatment can result in endometrial polyp-like structures within endometriosis. Selective receptor modulators (such as ulipristal acetate) can induce changes in foci of endometriosis similar to that seen in the endometrium ( Chapter 7 ) (Bateman et al.).
Glandular metaplasias are common in endometriotic glands and include tubal (ciliated), hobnail, and rarely, squamous and mucinous metaplasia.
Metaplasias are more common in ovarian endometriosis associated with an ovarian epithelial tumor than endometriosis without this association (see Atypical Endometriosis and Endometriosis-associated Tumors). Mucinous metaplasia, sometimes with papillary tufting, can abut an endometriosis-associated endocervical-type borderline mucinous tumor ( Chapter 13 ) (see Differential Diagnosis ).
In cecal and appendiceal endometriosis, the endometriotic epithelium is occasionally replaced by intestinal-type epithelium as a result of colonization or metaplasia, potentially mimicking an appendiceal mucinous neoplasm (Fu et al., Kim et al., Misdraji et al., Vyas et al.).
Unusual stromal changes:
Smooth muscle metaplasia occurs in up to 18% of cases of ovarian endometriosis (Fukunaga), usually within the walls of endometriotic cysts; florid examples (‘endomyometriosis’) can result in a uterus-like mass. A müllerian duct anomaly may also account for adnexal uterus-like masses ( Chapter 12 ) associated with genitourinary malformations. Endomyometriosis should be distinguished from the more common involvement of indigenous smooth muscle by endometriosis, as noted above.
Myxoid change in the endometriotic stroma, which may be more common during pregnancy, can potentially mimic metastatic mucinous adenocarcinoma or pseudomyxoma peritonei. The presence of typical endometrial glands and stroma facilitate the diagnosis.
Striking stromal elastosis can occasionally focally obliterate the endometriotic stroma, a finding that may be more common within endometriosis involving the muscularis of hollow viscera.
Bizarre atypia of endometriotic stromal cells. This finding is similar to that described in the stroma of occasional endometrial polyps and rarely in otherwise normal endometrial stromal cells ( Chapter 7 )
Reactive and inflammatory changes:
Epithelial atypia in endometriotic cysts (see Atypical Endometriosis).
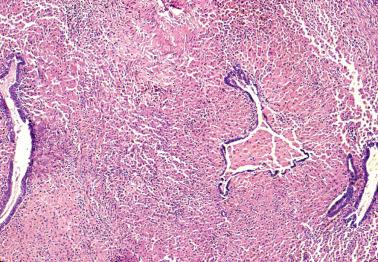
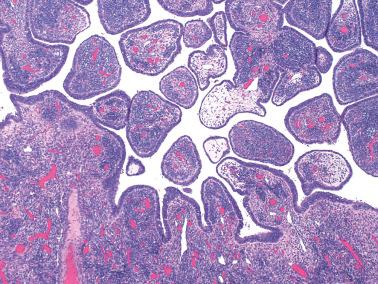
Mesothelial hyperplasia is a common response to endometriosis, especially in the walls and surfaces of endometriotic cysts and nearby peritoneal surfaces. Small tubules, papillae, nests, and parallel cords of bland mesothelial cells, sometimes within artifactual spaces or lymphatics, are embedded in reactive fibrous tissue, an appearance that may suggest an epithelial or mesothelial tumor. The proximity to endometriosis, the bland nuclear features, and a mesothelial phenotype ( Chapter 20 ) facilitate the diagnosis.
Necrotic pseudoxanthomatous nodules, likely representing burnt-out endometriotic foci, have a central necrotic zone surrounded by pseudoxanthoma cells (often in a palisaded arrangement), hyalinized fibrous tissue, or both; typical endometriotic foci are usually absent or sparse.
Reactive skeletal muscle regeneration may occur in abdominal wall endometriosis and consists of a tumor-like proliferation of round myoblast-like cells positive for desmin, myoD1, and myogenin.
Numerous neutrophils within an endometriotic cyst are usually due to bacterial infection.
Liesegang rings are rarely found in endometriotic cysts. These are round to oval, acellular laminated ring-like structures that are found in foci of chronic inflammation and/or necrosis.
Calcification and/or ossification may occur within endometriotic foci, particularly within the walls of long-standing endometriotic cysts.
Perineural, lymphatic, and vascular invasion have been rarely encountered in otherwise typical cases of endometriosis. Lymphatic invasion likely accounts for rare cases of endometriosis within lymph nodes.
Polypoid endometriosis (see gross findings).
The histologic findings are usually those of nonpolypoid endometriosis, but occasionally the appearance can resemble that of a eutopic endometrial polyp, including enhanced stromal and glandular p16 positivity.
Occasionally the microscopic findings may suggest a neoplasm, most often adenosarcoma (see Differential Diagnosis ).
Atypical endometriosis. This term has been used to refer to: (1) hyperplastic changes similar to those occurring in eutopic endometrium (sometimes secondary to an endogenous or exogenous estrogenic stimulus or tamoxifen therapy) and (2) epithelial atypia of the type frequently found in endometriotic cysts, as described earlier. This finding is considered further under the heading of ‘Endometriosis-associated Tumors’ below.
Associated lesions. Endometriosis can be intimately admixed with foci of peritoneal leiomyomatosis, glial implants of ovarian teratomas, or nodules of splenosis. Endometriosis-associated pseudoxanthomatous salpingitis is considered in Chapter 11 .
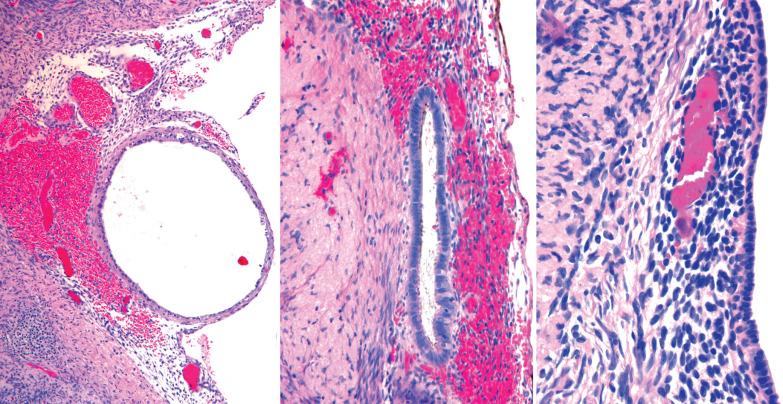
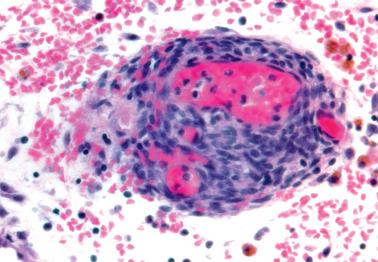
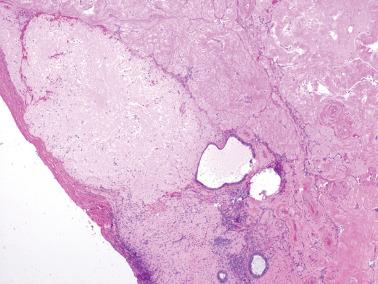
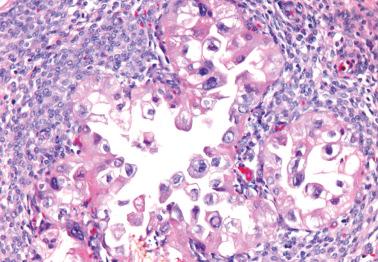
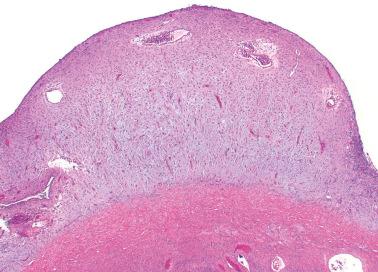
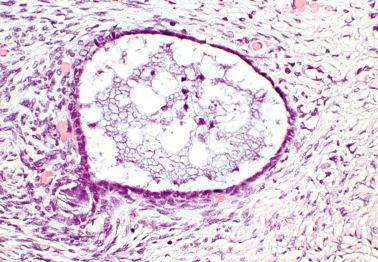
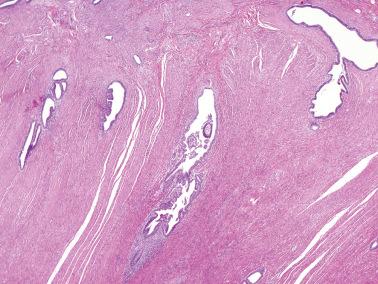
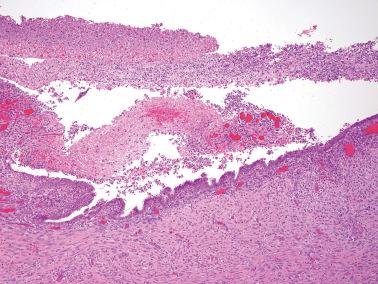
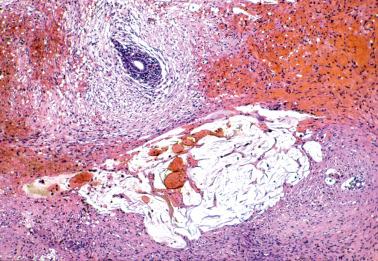
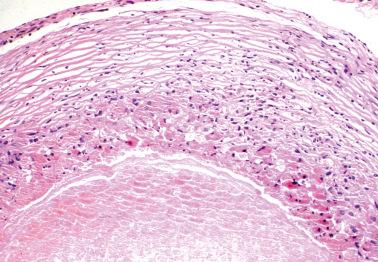
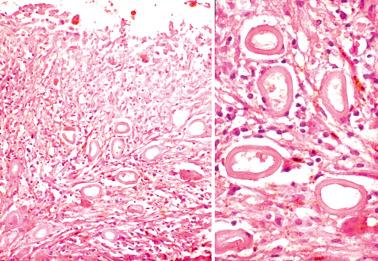
Ovarian inclusion glands and cysts. These, in contrast to many foci of ovarian endometriosis, are always within the cortical stroma although they may abut the surface. In contrast, endometriosis is often present on the surface sometimes projecting from it. Additionally, inclusion glands and cysts lack an investment of endometriotic stroma, although this finding can occasionally be subtle. The presence of stromal arterioles, extravasated erythrocytes, and foamy and/or pigmented histiocytes should suggest the possible endometriotic nature of the periglandular stroma, which can be supported by CD10-positivity.
Endosalpingiosis (see corresponding heading). Endometriotic stroma is definitionally absent. Unlike endometriosis, endosalpingiotic glands are usually ciliated, are often associated with psammoma bodies, and usually lack the extravasated erythrocytes and histiocytes typical of endometriosis.
Extrauterine low-grade endometrial stromal sarcoma (ESS) or metastatic endometrial ESS vs stromal endometriosis. Unlike ESS, stromal endometriosis rarely forms a mass and lacks the mitotic activity, sex-cord-like elements, and prominent invasion (including vascular invasion) of many ESSs. The presence of a uterine mass or a history of uterine ESS also obviously aids in this differential.
Extrauterine ESS with endometrioid glandular differentiation. Lesions reported as ‘aggressive’ endometriosis because of large size and prominent infiltration and vascular invasion are likely ESSs with glandular differentiation. In contrast to endometriosis, these tumors contain foci of more typical ESS devoid of glands, and in some cases, briskly mitotic stromal cells, sex-cord-like elements, and prominent vascular invasion.
Adenosarcoma. This diagnosis may be suggested in rare cases of otherwise typical endometriosis or polypoid endometriosis in which there are focal periglandular stromal cuffs and/or intraglandular stromal papillae. These findings, however, tend to be more focal than in adenosarcomas and the definitional stromal atypia of adenosarcomas is absent.
Müllerian borderline tumor of mixed cell type ( Chapter 14 ). The distinction between a focus of hyperplastic or atypically hyperplastic mucinous epithelium within an endometriotic cyst and an early borderline tumor of this type arising in an endometriotic cyst may be difficult and subjective. In such cases, fibrous-cored papillae with stromal neutrophils and marked epithelial stratification warrant a diagnosis of borderline tumor.
Kaposi's sarcoma vs stromal endometriosis. A grossly visible nodule or mass, a fascicular pattern, atypia and mitotic activity, hyaline globules, and reactivity for HHV8 indicate the former diagnosis.
Necrotic pseudoxanthomatous nodules (NPNs) versus other ovarian and peritoneal necrotic nodules, such as infectious granulomas, isolated palisading granulomas of the ovary, and granulomas related to diathermy. These granulomas have characteristic features and lack the numerous pseudoxanthoma cells of NPNs.
The exact frequency of cancer arising from pelvic endometriosis is unknown because the frequency of endometriosis in the general population is unknown, and because some cancers arising in endometriosis likely overgrow and obliterate the endometriosis.
Even when endometriosis and a müllerian-type tumor coexist in the same site, it is difficult to prove an endometriotic origin without histologic merging of the two lesions. The term ‘endometriosis-associated’ tumor is thus preferable in most cases.
Aside from the coexistence of endometriosis and ovarian cancer, an association between the two is also suggested by the increased risk of ovarian cancer after a diagnosis of ovarian endometriosis.
In studies of consecutive cases of endometriosis in one institution, a cancer was associated with endometriosis in 4% of cases of ovarian endometriosis (Prefumo et al.) and in 10% of cases of pelvic endometriosis (Stern et al.).
Compared to women with uncomplicated endometriosis, women with endometriosis-associated carcinomas tend to be younger (and premenopausal), obese, and to have used unopposed estrogens. Further, the endometriosis-associated tumors tend to be lower grade, lower stage, and more favorably prognostic than similar tumors unrelated to endometriosis.
Approximately 75% of tumors complicating endometriosis arise within the ovary. The most common extraovarian site is the rectovaginal septum; less frequent sites include the vagina, colon and rectum, urinary bladder, and other sites in the pelvis and abdomen.
Endometrioid carcinomas and CCCs respectively account for 75% and 15% of carcinomas arising within endometriosis, although a greater proportion of CCCs arise from endometriosis than endometrioid carcinomas.
found that ~22% of 442 ovarian cancers were associated with ovarian endometriosis.
Within this group, 40% of clear cell carcinomas (CCCs) and 31% of endometrioid carcinomas were endometriosis associated.
The endometriosis was atypical in 42% of cases vs 2% of cases of ovarian endometriosis not associated with carcinoma ( ).
Epithelial metaplasias (eosinophilic, ciliated, mucinous) were observed in all cases of atypical endometriosis but in only two-thirds of typical endometriosis.
In the same study, atypical ovarian endometriosis without an ovarian tumor was found in 7 (of 624) women, two of whom had a synchronous or subsequent extraovarian endometrioid carcinoma, suggesting a need for follow-up in patients with atypical endometriosis.
Vercellini et al. found that immunostaining for the oncofetal protein IMP3 aided recognition of what they considered preneoplastic atypia in endometriosis.
Endometrioid carcinomas arising in colonic endometriosis may clinically and pathologically mimic a primary colonic adenocarcinoma. An association with endometriosis, an atypical gross appearance, no mucosal involvement, low-grade nuclear features, squamous metaplasia, and a CK7+/ER+/CK20−/CDX2− immunoprofile favor or indicate endometrioid carcinoma. Rare CCCs arise from colonic endometriosis.
Other endometriosis-related epithelial tumors include ovarian and extraovarian examples of endometrioid cystadenoma, endometrioid adenofibroma, and borderline tumors of endocervical-like (‘seromucinous’) and mixed-cell-type.
ESSs may also arise from endometriosis. In a large study of extrauterine ESS, Masand et al. found that 48% were associated with endometriosis and 63% had multiple sites of involvement.
Including both endometriosis-related and unrelated tumors, the sites of involvement were abdomen/peritoneum (59%), bowel wall (44%), ovaries (40%), pelvis (32%), and vagina (10%).
The unusual location of the tumor, presentation, and occasional presence of unexpected histologic features or secondary changes (sex cord elements, smooth muscle, myxoid change, fibrosis, dedifferentiation) led to misdiagnosis in 25% of cases.
Of those with follow-up, 62% of patients had recurrence; final follow-up revealed that 55% were alive with no evidence of disease, 28% were alive with disease, and 17% were dead of disease.
Malignant mesodermal mixed tumors (MMMTs) and adenosarcomas (typical and with sarcomatous overgrowth) may occasionally arise from endometriosis.
Some peritoneal MMMTs may lack a demonstrable association with endometriosis. The latter may have been obliterated by the tumor or the tumor may have arisen directly from the secondary müllerian system.
Two studies found that 25% of endometriosis-associated colonic tumors were adenosarcomas. Endometriosis-associated adenosarcomas have a better survival than extragenital adenosarcomas without this association.
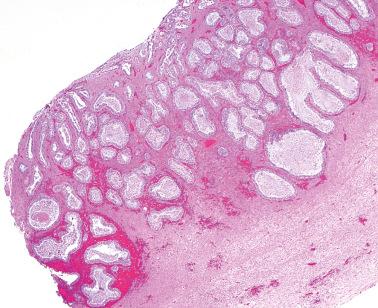
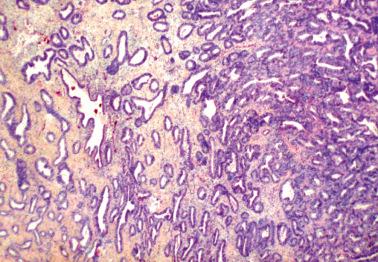
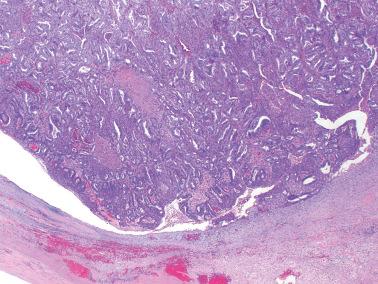
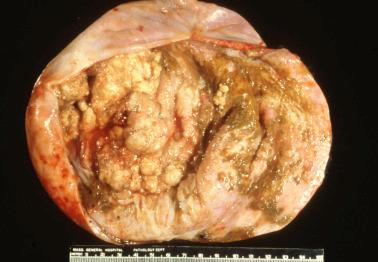
Molecular alterations common to both the endometriosis (especially atypical endometriosis) and synchronous carcinomas have included loss of heterozygosity, expression of p53 and c-erb-2, PTEN , PIC3CA , and ARID1A mutations, and decreased LINE-1 methylation.
Ayhan et al. found loss of ARID1A staining in 66% of endometriosis-associated ovarian CCCs and endometrioid carcinomas and in the adjacent (but not in the distant) endometriotic cyst epithelium.
Studying endometriosis-associated ovarian carcinomas, Matsumoto et al. found mutations in exon 3 of the β-catenin gene in 60% of endometrioid carcinomas (but in none of the CCCs), in 52% of the associated typical endometriosis, and in 73% of the atypical endometriosis. PIK3CA mutations were found in 31% and 35% of the endometrioid and CCCs respectively, and in some cases the coexisting atypical and atypical endometriosis.
Akahane et al. found p53 mutations in 30% of ovarian endometriosis-associated CCCs, but not in uncomplicated endometriosis or endometriosis-associated endometrioid carcinomas.
Senthong et al. found a stepwise decrease in LINE-1 methylation in the following order: endometriotic cysts, ovarian endometrioid carcinoma, and ovarian CCC.
Yamamoto et al. found increased Ki67 index and overexpression of Skp2 (a cell-cycle regulator) in atypical endometriosis (vs typical endometriosis) and CCC (vs atypical endometriosis).
Xiao et al. found loss of BAF250a expression, HNF-1β upregulation, and loss of ER and PR in endometriosis-associated CCCs, in atypical endometriosis, and even in areas of benign endometriosis.
found loss of MMR protein expression in 10% of endometriosis-associated carcinomas. Fuseya et al. found a stepwise decreased expression of MMR proteins in endometriosis, endometriosis-related ovarian carcinoma, and endometriosis-unrelated ovarian carcinoma. In endometriosis-associated carcinomas, decreased expression of MMR proteins and MSI were found in both the endometriosis and the tumor.
The molecular aspects of endometriosis-associated ovarian cancer have been reviewed in detail by Wei et al., and Maeda and Shih.
The differential diagnosis of endometriosis-associated tumors includes the occasional occurrence of peritoneal (or occasionally retroperitoneal) endometrioid neoplasms that are not demonstrably associated with endometriosis.
These tumors likely arise from the mesothelium or submesothelial stroma or possibly from foci of endometriosis that have been obliterated by the tumor.
Examples of these tumors have included endometrioid cystadenofibroma and cystadenocarcinoma, ESS, adenosarcoma, and MMMT. One MMMT was associated with florid vascular proliferation (glomeruloid microvascular proliferation).
Extrauterine ESSs often lack the JAZF1 and JJAZ1 gene fusion of uterine ESSs.
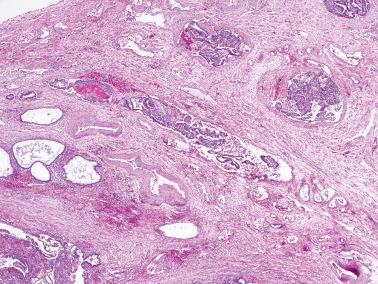
Peritoneal lesions of serous type include endosalpingiosis (a non-neoplastic lesion) and the full spectrum of serous neoplasms seen in the ovary.
This term refers to the presence of benign glands lined by tubal-type epithelium involving the peritoneum and subperitoneal tissues. Similar glands may involve retroperitoneal lymph nodes (‘müllerian inclusion glands’) (see Retroperitoneal Lymph Node Lesions ).
In addition to a secondary müllerian origin, other proposed origins for endosalpingiosis include implantation and/or lymphatic spread of tubal epithelial cells and maturation of peritoneal implants of serous borderline tumors (SBTs).
Endosalpingiosis is typically found in women of reproductive age (mean, 30 years), although occasionally it occurs after the menopause and rarely in men.
Endosalpingiosis is almost always an incidental finding on microscopic examination. Zinssner and Wheeler found endosalpingiosis in 12.5% of surgically removed omenta in a retrospective study and in 25% of omenta more thoroughly examined prospectively.
found an association between endosalpingiosis and endometriosis, uterine cancer, and ovarian cancer (specifically SBTs, CCCs, and invasive mucinous tumors).
Unusual presentations include multiple small cysts or a dominant cystic mass; fine pelvic calcifications on imaging; and psammoma bodies within cul-de-sac fluid, peritoneal washings, the tubal lumen, or cervical smears.
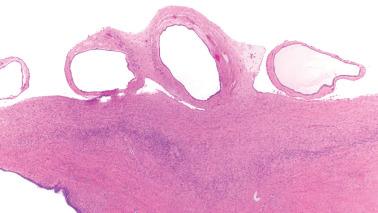
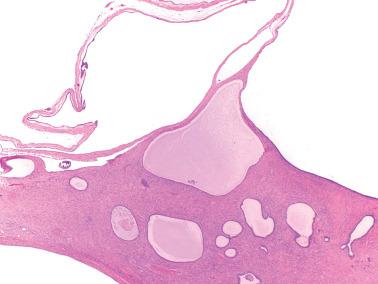
The most common sites are the serosa of the uterus, fallopian tubes, cul-de-sac, and omentum. Similar glands in the ovary are by convention referred to as surface epithelial inclusion glands ( Chapter 12 ) and in lymph nodes, as müllerian inclusion glands. Less frequent sites include the pelvic parietal peritoneum and serosa or subserosal tissues of the urinary bladder and bowel.
Although usually microscopic, occasionally multiple, usually <5 mm in diameter, opaque or translucent, fluid-filled cysts may be seen. Rarely, cystic masses involve the peritoneum, the wall of the uterus, or the appendix, mimicking a neoplasm.
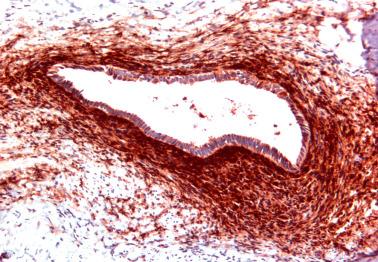
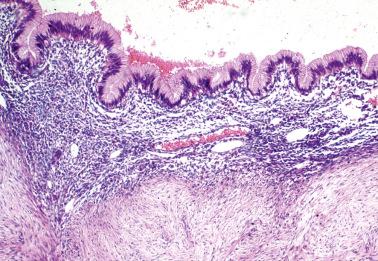
Glands of variable size and shape, sometimes cystic, are lined by a single layer of bland mitotically inactive, tubal-type epithelium that may include ciliated cells, nonciliated secretory cells, and ‘peg’ cells Mucin is often present in the apical cytoplasm and within the glandular lumens. Rarely cells with abundant cytoplasmic mucin are present.
The glands may exhibit occasional papillae and rarely a müllerian-papilloma-like proliferation ( Chapter 3 ).
Periglandular stroma is absent or consists of inconspicuous loose to fibrotic connective tissue, occasionally with a sparse mononuclear inflammatory infiltrate.
Psammoma bodies are common within gland lumens or the stroma. Psammoma bodies within subserosal fibrous tissue without epithelium may indicate atrophic endosalpingiosis.
The epithelial cells are typically reactive for ER, PR, PAX8, and WT-1, antigens found in tubal epithelium and serous tumors but not ovarian surface epithelium ( ). Carney et al. found that even nodal inclusions with nonspecific features can have a similar immunoprofile.
Satgunaseelan et al. reported a unique case of endosalpingiosis with perineural infiltration.
‘Atypical endosalpingiosis’ refers to endosalpingiosis with cellular stratification and cellular atypia (see Differential Diagnosis ).
Rare extraovarian serous tumors (borderline tumors, carcinomas) appear to arise from endosalpingiosis.
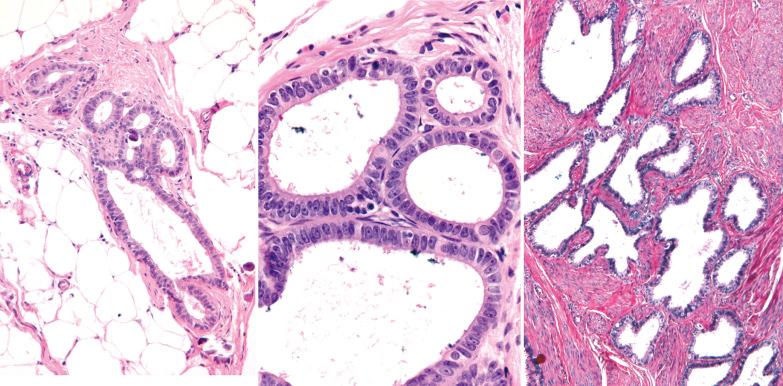
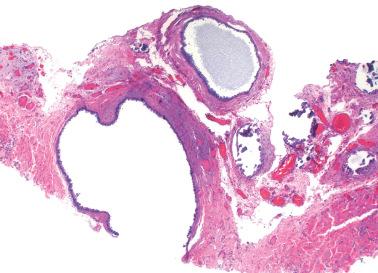
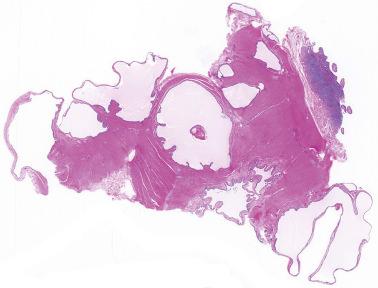
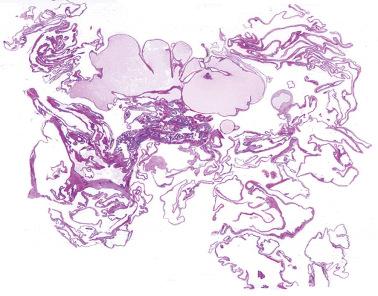
Extraovarian benign serous tumor. The distinction between an extraovarian serous cystadenoma and cystic endosalpingiosis is arbitrary, but a sizable solitary mass and/or an appreciable fibromatous component favor a neoplasm.
Implants of high-grade serous carcinoma. Rarely these implants can have a deceptively benign appearance on low-power examination potentially leading to underdiagnosis as endosalpingiosis. High-power examination will reveal focally malignant nuclear features excluding the latter.
Atypical endosalpingiosis. This lesion is in the differential diagnosis with peritoneal serous borderline tumors (see next section). Bell and Scully use the latter term if the ‘lesions composed of tubal-type epithelium exhibit papillarity, tufting, or detachment of cell clusters … even when they arise on a background of endosalpingiosis’.
‘Müllerianosis’. This lesion is characterized by an admixture of müllerian glandular epithelia (tubal, endocervical, endometrioid), with endocervical-like or serous glands usually predominating (see Endocervicosis ).
Mesonephric remnants. These are a common incidental microscopic finding in the broad ligament. Mesonephric tubules are typically located more deeply than endosalpingiosis, are lined by a single layer of nonciliated, low columnar to cuboidal cells, and are surrounded by a cuff of smooth muscle.
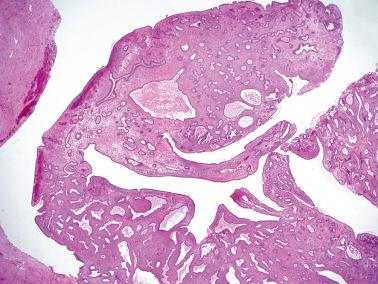
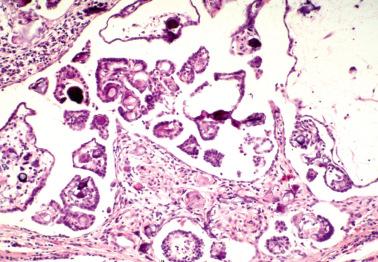
These tumors (SBTs) are characterized by usually widespread extraovarian peritoneal involvement and normal-sized ovaries that are free of disease or that have serosal involvement similar to that involving the extraovarian peritoneum.
The patients are typically of reproductive age, less commonly postmenopausal. Presenting features may include infertility, pelvic or abdominal pain, an adnexal mass, or small bowel obstruction. Some tumors are discovered incidentally at laparotomy for other conditions.
At operation, focal or diffuse miliary granules, fibrous adhesions, or both, involve the pelvic peritoneum and omentum, and less commonly, the abdominal peritoneum.
The lesions are superficial and resemble noninvasive implants of ovarian SBTs ( Chapter 13 ). Limited desmoplasia may be seen but the striking desmoplasia of desmoplastic noninvasive implants is rare. Conspicuous adhesions are common. Endosalpingiosis is present in 80% of cases.
The diagnosis of peritoneal SBT is appropriate only when the ovaries are uninvolved or only minimally involved by similar tumor.
The ovaries often show prominent inclusion glands and cysts or a benign serous tumor.
The differential diagnosis is with low-grade peritoneal serous carcinoma (see below).
The prognosis is favorable (similar to that of ovarian SBTs with noninvasive implants), even when the patients are treated conservatively.
Eighty-five percent of patients have had no clinical evidence of persistent or progressive disease on follow-up and most of the rest are well after resection of recurrent tumor.
Rarely the tumors may transform to an invasive low-grade peritoneal serous carcinoma, although in some such cases the latter may have been present but unsampled at the initial operation.
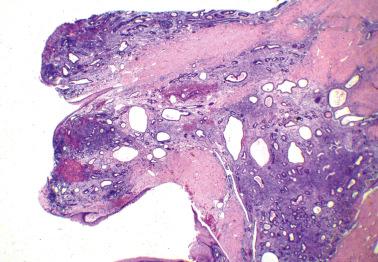
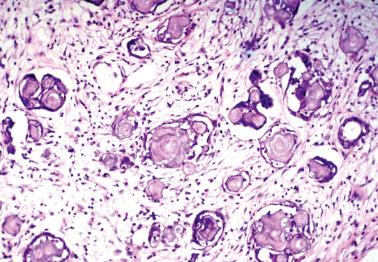
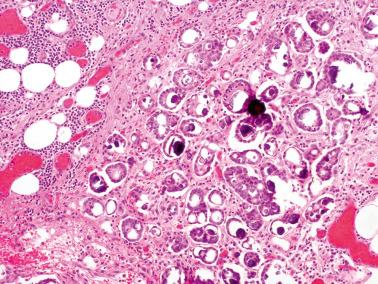
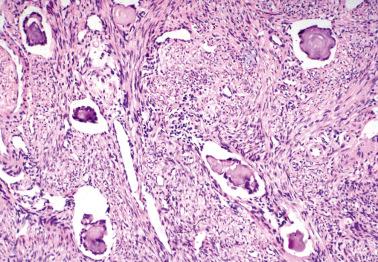
These tumors (LGPSCs) are composed of solid nests and micropapillae with low-grade (grade 1 or 2) nuclear features, and often exhibit invasion of normal underlying tissues and, occasionally, lymphatics.
Psammoma bodies are typically present and may vary from a few to massive in number. The latter have been referred to as psammocarcinoma, a term we now generally avoid as a descriptive opinion is clearer. Nonetheless, the dearth of viable epithelial cells appears to be associated with a favorable prognosis and accordingly the predominance of psammoma bodies should be noted.
In the largest study of LGPSC of usual type, the average age was 51.7 years (range 27.1–82.4); in a study of peritoneal psammocarcinomas, the average age was 40 years. Presenting features in both tumors are often abdominal pain, mass, or both, but in almost half the cases the tumor is an incidental finding. Operative and gross findings vary from nodules to adhesions to a dominant mass.
Despite a high rate of persistent disease at the completion of primary treatment, most patients have a long survival (~70% 5-year overall survival) (Schmeler et al.).
These tumors should be distinguished from peritoneal SBTs. The latter lack invasion and are not as cellular, being composed predominantly of papillae in contrast to the predominance of solid nests in LGPSCs. Adequate sampling is necessary to identify invasion, with highest yields of invasive foci in the omentum.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here