Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Severe primary dysmenorrhea, repeated episodes of ovulation and retrograde menstruation, and pelvic endometriosis visualized by laparoscopy probably represent the early and progressive stages of the same disease process.
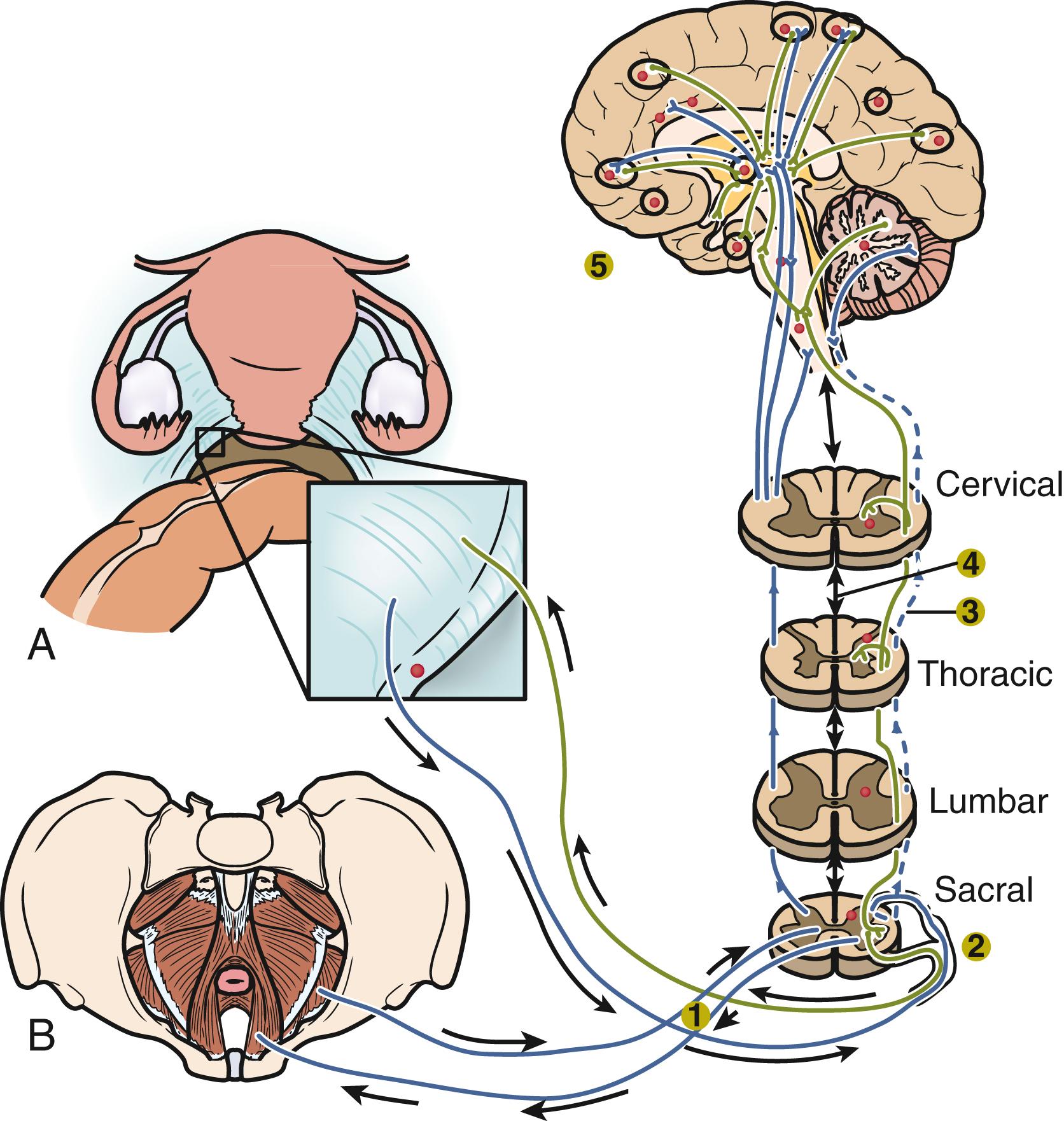
An average patient with endometriosis suffers from its symptoms for decades. Severe menstrual pelvic pain initially experienced during the teenage years gradually evolves in quality and severity as the inflammatory stimuli from pelvic disease persist and the peripheral and central nervous system is continually reconditioned, leading to the phenomenon of central sensitization.
The symptoms of endometriosis often disrupt the social, professional, academic, and economic potential of young women. Living with severe cyclic or continuous pelvic pain or the threat of its return, often for decades, can lead to anxiety and depression. Thus, the currently used and narrow anatomic definition of endometriosis neither reflects its true complexity or chronic nature nor addresses the potentially grim course of this disease in the absence of appropriate medical and surgical management.
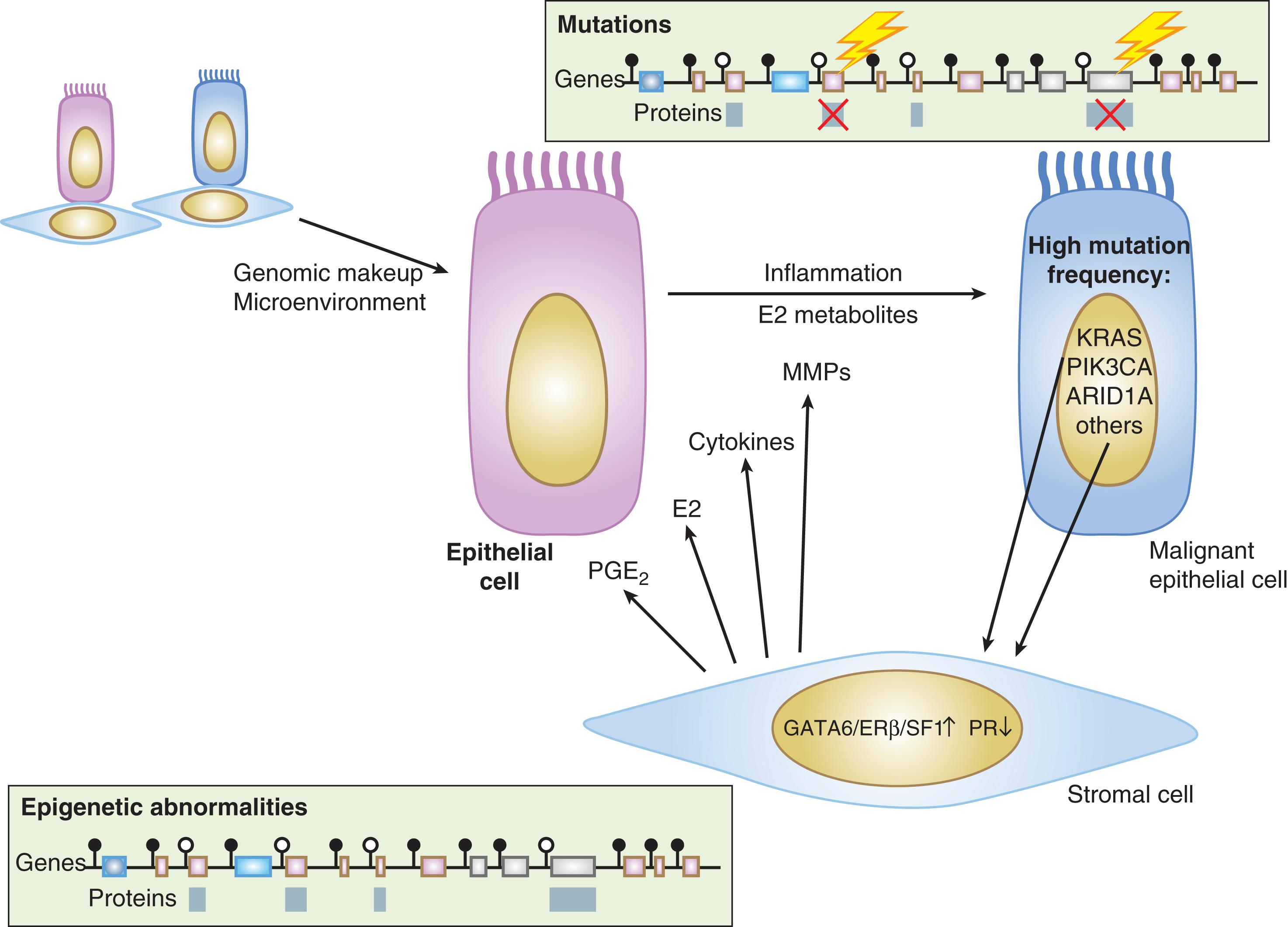
Adenomyosis, the presence of islands of endometrial tissue within the myometrium, is a related disorder with unique features and may coexist with endometriosis. Recent next-generation sequencing data suggest that both endometriosis and adenomyosis arise from clonally expanded endometrial cell populations carrying an epithelial cancer driver mutation.
The term endometriosis has been described conventionally as the presence of endometrial tissue outside of the uterine cavity, primarily on pelvic tissues and ovaries. , This narrow anatomic definition, however, is not sufficient to explain its natural history and duration, the full spectrum of its clinical features, the frequent recurrence of its symptoms, the underlying molecular pathophysiology, or its variable responsiveness to currently available management strategies. Unfortunately, the anatomic definition has led to confusion about treatment and even denial of hormonal or surgical treatment to endometriosis patients who do not have recognizable pelvic peritoneal lesions at the time of laparoscopy. Thus, the classical definition of endometriosis needs to be modernized to more of a patient-focused version that recognizes the cellular and molecular origins of the disease, its natural history from teenage years to menopause, its complex, chronic and systemic nature, the variety of tissues involved including the central nervous system and the need for long-term management. , , ,
Many clinicians, patients, and researchers now recognize endometriosis as a complex clinical syndrome characterized by an estrogen-dependent chronic inflammatory process that affects primarily pelvic tissues, including the ovaries, and is strongly linked to recurrent pelvic pain and persistent episodes of ovulation, menstruation, and cycling steroid hormones. , Endometriosis is the most common cause of chronic pelvic pain in reproductive-age women. It is also strongly associated with infertility.
In addition to common pelvic endometriosis (affecting pelvic peritoneal surfaces, subperitoneal fat, and ovaries), which comprises the vast majority of all cases of endometriosis, the disease may also affect the bladder, bowel (most commonly the rectum and appendix), deep pelvic nerves, ureters, anterior abdominal wall, abdominal skin, diaphragm, pleura, lungs, and the brain. , Thus, clinical findings can vary widely and include hematuria, hematochezia, pneumothorax, and seizures. The cyclic nature of these otherwise inexplicable symptoms and their association with menstruation (i.e., catamenial) suggests endometriosis as an underlying etiology. ,
The symptoms of endometriosis often severely disrupt the social, professional, academic, and economic potential of young women. , Living with severe cyclic or continuous pelvic pain or the threat of its return, often for decades, can lead to anxiety and depression. , Here, we will summarize the salient features of endometriosis, followed by a comprehensive definition of the disease process and the clinical and molecular advances that have been made over the past decade.
It is important for clinicians and researchers to develop a 360-degree perspective of endometriosis and take approaches to its diagnosis and treatment that will maximize benefits to the patient suffering from this disease. The following observations or postulates must be recognized and placed in a broader and unified pathological context.
(1) Severe primary dysmenorrhea, repeated episodes of ovulation and retrograde menstruation, and pelvic endometriosis visualized by laparoscopy probably represent the early and progressive stages of the same disease process ( Fig. 27.1 ). , , (2) An average patient with endometriosis suffers from its symptoms for decades. Severe menstrual pelvic pain initially experienced during the teenage years gradually evolves in quality and severity as the inflammatory stimuli from pelvic disease persist and the peripheral and central nervous system is continually reconditioned, leading to the phenomenon of central sensitization ( Fig. 27.2 ). , , (3) Retrograde menstruation associated with ovulatory cycles is the primary source of pelvic peritoneal endometriotic implants, rectovaginal nodules, and ovarian endometriomas, which arise primarily from cystic corpora lutea harboring menstrual tissue ( Figs. 27.1 and 27.3 ). (4) In the absence of any visible pelvic endometriotic implants, microscopic inflammatory processes in the pelvic peritoneum or eutopic endometrial tissue can exist and cause persistent pain that responds to treatments that suppress ovulation and estrogen production. , (5) Newly diagnosed patients with endometriosis significantly benefit from surgical or medical treatment; almost all treatment-naïve patients achieve pain relief. However, the response rate progressively and sharply decreases with each repeated treatment, , thereby making it more challenging to treat recurrent disease and its symptoms. (6) Any successful management strategy of endometriosis-associated pelvic pain should include long-term suppression of ovulatory meses.
It is also critical for clinicians to understand the following key mechanistic, cellular, and molecular findings that underlie endometriosis.
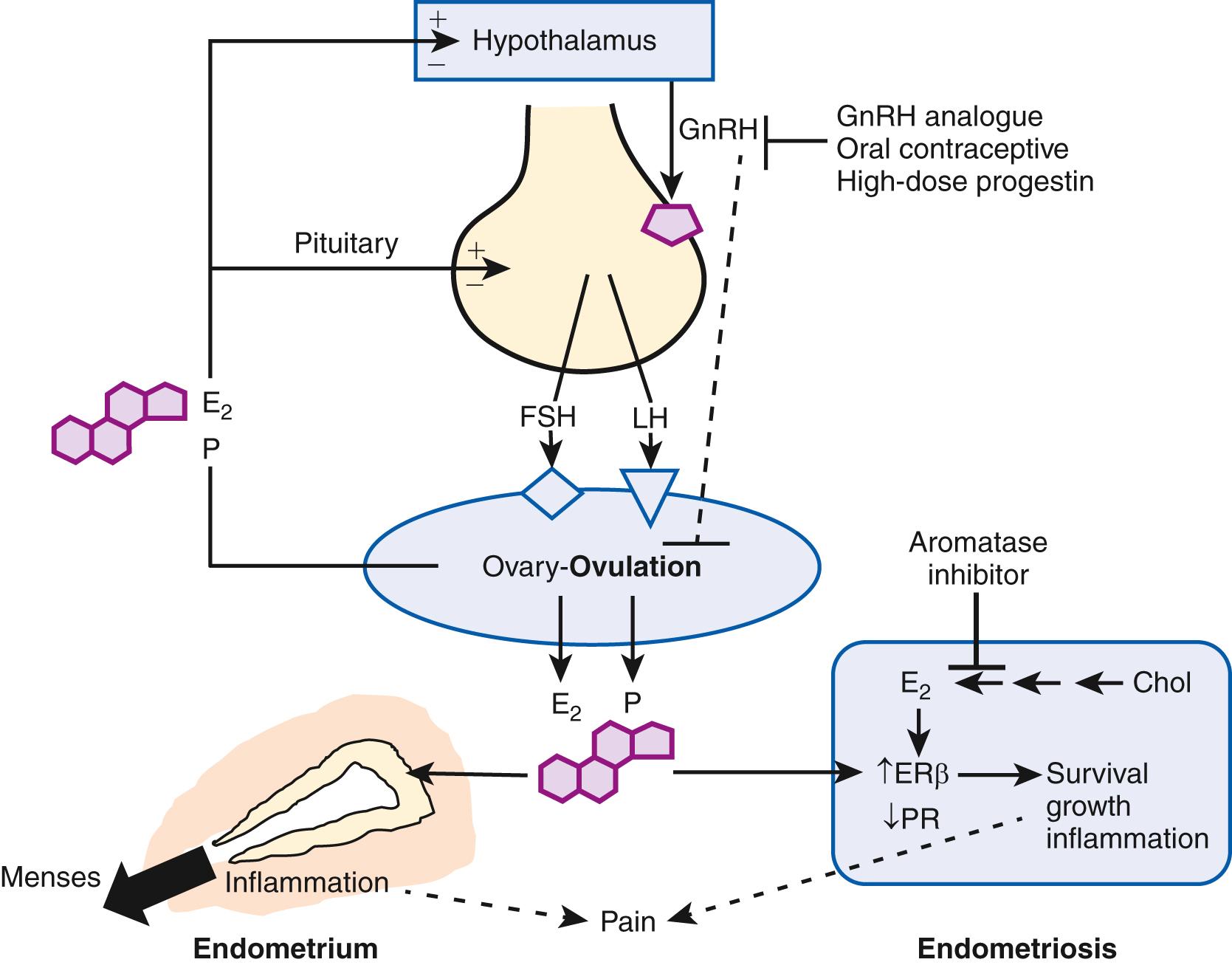
(1) Ovulatory menstruation is inextricably linked to the development of endometriosis, at least in its early stages ( Fig. 27.4 ). Species that do not menstruate do not develop endometriosis, and women without ovulatory menses do not develop endometriosis. Disruption of ovulatory menses in women with endometriosis is usually therapeutic ( Fig. 27.4 ). , (2) Endometriosis involves multiple tissues. Distinct cellular and molecular abnormalities involving inflammation and steroid responsiveness have been well described at least in two types of tissues: eutopic (intracavitary) endometrium and ectopic endometriotic tissue. It is possible that the list of affected tissues will grow. , Thus, surgical removal of ectopic pelvic endometriotic tissue, even by the most capable surgeons, will not cure endometriosis. (3) Histologically, the majority of endometriotic implants are composed primarily of stromal cells and contain a relatively smaller epithelial component that lacks the deep invaginations (glands) observed in eutopic endometrial tissue ( Fig. 27.3 ). (4) As of 2014, a series of paradigm-shifting next-generation sequencing studies consistently reported the mapping of distinct cancer-associated mutations in the epithelial cells of eutopic endometrium to the epithelial cells in ovarian endometriosis, extraovarian endometriosis, and adenomyosis. Each gland in the endometrial epithelium carried a distinct cancer-associated mutation or set of mutations. Although a number of mutations were detected in endometrial epithelial cells, KRAS was the most frequently and recurrently mutated gene in endometriosis and adenomyosis. It appears that clonal expansion of an endometrial epithelial cell population carrying a distinct mutation such as KRAS served as the source of endometriosis and adenomyosis. (5) In contrast to epithelial cells, stromal cells of endometrium or endometriosis were mutation-free. , When compared to endometrial stromal cells, stromal cells of endometriosis are epigenetically abnormal and demonstrate partial phenotypes of ovarian theca-granulosa cells and macrophages. Endometriotic stromal cells express the full cascade of steroidogenic enzymes such as aromatase and produce remarkable quantities of progesterone and estradiol from cholesterol. The endometriotic stromal cell also produces large amounts of immunologically active substances such as interleukin (IL)-1β, IL-6, tumor necrosis factor (TNF), regulated upon activation of normal T cell expressed and secreted (RANTES), and monocyte chemoattractant protein-1 (MCP-1). , , (6) The magnitude of the role of estrogen in endometriosis is somewhat similar to the role of insulin in diabetes. Estradiol is a master regulator of all key pathological processes in endometriosis and enhances lesion survival and inflammation leading to pain ( Fig. 27.4 ). In the absence of estradiol, endometrial tissue attachment to the peritoneum, lesion survival, production of inflammatory substances such as metalloproteinases, cytokines or prostaglandins and growth factors, and angiogenesis do not occur. Estradiol, primarily acting via estrogen receptor-β (ERβ), triggers pathways to enhance lesion survival, remodel pelvic peritoneal tissue, and produce inflammatory substances, which stimulate nociceptors in pelvic tissues and trigger pain ( Figs. 27.2 and 27.4 ). , Denial of estradiol to endometriosis either naturally (menopause) or pharmacologically (ovarian suppression or aromatase inhibition) causes regression of the disease and its symptoms ( Fig. 27.4 ). , , (7) Endometriosis is resistant to the effects of progesterone because of a deficiency of progesterone receptor (PR) in this tissue. In fact, endometriotic tissue produces large amounts of progesterone locally, which is consistent with PR deficiency or progesterone resistance. This partly explains why endometriotic tissue responds poorly to progesterone. On the other hand, synthetic antiprogestin compounds such as mifepristone and ulipristal acetate (which bind to lower levels of PR with higher affinity) are therapeutic in endometriosis. The beneficial effects of synthetic progestins are probably mediated primarily via ovarian suppression.
Adenomyosis, defined as the presence of islands of endometrial tissue surrounded by hypertrophic smooth muscle cells within the myometrium, is one of the most challenging uterine disorders in terms of diagnosis and management ( Fig. 27.5 ). Adenomyosis presents with pelvic pain, excessive uterine bleeding, anemia, and infertility. Although adenomyosis has been considered a variant of endometriosis (i.e., endometriosis interna), there are clinical and pathologic similarities and distinctions between the two entities. , In contrast to endometriosis, the frequency of adenomyosis is higher in parous women compared with nulliparas. , Likewise, women with a history of one or more spontaneous abortions were found to be at increased risk. , This may be explicable by a higher risk for endometrial-myometrial junction breakdown induced by pregnancy. The endometrial-myometrial junction may also be disrupted by repeated episodes of menstruation and associated myometrial contractions, leading the invagination of basal endometrium into the myometrium. , In fact, the risk is higher in women reporting heavy periods. , This view resonates with the involvement of tissue injury and repair as a mechanism for adenomyosis.
Despite certain distinctions, adenomyosis seems to be strongly associated with endometriosis. For example, one study showed that women with endometriosis also exhibit an irregular endometrial-myometrial junction and a higher rate of adenomyosis ( Fig. 27.5 ). Additionally, severe endometriosis is associated with adenomyosis with a deeper myometrial invasion. The lack of definitive molecular data has made it challenging to understand the associations between these uterine disorders. A new set of reports on targeted deep DNA sequencing-based analyses of these pathologies signals a paradigm shift in our ability to clarify the underlying disease mechanisms and the possible associations between them.
Nonsteroidal antiinflammatory drugs and hormone-based treatments for suppressing ovulatory menses and estrogen production such as progestins, oral contraceptives, gonadotropin-releasing hormone analogs, levonorgestrel-releasing intrauterine devices, aromatase inhibitors, danazol, and selective progesterone receptor modulators are currently used off-label to manage pain symptoms and abnormal uterine bleeding in adenomyosis. Gonadotropin-releasing hormone analogs are used before fertility treatments to help improve the chances of pregnancy in infertile women with adenomyosis. , Surgical treatment for diffuse adenomyosis has been limited to hysterectomy.
Endometriosis affects an estimated 1 in 10 women during their reproductive years (i.e., usually between the ages of 12 and 52), or approximately 200 million women worldwide. , However, it is more relevant to discuss the prevalence of endometriosis rather than its incidence because the disease can go undiagnosed for decades. , The underlying disease process can start as early as menarche, and last as long as four decades. , Though endometriosis may be suppressed via inhibition of ovulation and menses and pain or infertility may be managed by conservative surgical resection, the majority of these patients will not be cured of this disease. Endometriosis is the most common pelvic pathology found in women undergoing laparoscopy or laparotomy for chronic pelvic pain or infertility. ,
Retrograde menstruation is the most likely pathogenic mechanism for the development of endometriosis ( Fig. 27.1 ). , , The likelihood of implantation of retrograde-traveled endometrium may be influenced by the recent major increase in the number of menstruations due to earlier ages of menarche and progressively decreasing number of pregnancies per woman that naturally disrupt ovulatory cycles. Indeed, the risk of endometriosis appears to increase with a greater lifetime number of ovulatory cycles. Moreover, ovulation and the genesis of ovarian endometriosis have been linked, as endometriomas seem to develop from follicles immediately after ovulation and by the direct transition from hemorrhagic corpora lutea ( Fig. 27.1 ).
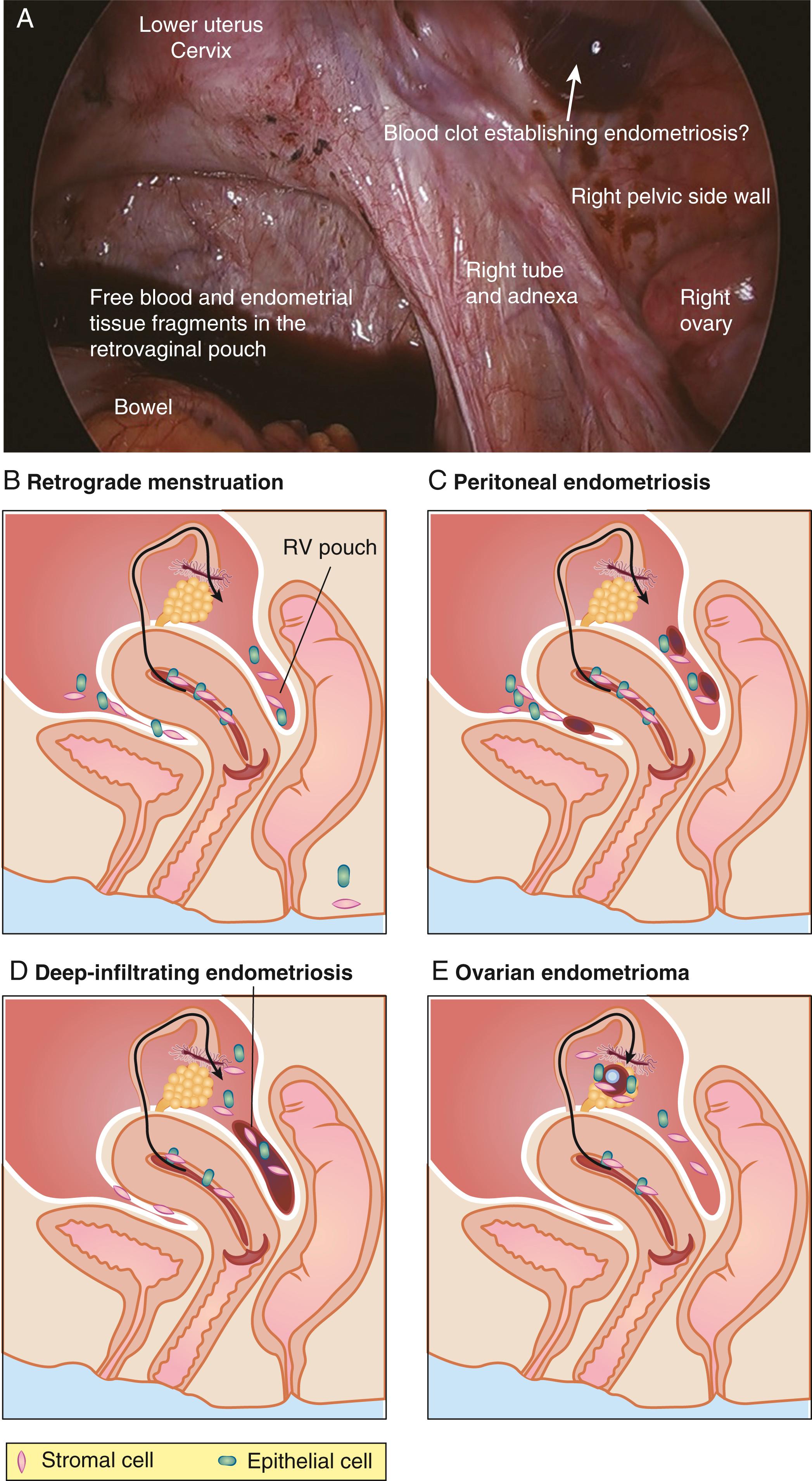
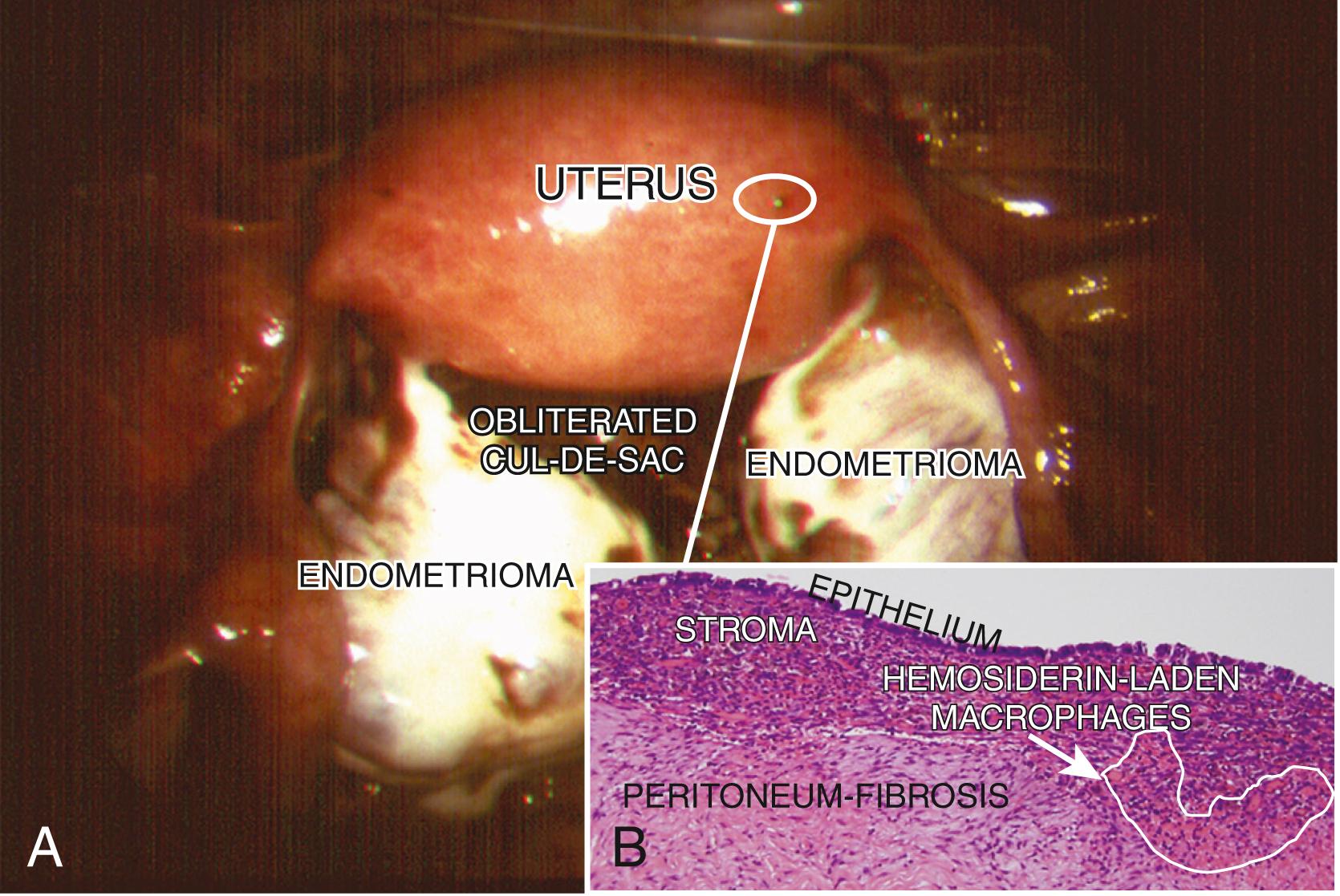
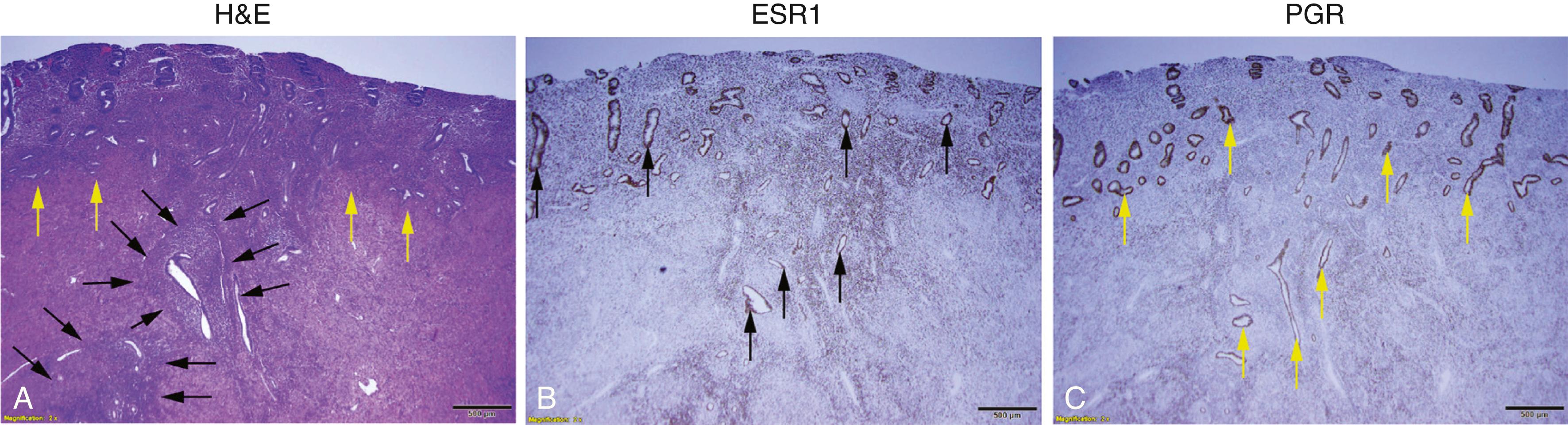
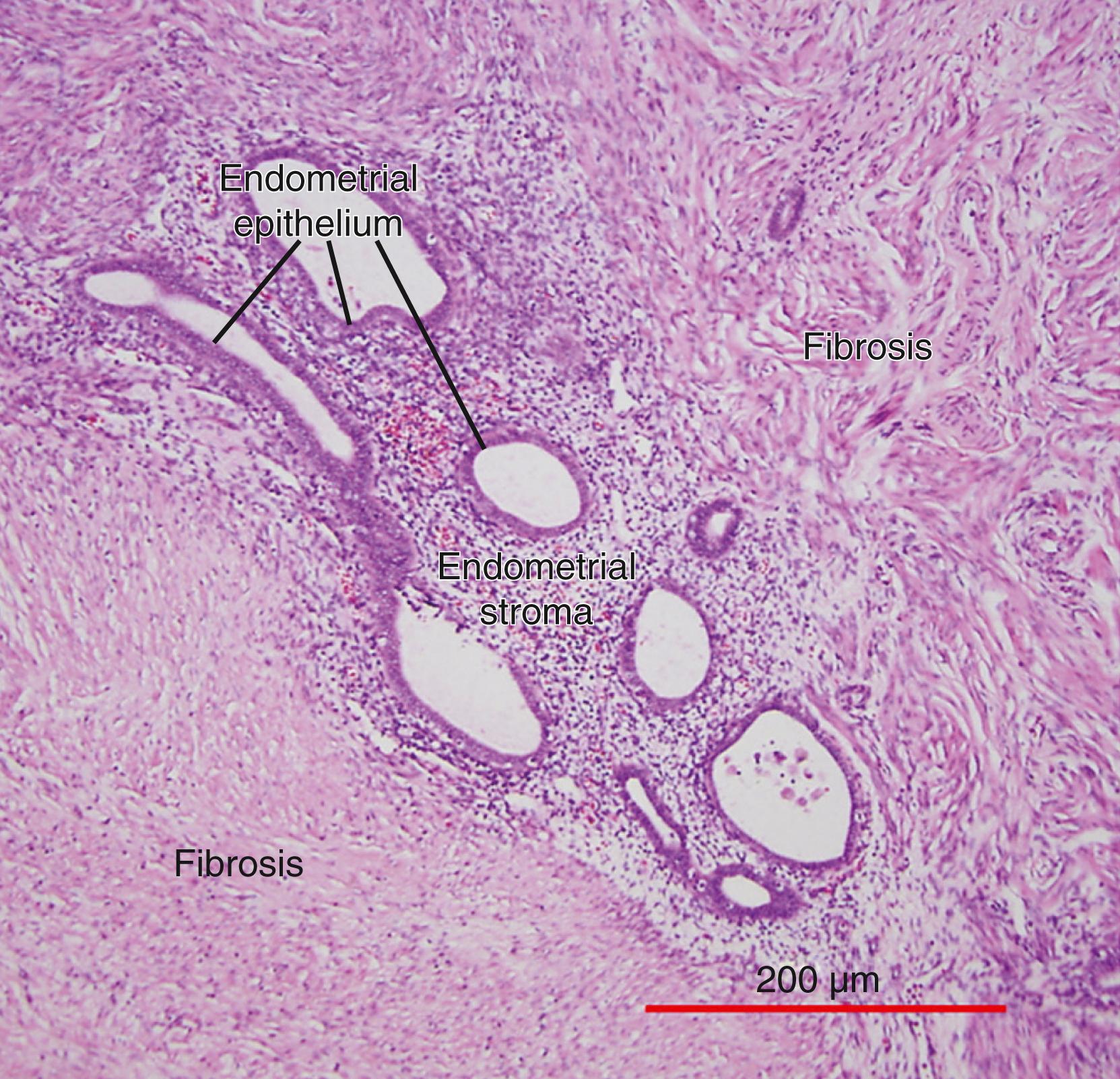
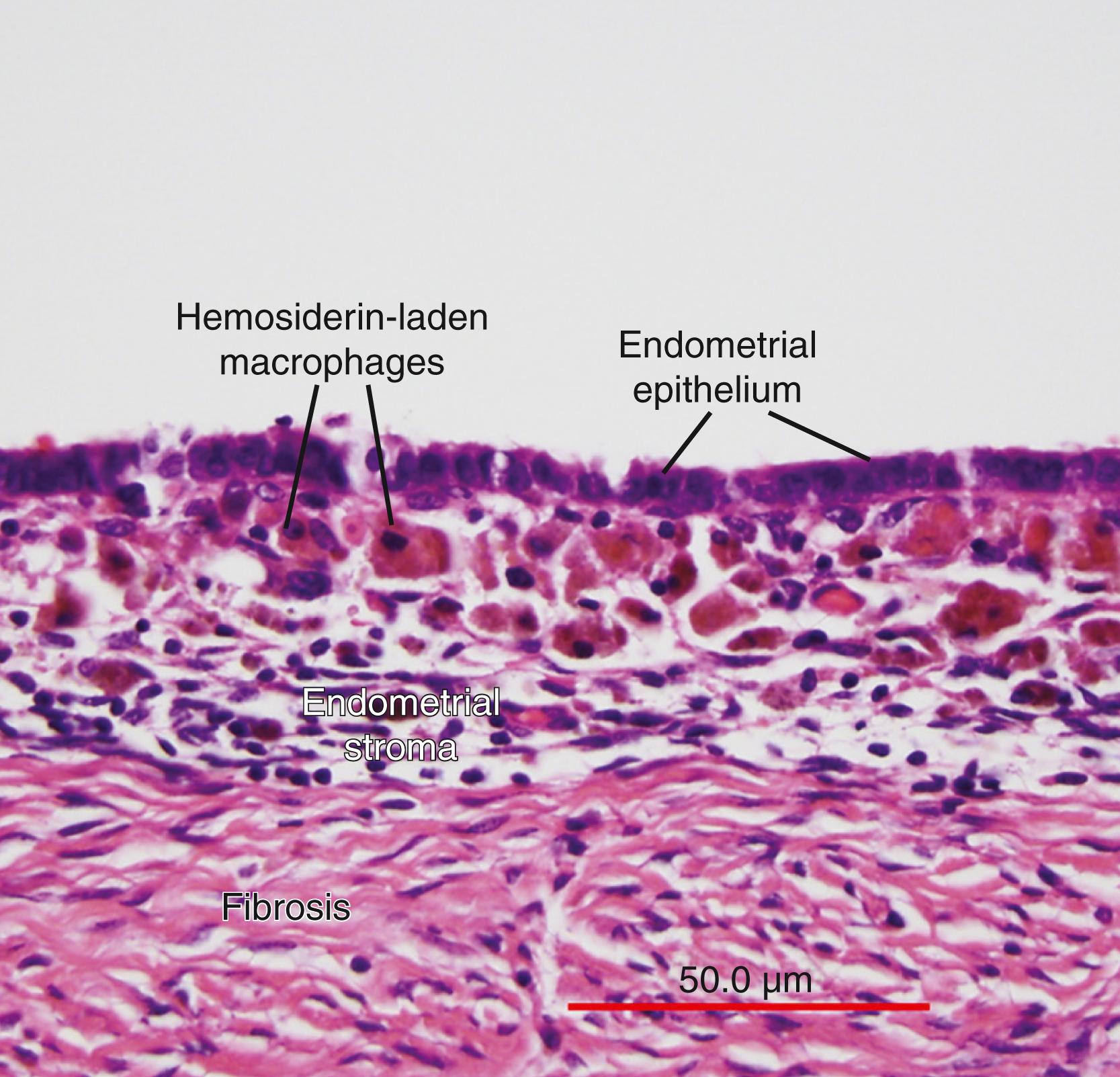
The term endometriosis initially referred to a histologic diagnosis but has eventually evolved to describe a syndrome as the clinical picture became clearer, expanding to include symptoms, laparoscopic findings, and molecular discoveries. Histologically, endometriosis refers to the presence of endometrium-like tissue outside of the uterine cavity, usually on or under the pelvic peritoneal surfaces or in the ovary. Histologic diagnosis is made upon the identification of three key elements: (1) endometrial stromal cells, (2) endometrial epithelial (glandular) cells, and (3) evidence of chronic bleeding in or adjacent to endometrium-like tissue, including sheets of red blood cells and blood pigment (hemosiderin)-containing macrophages. A pathologist usually requires two of these three findings to make a histologic diagnosis of endometriosis. Fibrosis comprised of fibroblasts and extracellular matrix is commonly observed surrounding endometriotic implants. It is not uncommon to receive a pathologic diagnosis stating, “fibrotic tissue consistent with endometriosis.” In this case, the pathologist probably found only endometrial stromal-like cells and fibrosis but not endometrial epithelial cells. Pelvic endometriosis is commonly classified by three common locations: (1) peritoneal endometriosis found on uterine serosa or pelvic peritoneal or subperitoneal tissue ( Fig. 27.6 ), (2) rectovaginal nodule or other forms of deep infiltrating endometriosis ( Fig. 27.7 ), and (3) ovarian endometrioma ( Fig. 27.8 ).
A number of mechanisms have been proposed regarding the histologic origins of pelvic endometriosis. Sampson and others postulated that fragments of menstrual endometrium pass retrograde through the fallopian tubes, then implant and persist on peritoneal surfaces. This has been demonstrated in primate models and observed naturally in humans; it is also supported by the observation that spontaneous endometriosis occurs exclusively in species that menstruate (i.e., female primates, including humans). Recent next-generation sequencing-based mapping of endometrial epithelial mutations to endometriosis or adenomyosis provided the most compelling evidence in support of Sampson’s hypothesis. None of the other proposed hypotheses have been demonstrated or supported by reproducible experimental data.
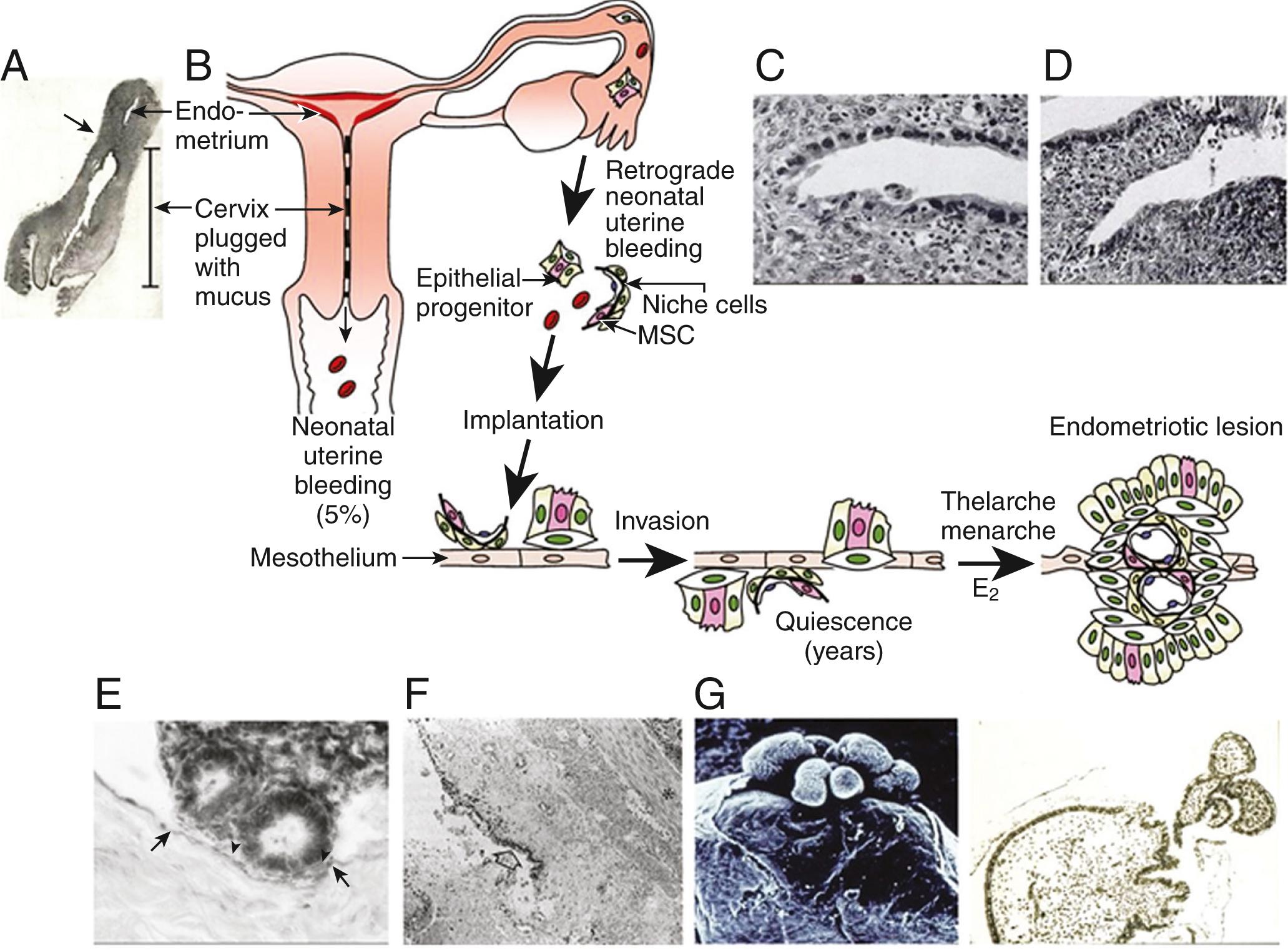
Sampson’s hypothesis of implantation of intracavitary endometrial tissue offers a plausible mechanism for the majority of abdominal endometriotic lesions but does not explain why only some women develop endometriosis. Although almost all women have reflux menstruation of endometrial tissue mixed with blood into the peritoneal cavity, endometriosis is encountered in only approximately 10% of cases. Two potential mechanisms may explain the successful implantation of refluxed endometrium to the peritoneal surface ( Fig. 27.9 ). First, eutopic endometrium or the tubal mucosa of women with endometriosis qualitatively exhibits multiple subtle but significant molecular abnormalities, including activation of oncogenic pathways or biosynthetic cascades, that favor increased production of estrogen, cytokines, prostaglandins, and metalloproteinases. , , When this biologically distinct tissue attaches to mesothelial cells, the magnitude of these abnormalities is amplified drastically to enhance implant survival ( Fig. 27.9 ). Second, larger quantities of otherwise normal endometrial tissue, including somatic stem cells, may arrive at the pelvic abdominal cavity during each menses in women with endometriosis compared with endometriosis-free women. It was also suggested that endometrial stem/progenitor cells may be involved in the pathogenesis of premenarchal and adolescent endometriosis through retrograde neonatal uterine bleeding due to maternal progesterone withdrawal at birth. Endometrial stem/progenitor cells would remain dormant beneath the peritoneum until increasing estradiol levels at menarche activate them to initiate clonal growths of ectopic endometrium and establish early-onset endometriosis prior to menstruation ( Fig. 27.10 ).
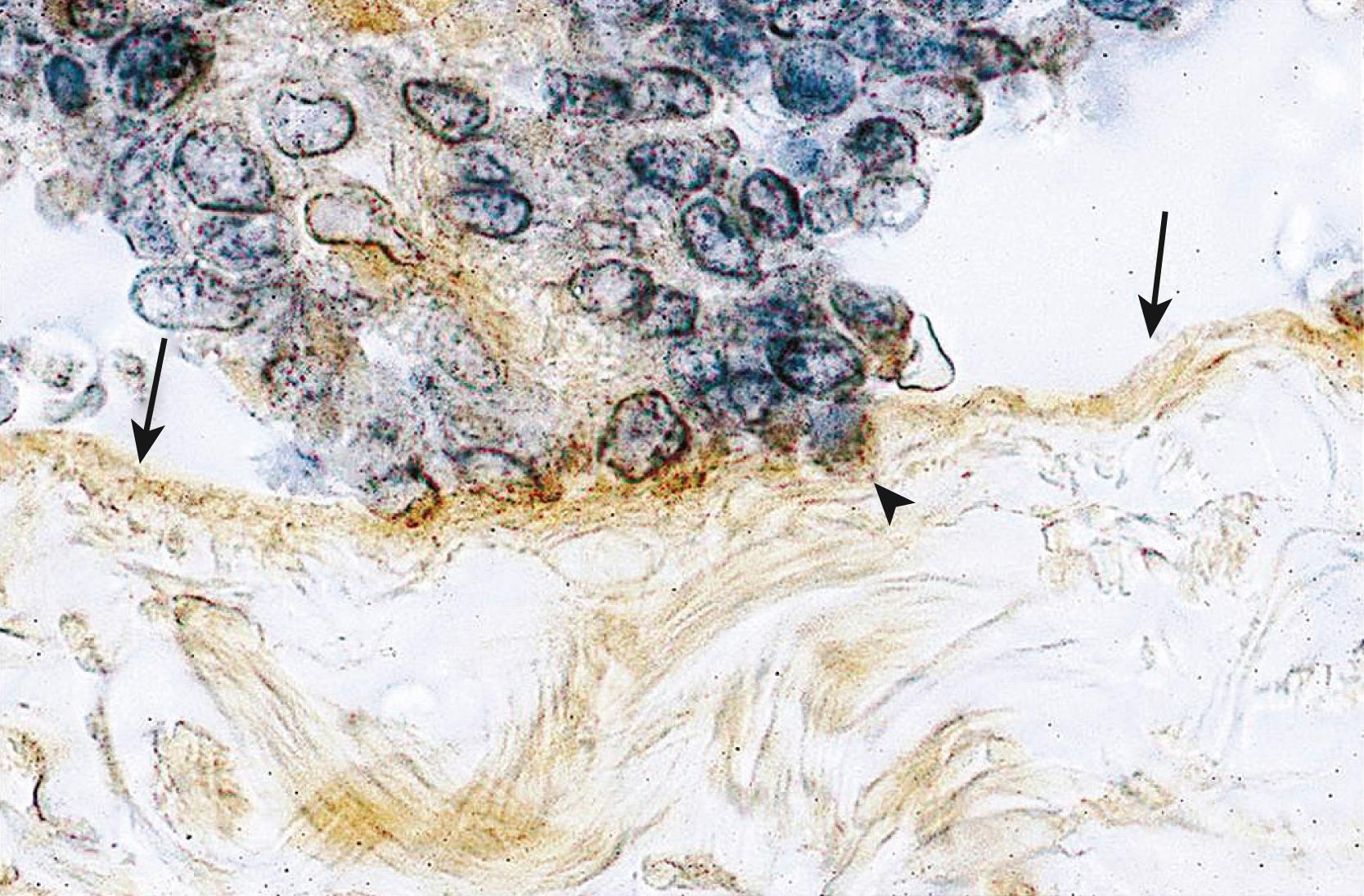
It has also been proposed that a defective immune system might fail to clear implants off the peritoneal surface. , , This argument, however, assumes that a healthy immune system should attack its native tissue, which is a variable phenomenon at best.
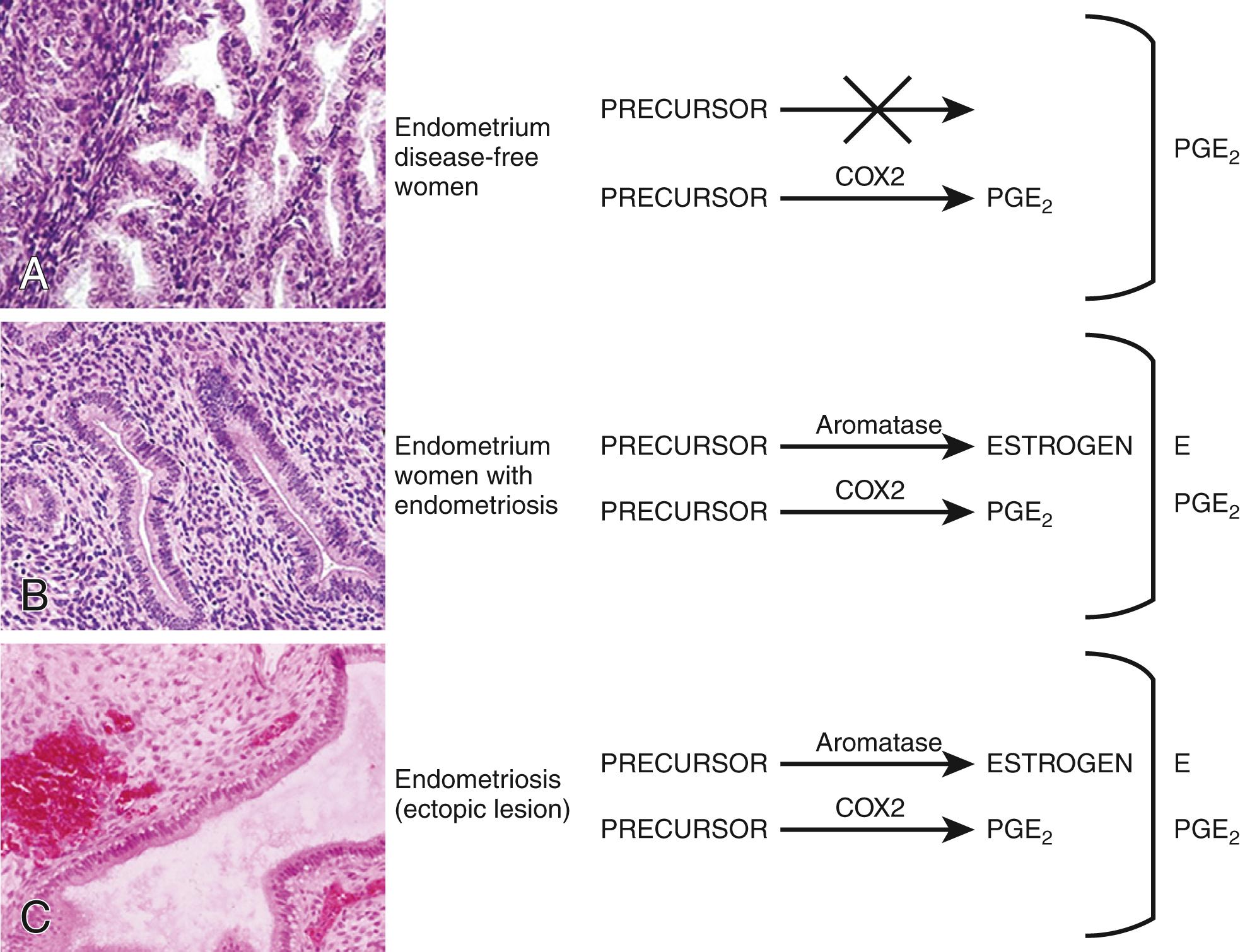
Molecular and cellular defects in eutopic endometrium. Histologically, the eutopic endometrium of women with endometriosis looks identical to that of endometriosis-free women. A considerable body of clinical and molecular evidence, however, supports the existence of qualitative and quantitative abnormalities in eutopic endometrial tissue of women with endometriosis. For example, stromal cells of eutopic endometrium of women with endometriosis contain strikingly higher levels of aromatase, COX2 and IL-6 mRNA, and protein, as well as higher levels of aromatase enzyme activity ( Fig. 27.11 ). , Gene expression profiling of eutopic endometrium in women with or without endometriosis confirmed these earlier observations and found dysregulation of selected genes that contribute to implantation. These included genes involved in embryonic attachment, embryo toxicity, immune dysfunction, and apoptotic responses, as well as genes that likely contribute to the pathogenesis of endometriosis, such as aromatase, progesterone receptors, and angiogenic factors.
Small nerve fibers were detected in the functional layer of eutopic endometrial tissue in all women with endometriosis but not in the eutopic endometrium of disease-free women. This may have important implications for understanding the generation of pain in these patients. For example, this may explain why endometriosis patients continue to experience pain after surgical resection of ectopic implants. Moreover, the pathologic presence of nerve fibers points to a general defect in the differentiation of somatic stem cells in the endometrium of patients with endometriosis (see below).
Intriguingly, two laboratories recently demonstrated that single endometrial glands carry distinct single or occasionally multiple epithelial mutations indicating the heterogeneous genomic architecture of the endometrium. , Some of these identical mutations could be mapped to endometriosis or adjacent adenomyosis in the same patient, further suggesting that clonal expansion of populations of epithelial cells with cancer-associated mutations and with possibly attached stromal cells may be a key underlying mechanism. Although PIK3CA is the most commonly mutated gene in endometrial glands, KRAS is the most frequently mutated gene in matched endometriosis or adenomyosis tissues, suggesting KRAS mutations confer survival and growth advantages. Although there was no difference between the eutopic endometrial glandular epithelial cells of women with or without endometriosis or adenomyosis with respect to the mutations in the PIK3CA or PPP2R1A genes, the frequency of a specific KRAS mutation (G12/G13) was significantly higher in the eutopic endometrium of women with endometriosis, adenomyosis or both.
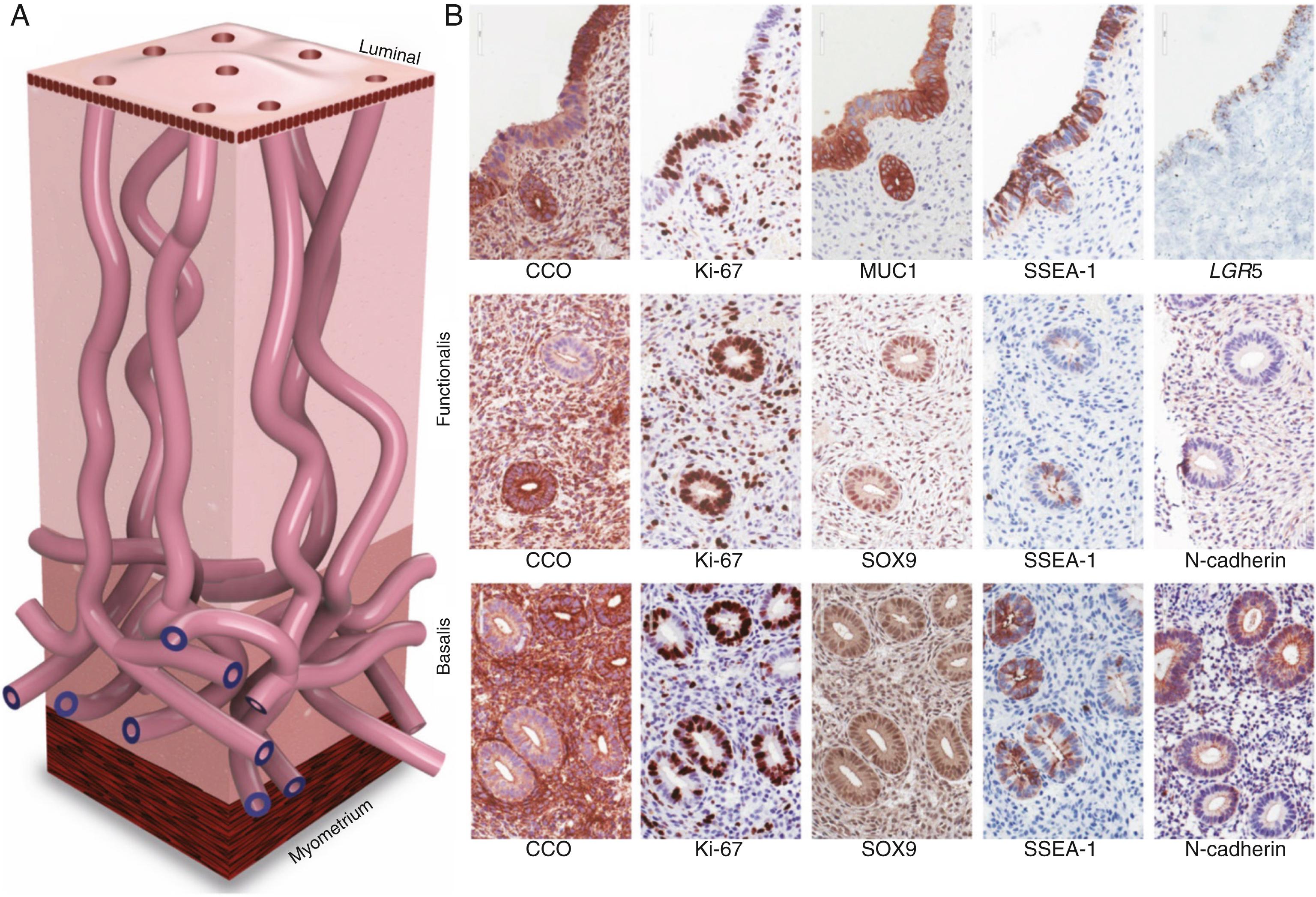
It is postulated that epithelial or stromal cells with progenitor properties located in the basalis layer of the endometrium are responsible for cyclic regeneration of this tissue under the influence of estrogen. If the transtubal migration and engraftment of viable endometrial fragments to the peritoneal mesothelium or an ovarian cyst is the primary mechanism of endometriosis, then progenitor/stem cell populations must also exist in these fragments of the functionalis layer. In fact, compared with healthy women without endometriosis, the functionalis layer of the eutopic endometrium of endometriosis patients contains higher numbers of epithelial cells with specific markers (SSEA1+, nSOX9+) for the secretory basalis endometrium. Moreover, these epithelial cells from the eutopic endometrium of women with endometriosis molecularly demonstrated established stem cell markers and functionally formed endometriotic gland-like structures in vitro .
As for endometrial stromal cells, mesenchymal stem cells have also been isolated from endometrial tissue and characterized; they have similar properties to bone marrow mesenchymal stem cells. Selective markers for mesenchymal stem cell enrichment have been identified: CD146, platelet-derived growth factor receptor-β (PDGFRβ), and sushi domain containing-2 (SUSD2) reveal a perivascular location of MSCs and suggest a pericyte identity of these cells in endometrial basalis and functionalis vessels. As for epithelial cells, the existence of progenitor/stem cells capable of regenerating the entire complement of glandular epithelial lineages was shown using somatic mitochondrial or nuclear DNA mutations as clonal markers [32476144; 32350471; 30110635; 31857578]. Vertical tracking of each mutation suggested that distinct stem cell populations reside in basalis glands ( Fig. 27.12 ) [32476144; 32350741; 31857578]. Molecular evidence suggests that mesenchymal stem cells of eutopic endometrium may contribute to endometriotic tissue. In summary, recent discoveries of both endometrial epithelial mutations and markers for endometrial stem/progenitor cells have enabled the examination of the role of eutopic endometrial cellular populations in endometriosis and adenomyosis more precisely. , , ,
The endometriotic stromal cell that dominates in quantity and exhibits the central pathologic processes in endometriosis plays a central role in its biology ( Figs. 27.3 , 27.6–27.8 ). The endometriotic stromal cell exhibits most of the key and clinically relevant molecular abnormalities, such as estradiol production, progesterone resistance, cytokine production, and prostaglandin production. It should be pointed out, however, that the endometrial or endometriotic stromal cells can readily be maintained in primary culture to study their biology, whereas it is more challenging to culture epithelial cells. , This limitation has led to a disproportionate number of mechanistic molecular studies employing endometrial or endometriotic stromal cells and could have created a scientific bias against the potential roles of other cell types. It is anticipated that the recent discovery of recurrent cancer-driver mutations in endometriotic epithelial cells, particularly the activating mutations of KRAS , will drastically change the current paradigms. Nevertheless, the following conclusions resonate with the existing data, histologic sections of endometriotic implants, and clinical observations.
Apoptosis is clearly decreased in endometriotic stromal or epithelial cells compared with eutopic endometrial tissues. , This may be linked to pathologic SF1 (estradiol biosynthesis) and ERβ expression in stromal cells. In endometriotic stromal cells, ERβ mediates the antiapoptotic effects of estradiol. In endometriotic epithelial cells, it is likely that mutated KRAS or other driver mutations may directly contribute to decreased apoptosis. Moreover, the paracrine products of these mutated cells may influence the function of adjacent stromal cells.
A central process in endometriosis is deficient differentiation (decidualization) of the stromal cell. , This has been linked to progesterone resistance. , Although the first molecular evidence of progesterone resistance was observed in the endometriotic epithelial cell, deficient stromal decidualization was eventually found to be a central mechanism (see “Progesterone Resistance” below). , The notion of deficient differentiation also resonates with the proposed roles of epigenetically misprogrammed mesenchymal stem cells in endometriosis. ,
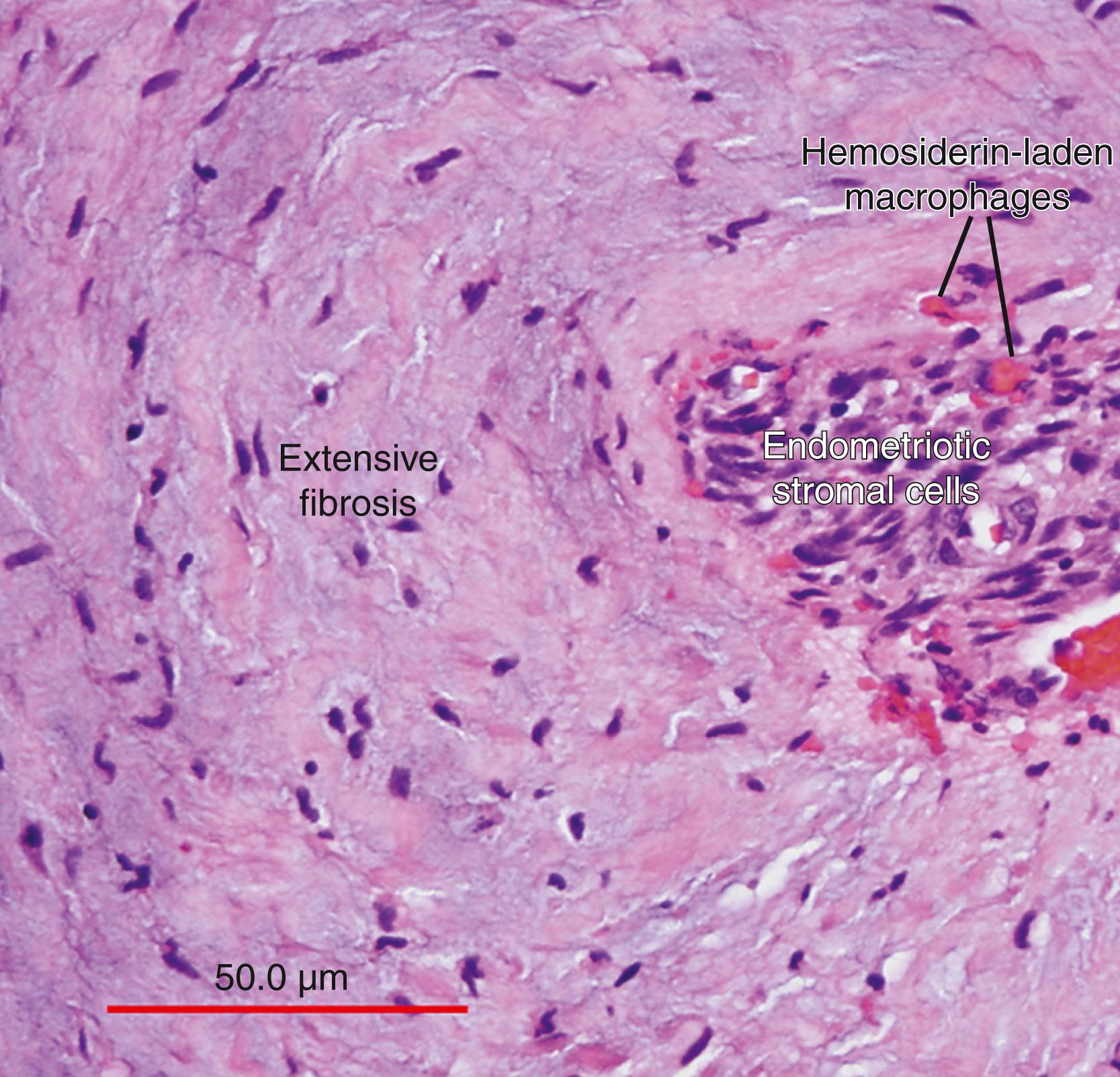
Inflammation is the most clinically relevant pathologic process in endometriosis. It can lead to pain, remodeling of neighboring tissues, fibrosis, adhesion formation, and infertility. The production of cytokines, prostaglandins, and infiltration of immune cells are some of the hallmarks of inflammation. Interestingly, the endometriotic stromal cell per se is one of the major sources of cytokines and prostaglandins. , Recurrent episodes of bleeding and an attempt of macrophages to remove the blood pigments also contribute to the inflammatory process, fibrosis, and adhesion formation ( Fig. 27.3 ). Activation and proliferation of subperitoneal tissue fibroblasts and extracellular matrix formation are thought to be an important source of fibrosis and tissue remodeling observed around the endometriotic lesions. Estradiol is a key inducer of inflammatory processes in endometriosis. , , , ,
Some invasive activity followed by proliferation seems to be necessary for the initial attachment of endometrial tissue fragments to the pelvic peritoneum and the establishment of subperitoneal endometriotic lesions. This is a complex process mediated by a number of molecular pathways. One of the best-studied pathways involves high metalloproteinase activity in endometriotic implants. Either proliferation or invasion, however, contributes to a much lesser degree to the pathology of endometriosis compared with malignant (e.g., endometrial cancer) or benign (e.g., uterine leiomyoma) neoplasms.
Studies on the heritability of endometriosis attempt to answer the question of whether this disease can be attributed to variation of genetic factors as opposed to variation of environmental or acquired effects. These include parent-offspring and twin studies, genetic linkage analysis, and a number of complex statistical methods used to analyze genome-wide association studies (GWAS). The risk of endometriosis seems to be transmitted in families in a polygenic manner. In one study, the mothers and sisters of women with severe endometriosis had a seven fold higher likelihood of suffering from endometriosis compared with primary female relatives of their partners. Consistent with this observation is the finding that familial cases of endometriosis tend to be more severe and have an earlier onset of symptoms than sporadic cases. Based on a study of 3096 twins, the heritability of endometriosis, or the proportion of disease variance due to genetic factors, has been estimated at around 52%.
The studies on familial endometriosis point to genetic transmission of the disease. The mode of transmission, however, seems to be complex, as seen in other chronic inflammatory conditions. Studies on a number of “off-the-shelf” candidate gene loci or case-control genetic association studies have not revealed any function-altering germ-line mutations in familial endometriosis or in any endometriosis phenotype. , Genetic linkage analysis of 1176 affected sister-pair families showed a significant association of endometriosis with chromosome 10q26. Subsequent fine-mapping of this region suggested a possible association of common variants near the CYP2C19 gene, but no mutations linked to endometriosis could be demonstrated. ,
GWAS typically focus on associations between single-nucleotide polymorphisms (SNPs) and traits such as major human diseases. Since 2010, a number of widely acknowledged endometriosis GWASs have been published; these studies employed datasets involving thousands of endometriosis cases and controls, primarily women of Japanese or of European ancestry. A meta-analysis of these GWAS datasets confirmed their findings and revealed additional loci. Together, these studies have provided significant evidence for the association of loci near: CDKN2B-AS1 (cyclin-dependent kinase inhibitor 2B antisense RNA), an intergenic region on chromosome 7p15.2, WNT4 (wingless-type MMTV integration site family member 4); VEZT (vezatin), an intergenic region on 2p14; and ID4 (inhibitor of DNA binding 4). Some of these loci had stronger effect sizes among Stage III-IV cases. Another GWAS study suggested common genetic origins between uterine fibroids and endometriosis.114 In a complex disease such as endometriosis, the majority of these SNIP-based associations are possibly too weak to reveal targetable gene products or causal mechanisms in part because they do not take into account cell-specific events, but further analyses of the GWAS data may provide insight into biologic networks that may prove to be important in the future.
As of 2011, a number of groundbreaking whole exome and whole genome sequencing (WES/WGS) studies of uterine disorders (followed by targeted deep sequencing) significantly improved our understanding of the mechanisms underlying uterine leiomyomas, endometriosis, and adenomyosis ( Table 27.1 ). , , These next-generation sequencing (NGS) studies not only verified the previously postulated role of eutopic endometrium in endometriosis or adenomyosis but also provided a compelling link between eutopic endometrial cells and ovarian cancer. , , , , ,
| Mutations | EE | Adenomyosis | Endometriosis | Leiomyoma |
|---|---|---|---|---|
| KRAS | + | +++++ | +++++ | |
| PIK3CA | +++++ | + | ++ | |
| PPP2R1A | + | + | + | |
| ARID1A | + | + | ||
| MED12 | +++++ |
Remarkably, almost all recurrent mutations were found in micro-dissected epithelial cells of eutopic endometrium, endometriosis, and adenomyosis, whereas the abundantly present stromal cells in these tissues were mutation-free. In approximately two-thirds of all uterine leiomyomas, the smooth muscle cell, which comprises the majority of the tumor cells, carried an identical mutation in a single gene ( MED12 ). This supported the concept that a leiomyoma arises from the clonal expansion of a mutated progenitor myometrial smooth muscle cell ( Table 27.1 ). , , In contrast, endometriosis and adenomyosis appeared to originate from the ectopic proliferation and expansion of multiple mutated epithelial cell clones that also contain an attached stromal cell population. In the invaginating deep crypts of clinically normal eutopic endometrial glands, multiple epithelial cell clones with distinct driver mutations often originate during the first decades of life and progressively colonize the epithelial lining of the endometrium in a mosaic-like manner ( Table 27.1 ). In the endometrium, endometriosis, and adenomyosis, the most frequent genetic alterations in PIK3CA, KRAS , and PPP2R1A , were predicted to be activating-type mutations, whereas ARID1A mutations lead to a loss of function ( Table 27.1 ).
PIK3CA was found to be the most frequently mutated gene in the eutopic endometrial glands examined ( Table 27.1 ). , Substantially less frequent mutations within a multitude of other cancer-associated genes, including KRAS , were also identified in the eutopic endometrium. , In contrast to eutopic endometrium, KRAS mutations comprised the majority of genetic alterations in endometriosis and adenomyosis. Moreover, identical mutations of KRAS and other cancer-associated genes were meticulously mapped in matched deep-infiltrating endometriosis or ovarian endometriomas and the eutopic endometrial tissues obtained from the same patients. These genomic analyses collectively suggest that an increase in expanded KRAS -mutated clones in eutopic endometrial tissue may be an early step in the molecular pathogenesis of endometriosis. During periodic retrograde travel of menstrual endometrial fragments, selection and survival of KRAS-mutated clones may initiate endometriosis in the ovary and the peritoneal surfaces ( Fig. 27.13 ). All mutations in the KRAS gene were located at amino acids 12, 13, or 61 and led to impaired guanosine triphosphate (GTP) hydrolysis by the KRAS GTPase-accelerating proteins (GAPs). This in turn led to constitutive activation of GTP-bound RAS. Constitutively activated mutant KRAS may induce a number of downstream pathways including PI3K-PDK1-AKT and RAF-MEK1/2-ERK1/2, favoring cell proliferation and survival. , Moreover, KRAS, Sirtuin 1 (SIRT1) and B-cell lymphoma 6 (BCL6) were reported to be coordinately overexpressed in eutopic endometrium of women with endometriosis and contribute to progesterone resistance in endometriosis. Although therapeutic targeting of these pathways in cancers with KRAS mutations has been disappointing, more promising progress has been made in targeting the KRAS protein directly. , The possible future use of these therapeutic strategies in endometriosis will depend on their clinical benefit and adverse effect profiles.
Epigenetic mechanisms refer to changes to the genome that do not involve a change in the DNA sequence. These mechanisms include but are not limited to DNA methylation and histone modification, each of which modifies how genes are expressed. These epigenetic influences may last through cell divisions for the duration of the cell’s life and may also last for multiple generations.
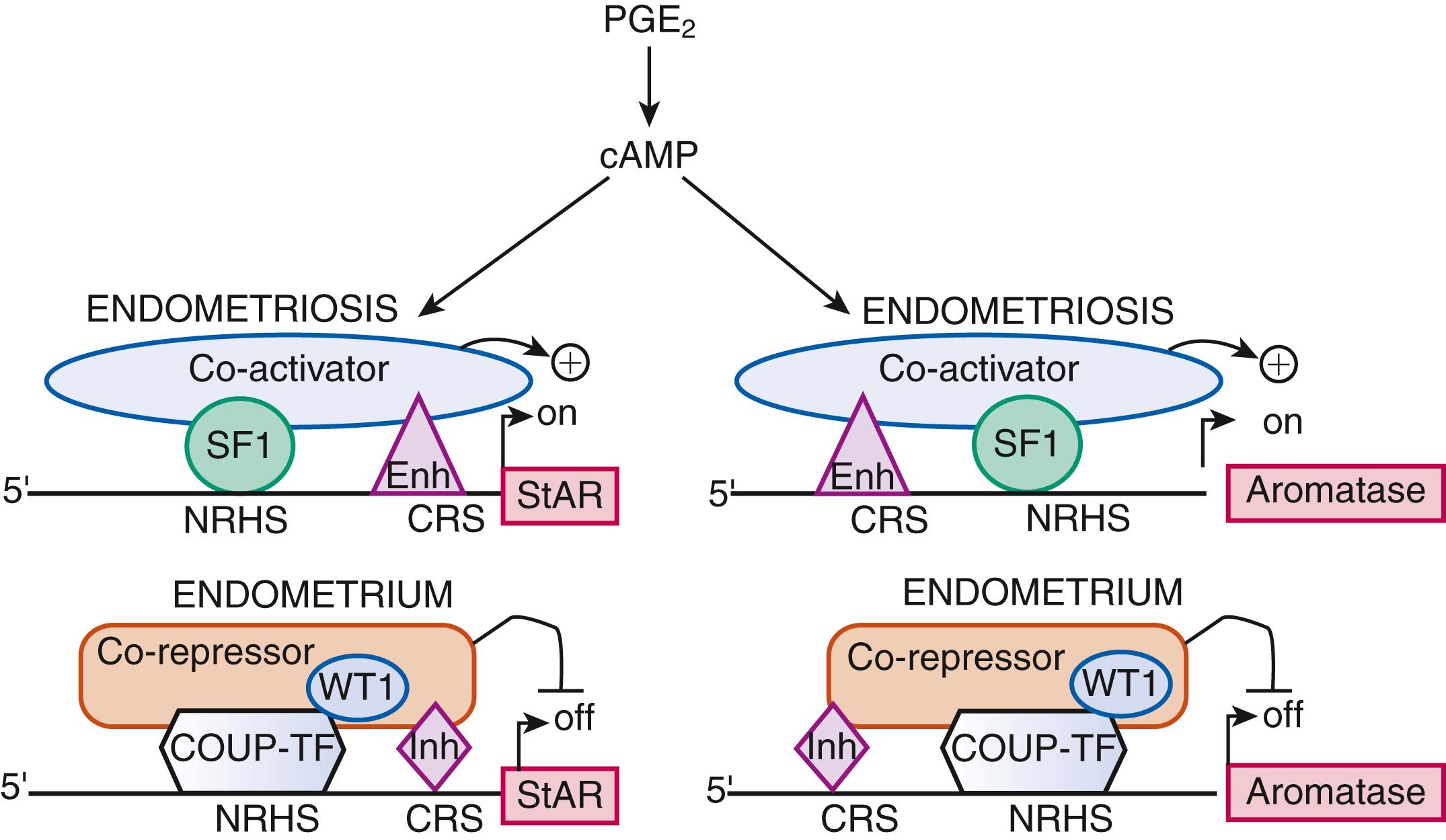
Gene expression . There are a number of general mechanisms responsible for gene expression in various cell types. , First, certain genes can be expressed in a highly specialized cell type because a specific set of necessary signaling pathways and downstream transcription factors are selectively present in this particular cell. For example, the endometriotic stromal cell specifically expresses the prostaglandin (PG) E 2 receptor EP2 that activates the cAMP-dependent signaling pathway and its downstream effector steroidogenic factor-1 (SF1, or NR5A1), which binds to the promoters of the steroid-producing genes, including steroid acute response protein (StAR) and aromatase ( Fig. 27.14 ). The presence of the transcription factor SF1 in endometriotic stromal cells is essential for the high levels of StAR and aromatase expression. Thus, the specific availability of a ligand (PGE 2 ), its receptor (EP2), and a transcription factor (SF1), which acts as the downstream mediator of this signaling pathway, determine the expression (transcription) of the StAR and aromatase genes, whose promoters are not strongly regulated by epigenetic alterations.
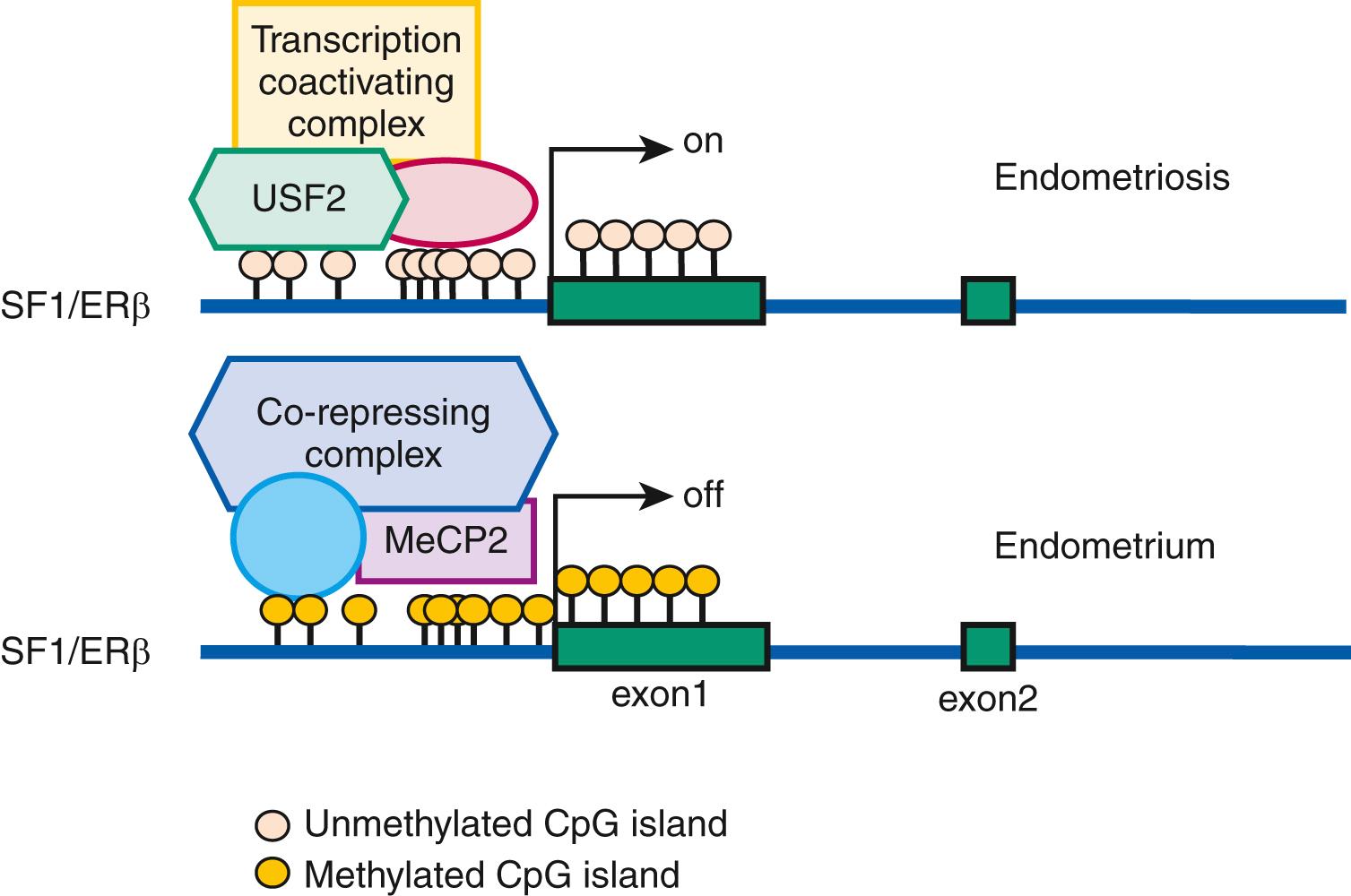
On the other hand, certain transcription factors (e.g., SF1 and ERβ) are either present or absent in a cell type and their protein levels are ordinarily not induced by a specific stimulus. The expression of genes that encode them may be regulated by less modifiable mechanisms such as DNA methylation of specific cytosine-(phosphate)-guanine (CpG) sequences ( Fig. 27.15 ). , Under physiologic circumstances, a dense cluster of CpGs (an “island”) within the promoter region of the SF1 gene is not methylated and therefore not repressed in ovarian granulosa cells that contain abundant SF1 and its target aromatase, whereas the SF1 promoter is methylated and repressed in normal endometrial stromal cells that do not express SF1 or aromatase ( Fig. 27.15 ). , , However, the endometriotic stromal cell manifests a partial characteristic of the ovarian granulosa cell to express SF1, which binds to the promoters of key steroidogenic genes under the stimulation of PGE 2 and cAMP production in a coordinated fashion. This effectively catalyzes the local conversion of cholesterol to progesterone and estradiol in a stepwise fashion in endometriosis. , ,
Epigenetic regulation of gene expression and protein production is the hallmark of stem cell function and the differentiation of progenitor/stem cells to more mature cell populations giving rise to specialized organ function. The proposed key roles of endometrial tissue stem cells in the development of endometriosis (see above) underscore the indispensable function of epigenetic mechanisms in the pathogenesis of endometriosis.
DNA methylation . Endometriotic cells express variable levels of the DNA methyltransferase enzymes (DNMTs), which introduce and maintain DNA methylation of cytosine in CpG dinucleotides. Several investigators have explored pathologic gene regulation in endometriosis related to differential DNA methylation between the eutopic endometrium and endometriosis, as well as between the eutopic tissues of women with or without endometriosis. , Abnormal DNA methylation in endometriosis affects the expression of several genes, including homeobox A10 ( HOXA10 ), estrogen receptor-β (ERβ, ESR2 ), and SF1 ( NR5A1 ), which alter steroid signaling and responsiveness, and are critically involved in development and decidualization ( Fig. 27.15 ). , , It was also proposed that the inflammatory environment in the lower abdomen or prolonged exposure to estradiol or progesterone regulated endometrial gene expression through altered DNA methylation. ,
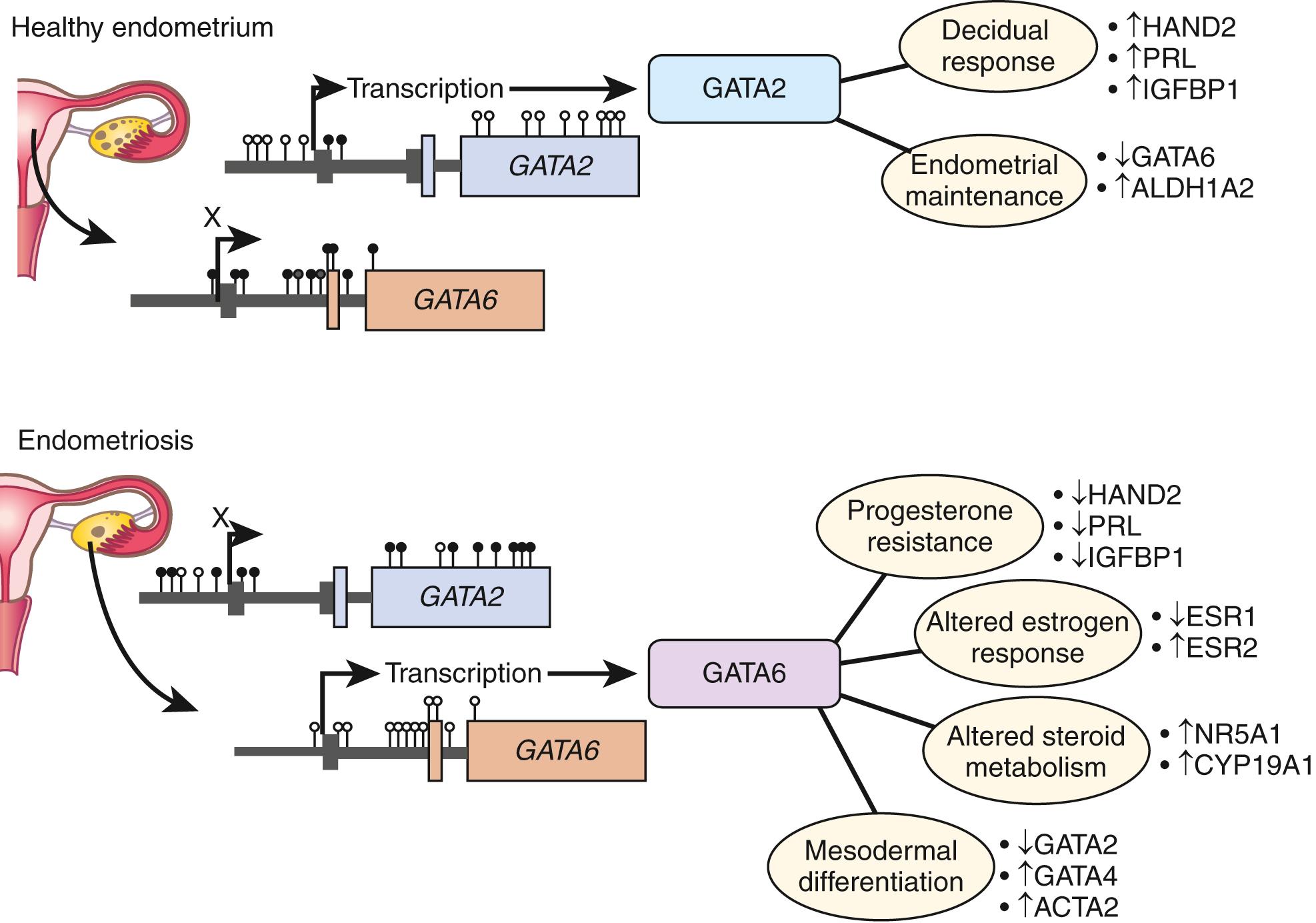
Genome-wide differences in DNA methylation between healthy human endometrial and endometriotic stromal cells have been mapped and correlated with gene expression using an interaction analysis strategy. Significant differences in methylation were mapped to some 400 genes, which included a disproportionally large number of transcription factors implicated in the pathology of endometriosis and decidualization. Differential methylation affected the HOX gene clusters, nuclear receptor genes, and intriguingly, the GATA family of transcription factors.
Functional analysis of the GATA family revealed that GATA2 regulates key genes necessary for the hormone-driven differentiation of normal endometrial stromal cells but is hypermethylated and repressed in endometriotic cells. GATA6 , which is hypomethylated and abundant in endometriotic cells, potently blocks hormone sensitivity, represses GATA2 , induces markers of endometriosis such as aromatase, SF1, and ERβ, and represses ERɑ and PR when expressed in healthy endometrial cells ( Fig. 27.16 ). Intriguingly, ectopic expression of GATA6 in a healthy endometrial stromal cell transformed it to an endometriotic cell that demonstrates progesterone resistance and estradiol production. , The unique epigenetic fingerprint in endometriosis suggests that DNA methylation is an integral component of the disease and identifies a key role for the GATA family as key regulators of uterine physiology—aberrant DNA methylation in endometriotic cells correlates with a shift in GATA isoform expression that facilitates progesterone resistance and disease progression ( Fig. 27.16 ). , ,
Nuclear receptors and DNA methylation. As described earlier, the levels of several nuclear receptors, including SF1, ERα, ERβ, and PR, are significantly different in endometriotic tissue compared with endometrium. The practical absence of the orphan (without a known ligand) nuclear receptor SF1 in endometrium and its 12,000-fold higher presence in endometriosis is, in part, determined by a classic CpG island at its promoter that is heavily methylated in endometrial stromal cells and unmethylated in endometriotic stromal cells ( Fig. 27.15 ). In endometrial cells, the silencer-type transcription factor methyl-CpG-binding domain protein 2 is recruited to the methylated SF1 promoter and prevents its interaction with transcriptional activators ( Fig. 27.15 ). In endometriotic cells, the transcription factor upstream stimulatory factor-2 (USF2) binds to the unmethylated SF1 promoter to activate it. In vivo , USF2 levels are strikingly higher in endometriosis compared with endometrium. Thus, differential SF1 expression in endometriosis versus endometrium is primarily controlled by an epigenetic mechanism that permits binding of activator versus inhibitor complexes to its promoter. ,
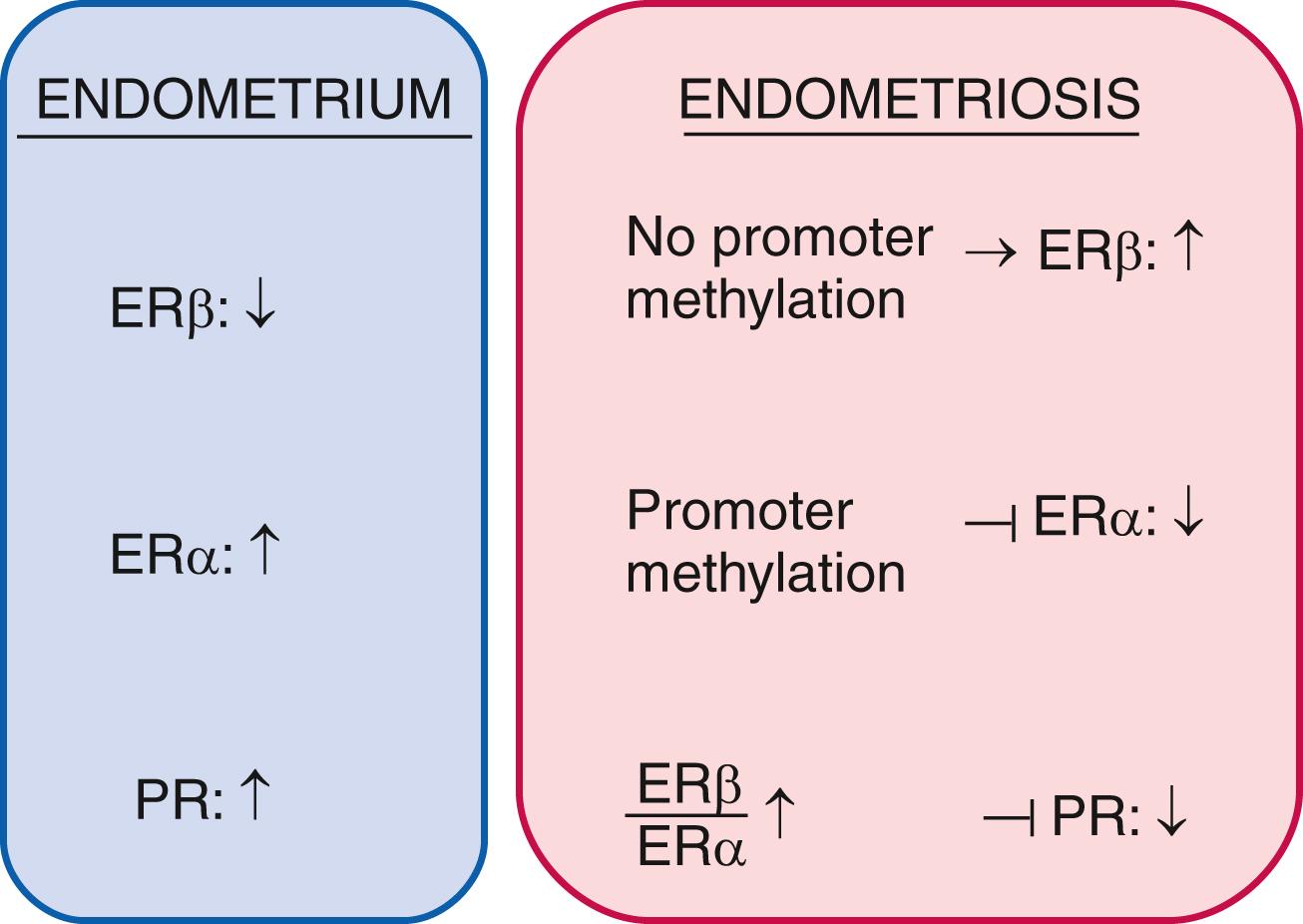
Among estrogen receptors, ERβ (ESR2) levels are 142-fold higher, whereas ERα levels are 9-fold lower in endometriotic tissue compared with endometrium ( Fig. 27.15 ). Hypomethylation of a CpG island at the promoter region of the ERβ gene allows high levels of expression in endometriotic stromal cells, and hypermethylation silences it in endometrial stromal cells ( Fig. 27.15 ). In contrast, the ERα (ESR1) promoter is appropriately unmethylated in eutopic endometrium and heavily methylated in endometriosis. Consequently, ERɑ levels are strikingly lower in endometriotic versus endometrial stromal cells. It is thus conceivable that the pathologically high ERβ:ERα ratio in endometriotic stromal cells perturbs estradiol induction of the PR gene giving rise to low PR expression in endometriosis ( Fig. 27.17 ).
MicroRNAs. MicroRNAs (miRNAs) are endogenous, small, highly conserved, noncoding RNAs that negatively control gene expression by targeting mRNAs, thus providing another layer of epigenetic regulation. In the eutopic endometrial tissues of women with or without endometriosis, global miRNA profiling showed dysregulation of the miR-9 and miR-34 miRNA families. HOXA10 is aberrantly regulated in the endometrium of women with endometriosis due to increased levels of both miR135a and miR135b, which target this gene. An altered microRNA profile in the endometrium may also suppress genes required for implantation. Aromatase inhibitor treatment significantly increases expression of let-7f in endometrial stromal cells from patients with endometriosis, whereas increased let-7f expression effectively reduces the migration of endometrial cells. Another study revealed that 10 microRNA species including miR-202 were up-regulated, whereas 12 microRNAs (e.g., miR-504) were down-regulated in ovarian endometriomas compared with endometrium. These studies collectively suggest clinically relevant biological roles of miRNAs in the pathology of endometriosis.
Histone modification . Histones comprise the backbone of chromatin and are intricately intertwined with DNA to regulate its programming. Posttranslational modification of histones by a number of key enzymes is indispensable for epigenetic regulation of gene expression. The family of histone deacetylases (HDAC) plays a key role in this regulation. In fact, HDAC3 is attenuated in the eutopic endometrium of infertile patients with endometriosis. HDAC3 loss caused a defect of decidualization through the aberrant transcription of collagen1A1 and1A2 genes. It is anticipated that other examples of histone modification defects will be discovered in endometrial and endometriotic cells in the near future.
Estrogen production plays a key role in the pathology of endometriosis because its inhibition by gonadotropin-releasing hormone analogs (GnRHa), oral contraceptives, progestins, and aromatase inhibitors significantly reduces pelvic disease and pain ( Fig. 27.18 A ). StAR facilitates the initial step of estrogen formation, the entry of cytosolic cholesterol into the mitochondrion. Then, six enzymes (side chain cleavage enzyme, 3β-hydroxysteroid dehydrogenase-2, 17-hydroxylase/17-20-lyase, aromatase, and 17β-hydroxysteroid dehydrogenase-1 [HSD17B1]) catalyze the conversion of cholesterol to biologically active estradiol ( Fig. 27.18 B). The key step , the conversion of C 19 steroids to estrogens, is catalyzed by aromatase, inhibition of which effectively eliminates all estrogen production ( Fig. 27.18 B).
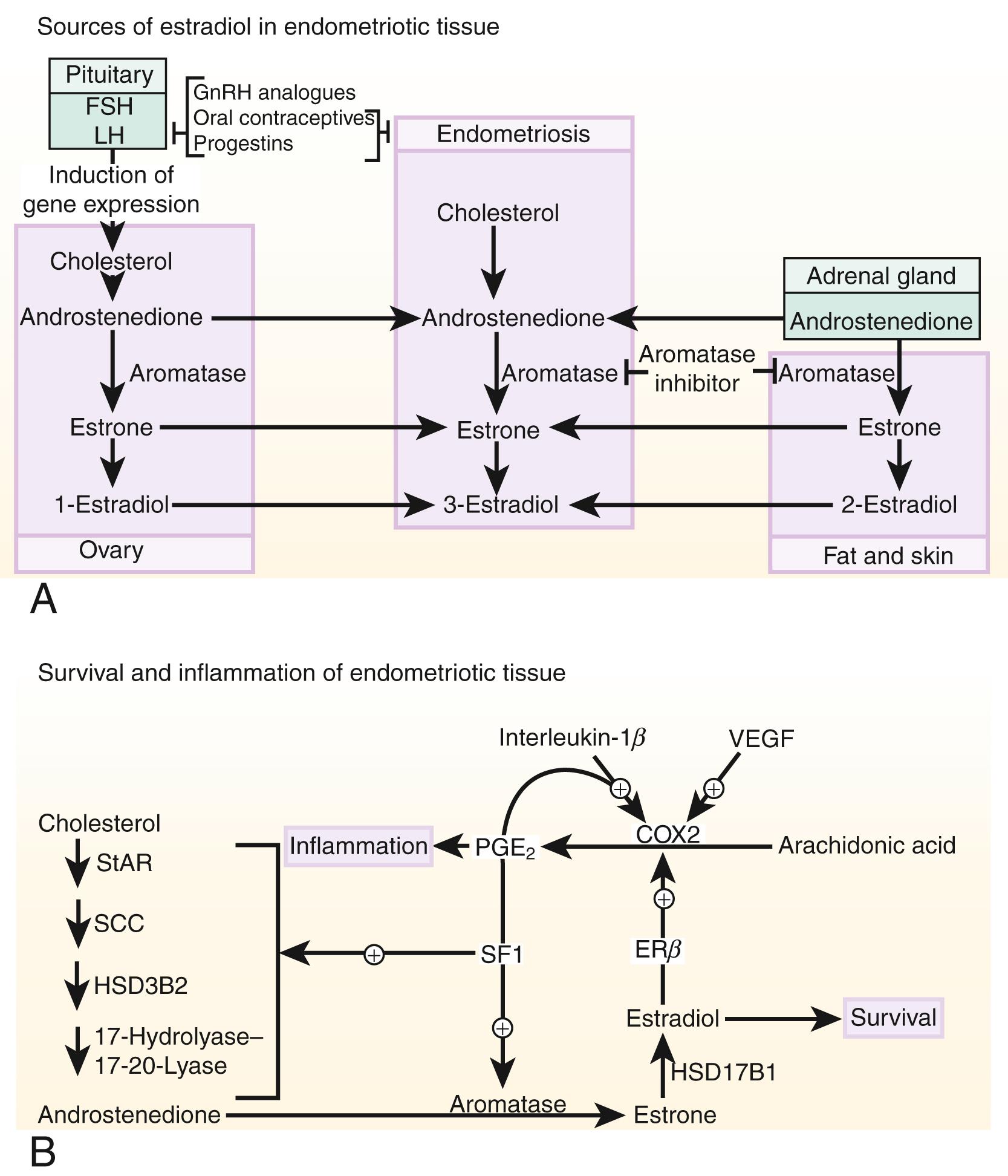
Origins of estrogen in endometriosis . Three major body sites produce estrogen in women with endometriosis. First, estradiol secreted by the ovary reaches endometriotic tissue via circulation. Moreover, follicular rupture at each ovulation causes spillage of extraordinarily large amounts of estradiol directly onto pelvic implants. Second, aromatase enzyme produced by peripheral adipose and skin tissue catalyzes the conversion of circulating androstenedione to estrone that may reach endometriotic tissue via the circulation and then is converted locally to estradiol. Finally, cholesterol can be converted to estradiol locally in endometriotic tissue, which expresses the complete set of steroidogenic genes, including aromatase. , Peripheral and/or local aromatase activity in endometriosis may be particularly important because a combination of a GnRHa plus an aromatase inhibitor is significantly superior to GnRHa only for providing long-lasting pain relief in women with endometriosis ( Fig. 27.18 B).
Connection between estrogen and inflammation. Cell survival and inflammation are responsible for chronic pelvic pain and infertility in endometriosis. Estrogen enhances the survival or persistence of endometriotic tissue, whereas prostaglandins and cytokines mediate pain, inflammation, and infertility. , A link between inflammation and estrogen production in endometriosis involves a positive feedback cycle that favors overexpression of key steroidogenic genes, most notably aromatase, overexpression of cyclooxygenase (COX) 2, and continuous local production of estradiol and PGE 2 in endometriotic tissue ( Fig. 27.18 B). ,
PGE 2 stimulates estradiol formation. PGE 2 coordinately stimulates the expression of all necessary steroidogenic genes, enabling the endometrial stromal cell to synthesize estradiol from cholesterol de novo . Among the six steroidogenic genes, the regulation of StAR and aromatase has been characterized extensively ( Figs. 27.14 and 27.18B ).
Both endometriotic and endometrial stromal cells express similar levels of the PGE 2 receptor subtypes EP 1 , EP 2 , EP 3 , and EP 4 . Activation of the EP 2 receptor raises intracellular cAMP, which is responsible for mediating induction of StAR and aromatase expression by PGE 2 in endometriotic stromal cells. , , In PGE 2 -treated endometriotic cells, SF1 binds coordinately and assembles an enhancer transcriptional complex at the promoters of the StAR and aromatase genes to induce their expression ( Figs. 27.14 and 27.18 B).
The redundant presence of a number of transcriptional inhibitors of StAR and aromatase promoters in endometrial cells provides a fail-safe system for silencing these steroidogenic genes. These repressors are chicken ovalbumin upstream transcription factor (COUP-TF), Wilms’ tumor-1 (WT1), and CAAT/enhancer binding protein-β (C/EBPβ). Levels of WT1 and C/EBPβ are much higher in normal endometrium than in endometriosis. In the absence of SF1, a transcriptional complex comprised of these repressors occupies the steroidogenic promoters and suppresses them in endometrial cells ( Fig. 27.14 ).
In summary, upon PGE 2 induction, coordinated recruitment of SF1 to the promoters of the essential steroidogenic genes is the key event driving local estradiol synthesis in endometriotic stromal cells ( Figs. 27.14 and 27.18 B). Because aromatase inhibition blocks all estradiol biosynthesis, it is a suitable therapeutic target among the steroidogenic enzymes. Aromatase inhibitors diminish or eradicate endometriotic implants and associated pain refractory to currently available treatments.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here