Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Pregnancy is a diabetogenic state. Control of maternal glucose metabolism is shared by the mother and the fetoplacental unit. Changes such as insulin resistance and reduced peripheral glucose uptake provide a continuous supply of glucose for the developing fetus.
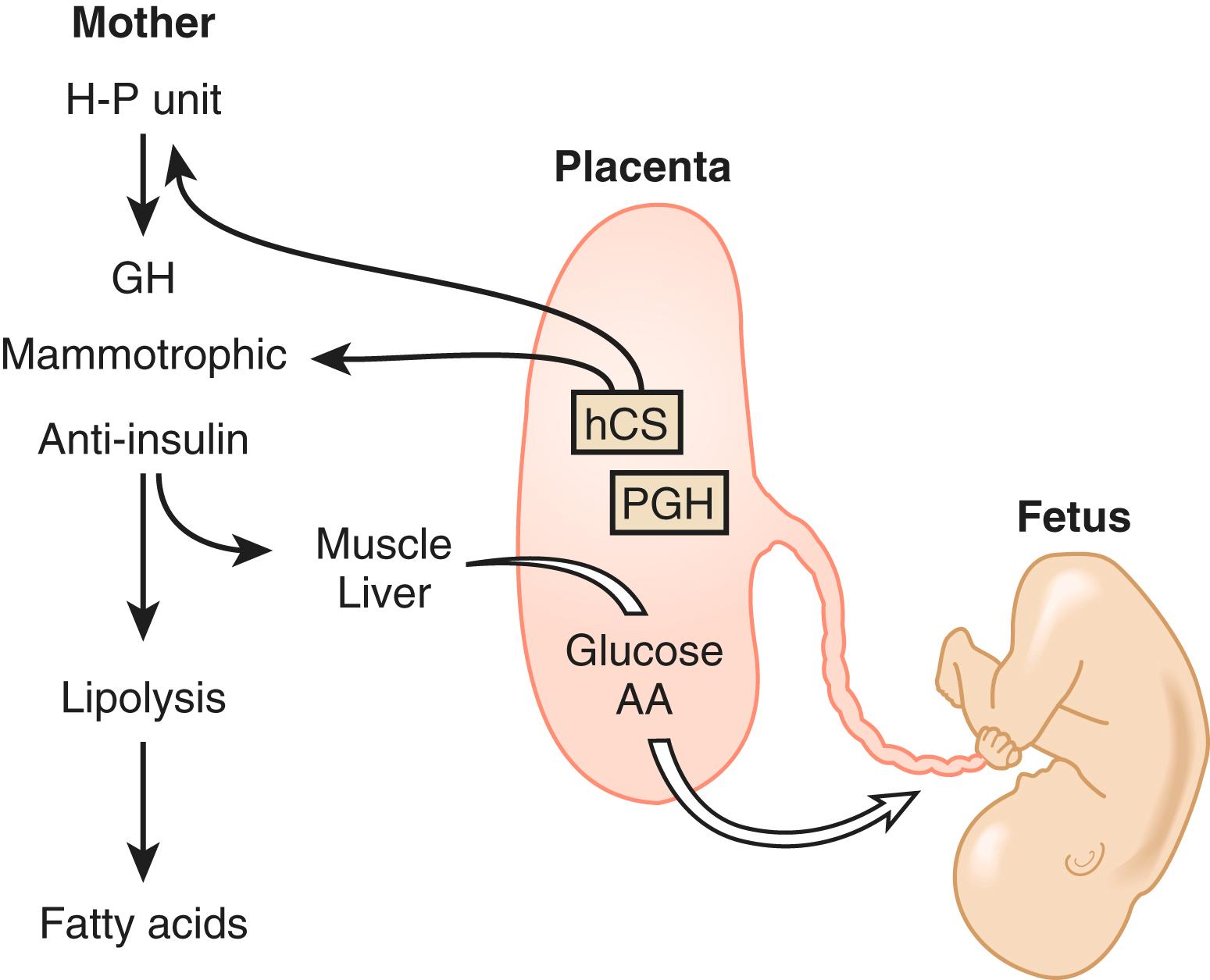
The fetoplacental unit is responsible for pregnancy-induced insulin resistance, primarily through the production of antiinsulin hormones including growth hormone, human chorionic somatomammotropins, cortisol, and progesterone.
Normal pregnancy can be regarded as a diabetogenic (prodiabetic) state with evidence of insulin resistance, maternal hyperinsulinism, and reduced peripheral uptake of glucose. These endocrine alterations, which result primarily from the production of antiinsulin hormones from the placenta (see Chapter 12 ), are designed to ensure a continuous supply of glucose for the developing and growing fetus. Therefore, in pregnancy, the control of maternal glucose metabolism is shared by the mother and the fetoplacental unit. The endocrine and molecular mechanisms by which the fetoplacental unit is able to reset the carbohydrate homeostatic equilibrium in the mother are not clear, but likely involve the action of several placental hormones. These hormones include growth hormone (GH), human chorionic somatomammotropins (hCS, placental lactogens), corticotropin-releasing hormone (CRH), cortisol, and progesterone.
Major functional changes occur in insulin production and action during pregnancy. β-cells in the islets of Langerhans within the pancreas—the cells responsible for insulin production—undergo hyperplasia, leading to insulin hypersecretion and a 200% to 250% increase in circulating insulin levels throughout pregnancy. It is this mechanism, along with the hemodilution of pregnancy and the initial enhanced responsiveness of cells to circulating levels of insulin, that is likely responsible for the fasting hypoglycemia seen in early pregnancy. The increased insulin sensitivity of early gestation promotes the uptake of glucose into adipose tissue to increase stores for later in gestation. However, as pregnancy progresses, peripheral resistance to insulin increases. In an attempt to overcome this resistance, the pancreas further increases insulin secretion. This compensatory response serves to return circulating maternal glucose levels to the normal range but results also in chronically elevated insulin levels (both in the fasting and fed state), in the postprandial hyperglycemia that characterizes normal pregnancy, and in islet cell hyperplasia.
Beta cell hyperplasia and expansion are controlled, in part, by prolactin and hCS, which both cause an increase in the number of pancreatic β-cells in pregnancy. Data from mice have provided some insights into the mechanisms regulating β-cell hyperplasia. Kim and colleagues found that serotonin acts downstream of lactogenic hormone signaling via the prolactin receptor to stimulate β-cell proliferation. Karnik and colleagues reported that repression of menin—the protein product of the MEN1 gene—is necessary for normal B-cell proliferation, and that prolactin represses menin levels in pancreatic islets.
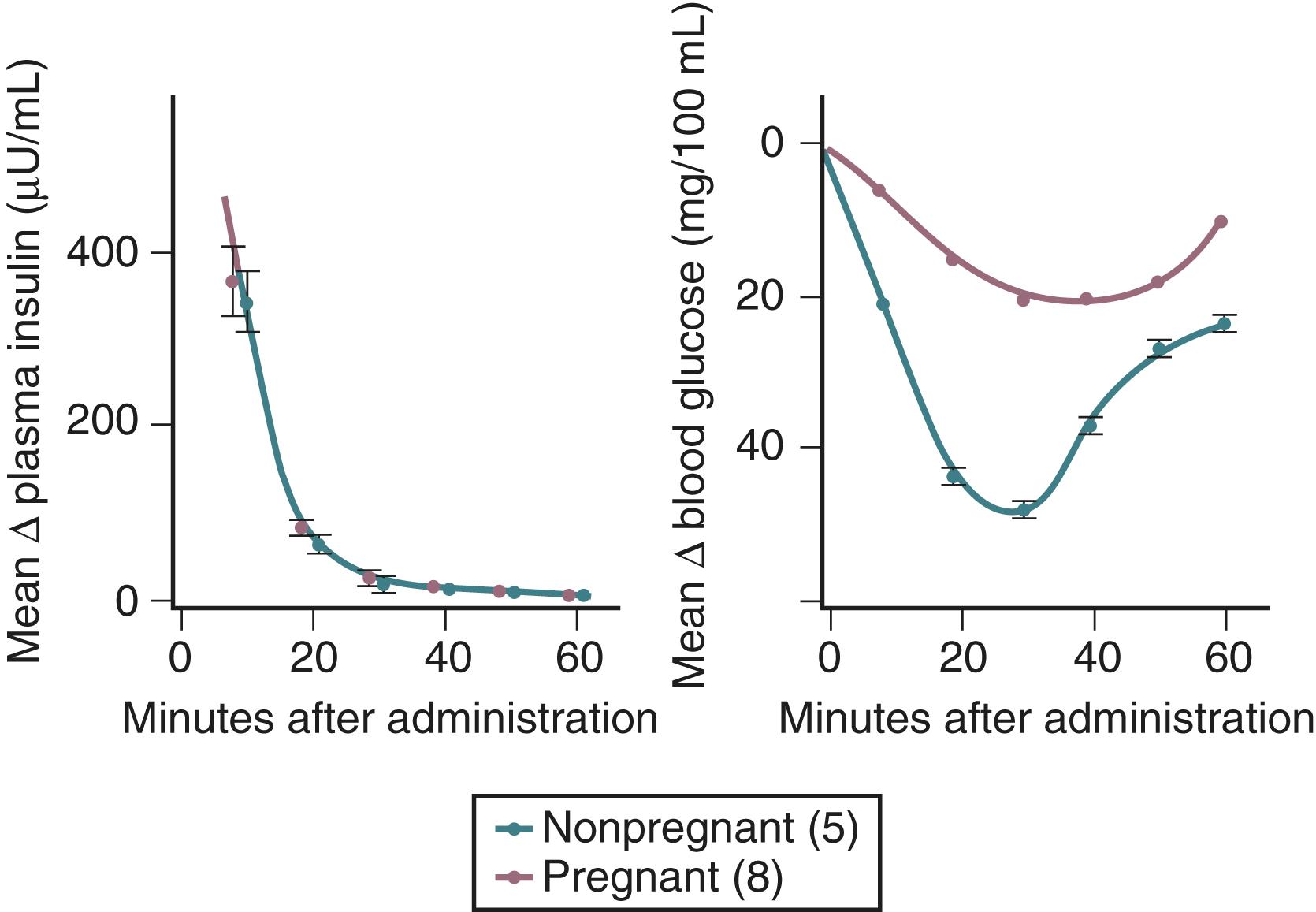
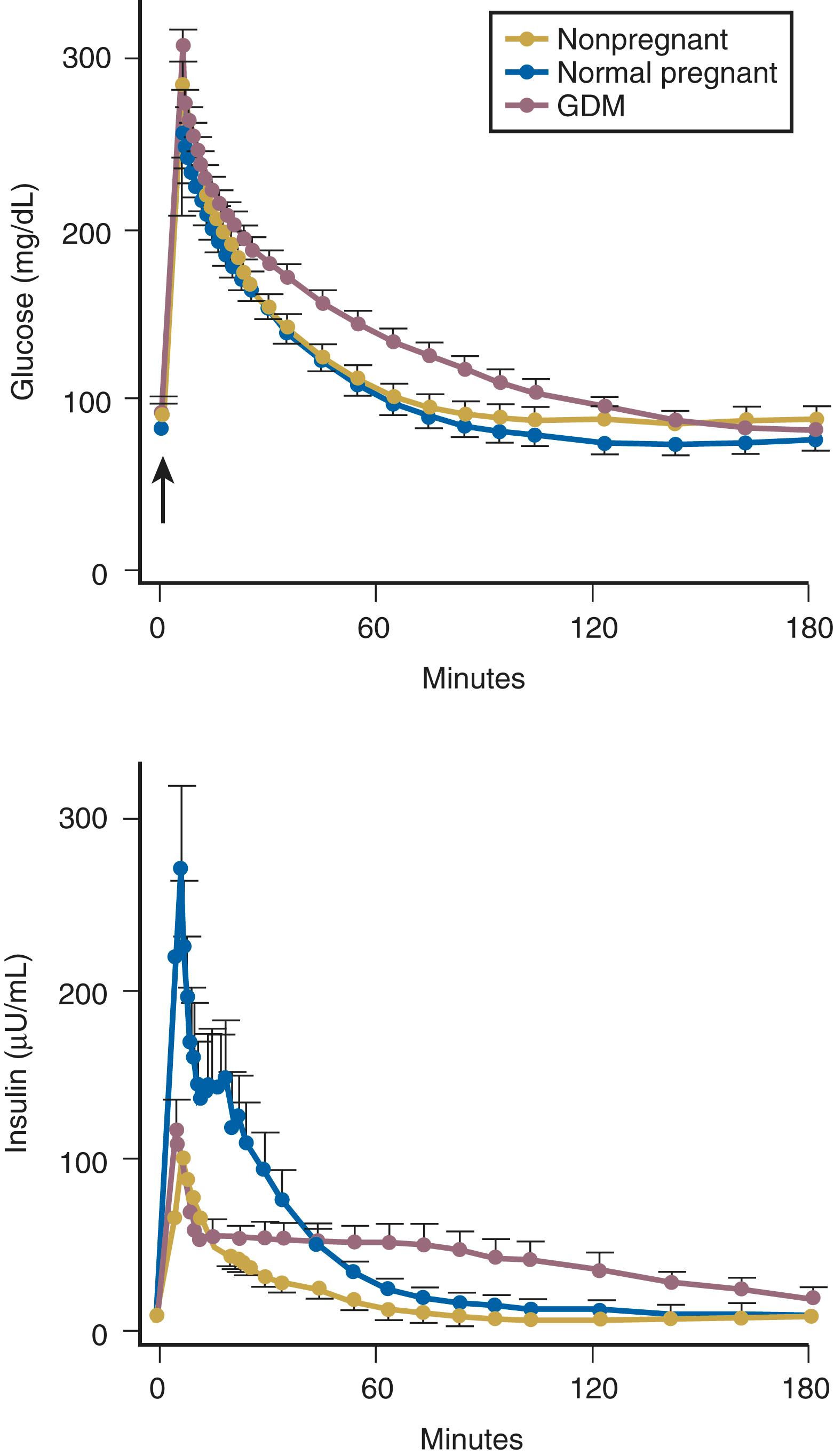
Insulin resistance refers to a decrease in the ability of a fixed concentration of circulating insulin to stimulate peripheral glucose uptake in adipocytes and muscle cells. The condition can be demonstrated either by the insulin tolerance test or by glucose loading tests. An insulin tolerance test involves the injection of a standard dose of insulin followed by serial blood glucose measurements. The clearance of insulin from the circulation is not altered by pregnancy ( Fig. 29.1 ). The half-life of insulin is approximately seven minutes both before and during pregnancy. However, in pregnant subjects, the administration of insulin results in a smaller decline in circulating glucose than that seen in nonpregnant subjects (see Fig. 29.1 ). Furthermore, in pregnancy, intravenous ( Fig. 29.2 ) or oral ( Fig. 29.3 ) administration of glucose causes significant hyperinsulinemia as compared with the nonpregnant state, resulting in relative hyperinsulinemia after meals. Taken together, these data provide evidence in support of pregnancy being an insulin-resistant state.
The molecular mechanisms responsible for insulin resistance in pregnancy are not well understood, but several factors are likely involved. Although insulin receptor kinase activity does not appear to be affected by pregnancy, the numbers of high-affinity insulin receptors on the surface of adipocytes are threefold lower in pregnancy than in nonpregnant women. , The glucose transport system also appears to be perturbed in pregnancy, with a threefold reduction in insulin-stimulated glucose transport as compared with nonpregnant controls.
The movement of glucose into adipocytes and skeletal muscle cells is mediated by the glucose transport proteins, GLUT-1 and GLUT-4. GLUT-1 is responsible for basal glucose transport and is not responsive to insulin. Insulin increases glucose uptake in cells by stimulating the translocation of GLUT-4 from intracellular sites to the cell surface. , Up to 75% of insulin-dependent glucose disposal occurs in skeletal muscle, whereas adipose tissue accounts for only a small fraction. In some pregnancies complicated by gestational diabetes, GLUT-4 is markedly reduced and fails to translocate to the cell surface with insulin stimulation, leading to a reduction in glucose transport in both the basal and insulin-stimulated states. Taken together, these data suggest that the peripheral insulin resistance that characterizes pregnancy likely results from several integrated mechanisms, including a decrease in insulin receptor number, a “postreceptor” defect in insulin action, and alterations in glucose transport systems. The postreceptor mechanisms that contribute to insulin resistance in late pregnancy occur in skeletal muscle at the β-subunit of the insulin receptor, at the level of insulin receptor substrate-1 (IRS-1), and in the cytoplasm, where increased free intracytoplasmic p85α regulatory subunit of phosphatidylinositol 3-kinase leading to decreased ability of insulin to stimulate the association of catalytic proteins with IRS-1. , Together, these alterations in insulin signaling likely result in less glucose uptake in skeletal muscle.
Impaired suppression of nonesterified fatty acids (NEFAs) in pregnant women suggests another mechanism for insulin resistance in pregnancy. NEFAs have been demonstrated to impair insulin-stimulated hepatic glucose uptake and whole body glucose disposal. Impaired NEFA suppression to endogenous insulin has been observed in a pregnant population subjected to a hyperinsulinemic clamp. Thus, inappropriately elevated NEFA levels may play a role in the insulin resistance observed in pregnancy. Dysregulation of NEFA has been implicated in both insulin resistance in normal pregnancy and decreased insulin secretion in gestational diabetes and has been posited as a pathophysiological link between gestational diabetes and the subsequent development of type 2 diabetes (DM2).
Although the molecular mechanisms have yet to be fully elucidated, the fetoplacental unit is clearly responsible for the pregnancy-induced insulin resistance, exerting its effect largely through the production of counterregulatory (antiinsulin) hormones. Insulin promotes the uptake of glucose by adipocytes and muscle cells. Counterregulatory hormones inhibit insulin-mediated glucose uptake by adipocytes and muscle cells, acting largely at a postreceptor level. Such hormones include, among others, GH, hCS, cortisol, and progesterone.
Placental GH differs from pituitary GH by 13 amino acid (191 nucleotide) substitutions and circulates in at least three isoforms: 22-, 24-, and 26-kDa. Human chorionic somatomammotropins are single-chain proteins produced largely by the syncytiotrophoblast with a high degree of sequence homology to both GH and prolactin. In primates, hCS genes appear to have evolved from a precursor GH gene; in nonprimate species, on the other hand, the placental lactogens appear to have evolved from a precursor prolactin gene. Because of these differences in evolution, we have chosen to use the term “human chorionic somatomammotropins” to refer to these genes collectively. The dominant isoform of hCS is 191 amino acids in length, with a molecular mass of 23 kDa , hCS binds with high affinity to prolactin (PRL) receptors but with low affinity to GH receptors, suggesting that it functions largely as a lactogen rather than as a somatogen in pregnancy. Conversely, placental GH binds with high affinity to GH receptors but with low affinity to PRL receptors. Thus, by midgestation, the endocrinological milieu is one of high levels of two lactogenic hormones (PRL and hCS) and placental GH, which acts almost exclusively as a somatogen. hCS is also secreted directly into the fetal circulation but is present at much lower levels than fetal PRL. Placental GH, however, is detectable only in maternal blood. ,
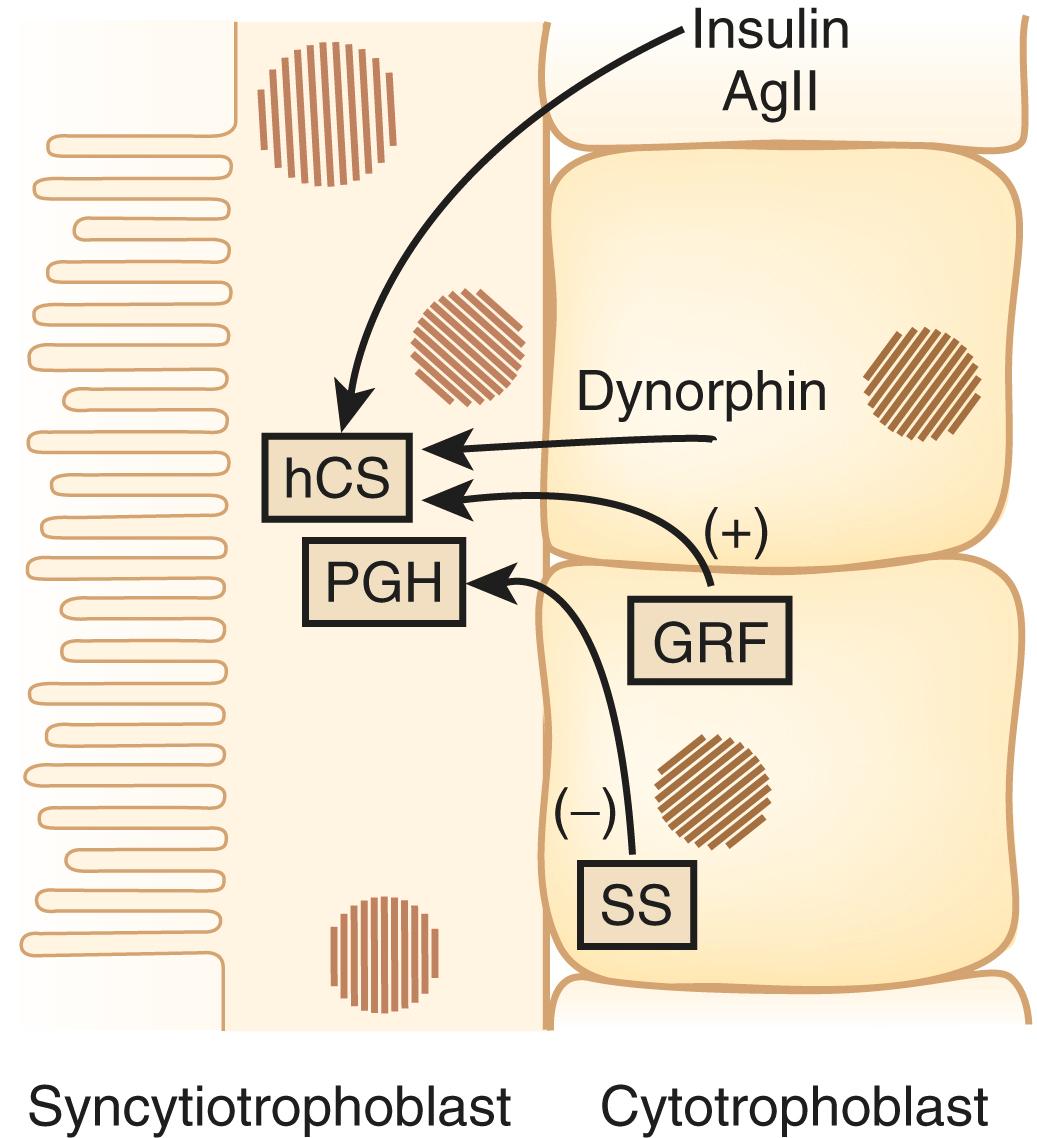
The factors that regulate GH and hCS synthesis and secretion are not fully understood, but somatostatin and GH-releasing hormone produced by the cytotrophoblast may play inhibitory and stimulatory roles, respectively. Additional regulation of hCS may be provided by insulin and angiotensin II, which both stimulate hCS release, , as well as by dynorphin ( Fig. 29.4 ). This placental endocrine and paracrine-autocrine regulatory system is similar to that observed in the hypothalamic-pituitary axis, which has led Dr. Samuel Yen to refer to the placenta as “the third brain.”
The genes coding for GH and hCS are clustered together in a single region of chromosome 17 in the following order (5′ to 3′): hGH-N (pituitary GH gene), hCS L, hCS-A, hGH-V (placental GH gene), and hCS-B. The pattern of expression of these genes is tissue-specific and changes throughout gestation. For example, the placenta does not express the hGH-N (pituitary GH) gene. Pituitary GH is secreted in a pulsatile fashion from the maternal anterior pituitary and can be measured in the maternal serum throughout the first trimester of pregnancy. Thereafter, however, pituitary GH secretion progressively declines. By the third trimester of pregnancy, pituitary GH secretion is effectively suppressed and cannot be rescued by induction of a hypoglycemic stimulus or by amino acid infusions (which will be discussed later in this chapter). , In contrast, circulating concentrations of placental GH—encoded by the hGH-V (placental GH) gene and expressed exclusively in the placenta—increase progressively throughout the second and third trimesters of pregnancy. , Similar differences are seen in the expression of the hCS genes. For example, at 8 weeks’ gestation, the hCS-A and hCS-B genes are expressed equally in the placenta; however, at term, expression of hCS-A is five times greater than that of hCS-B. Results of radioreceptor assay studies suggest that the relative contributions to circulating GH-like activity in pregnancy at term are 85% from hGH-V (placental GH), 12% from hCS, and less than 3% from hGH-N (pituitary GH). Multiple genes, multiple mRNA species (as in the hCS-L and hGH-V genes, which generate two distinct mRNA transcripts on the basis of alternative splice-acceptor sites for each gene, and heterogeneity in posttranslational processing result in many isoforms of these key placental hormones. The potential teleological advantages of having multiple placental GH-like genes are to ensure that the placenta can generate sufficient quantities of GH-like hormone to regulate maternal and fetal metabolism and to minimize the risk of pregnancy failure due to a functional “knockout” of any single gene.
During pregnancy, there is a major transition in the locus of control of the GH axis from the maternal hypothalamic-pituitary unit to the placenta. Circulating levels of placental GH and hCS increase throughout pregnancy. These proteins act through cell surface receptors (GH and prolactin receptors belong to a superfamily of cytokine receptors that share a high degree of sequence homology to stimulate the production of insulin-like growth factor-1 (IGF-1). Circulating concentrations of IGF-1 in the maternal serum increase throughout pregnancy, reaching a peak near term. , This increase in IGF-1 has also been observed in a pregnant dwarf with complete pituitary GH deficiency, suggesting that placental hormones may mediate this effect. Concentrations of IGF-binding protein-1 (IGFBP-1) rise in the first trimester, reach a peak at approximately 12 to 14 weeks of gestation, and thereafter remain stable for the remainder of pregnancy. The level of unbound (bioavailable) IGF-1 therefore increases as pregnancy progresses and likely contributes to the suppression of hGH-N (pituitary GH) gene expression in the latter half of gestation.
In circulation, GH can exist in a free form or bound to a GH-binding protein (GHBP). Approximately 30% of circulating GH is bound to GHBP and therefore not biologically active. Veldhuis and colleagues proposed that GHBP may serve as a buffer to prevent the level of free (bioavailable) GH from falling too low between secretory pulses. GHBP is the ectodomain of a larger cellular GH receptor and is released into the circulation after proteolytic cleavage of the parent molecule. It is likely, therefore, that circulating concentrations of GHBP parallel that of the cellular GH receptor in important target organs like the liver. This relationship allows for a balance between GH action (mediated through the GH receptor) and inactivation (by binding to the GHBP). The greater the levels of circulating GHBP, the greater the concentration of cellular GH receptors and the greater the sensitivity of cells to the actions of GH. GHBP concentrations tend to decline as gestation advances, although the reason for this change and its physiological significance remains unclear.
This system is not a prerequisite for pregnancy success, because a normal pregnancy is possible in women with Laron dwarfism in which both the GH receptor and GHBP are absent. However, aberrations in this system may be associated with pregnancy-related complications. For example, GHBP levels have been shown to be significantly higher in women with gestational diabetes compared with nondiabetic pregnant women. This observation suggests that the concentration of GH receptors is also increased in women with gestational diabetes, leading to a degree of sensitization to the effects of GH and hCS. Increased sensitivity to the effects of circulating GH could explain many of the endocrine changes observed in gestational diabetes, including insulin resistance, higher serum glucose levels, and an increased incidence of fetal macrosomia.
Differential levels of placental GH and hCS have been noted in normal and pathological pregnancies. Low maternal levels of placental GH and hCS have been noted in pregnancies complicated by hypertension, preeclampsia, and intrauterine growth restriction. , Conversely, high levels of hCS have been noted in the blood of pregnant mothers with gestational diabetes. , Placental gene expression profiling has demonstrated different mRNA expression profiles of placental GH and hCS in pregnancies affected by preeclampsia and GDM, compared to normal pregnancies. The same investigators also demonstrated downregulation of the entire GH/hCS cluster in the placenta in pregnancies with small-for-gestational-age newborns, with significantly increased expression of hCS mRNA placental transcripts in pregnancies resulting in large-for-gestational-age newborns. Uteroplacental insufficiency, as seen in preeclampsia and other maternal hypertensive disorders, may lead directly to decreased placental GH expression, resulting in decreased maternal lipolysis and decreased maternal IGF-I.
Circulating levels of placental GH (hGH-V), IGF-1, and the IGFBPs appear to correlate with birth weight. For example, a decrease in both placental GH and IGF-1 levels has been associated with fetal growth restriction (FGR). The decrease in placental GH levels is due both to a decrease in placental mass and a decrease in the density of placental GH-secreting cells. There is also a strong inverse correlation between maternal IGFBP-1 concentrations and birth weight in both term and preterm pregnancies. , The higher the IGFBP-1 concentration, the lower the circulating level of unbound (bioavailable) IGF-1 and the lower the birth weight. Moreover, several investigators have reported a positive correlation between birth weight and circulating concentrations of IGF-1 in the fetus and neonate. , Reece and colleagues reported that IGF-1 concentrations were significantly lower in neonates below the mean birth weight for gestational age than levels in neonates above the mean (mean ± SEM: 40 ± 11 versus 86 ± 6 ng/mL, respectively), with no differences in IGF-2 concentrations. Similar findings were reported by Lassarre and colleagues. Conversely, maternal hyperglycemia may increase placental and fetal weight via induction of IGF-2 and fetal hyperinsulinemia. Increased maternal fat stores may reduce plasma adiponectin and thus increase hCS expression via decreased adiponectin suppression. Increased hCS in the fetal circulation may promote fetal hyperinsulinemia via induction of β-cell replication, increasing fetal weight gain. , , Taken together, these data suggest that GH, hCS, IGF-1, as well as their binding proteins (GHBP and the IGFBPs), may play an important role in governing fetal growth and pregnancy outcome and that these endocrine factors may be regulated by both the mother and the fetoplacental unit.
Cortisol is a potent diabetogenic hormone. It promotes lipolysis in adipocytes and protein breakdown in muscle, leading to an increase in circulating free fatty acids and amino acids. Levels of adrenocorticotropic hormone (ACTH) and cortisol increase in pregnancy. The increase in ACTH is due, at least in part, to an increase in CRH production by the placenta (discussed later in this chapter). Much of the increase in total cortisol concentration in pregnancy is due to the excessive production of corticosteroid-binding globulin by the liver under the influence of estrogen. However, there is also a significant increase in urinary free cortisol excretion during pregnancy, suggesting that circulating levels of free cortisol may also be increased. The relative contribution of an increase in circulating free cortisol to the insulin resistance of pregnancy is unclear.
High concentrations of progesterone have been shown to cause insulin resistance in cells in culture and in laboratory animals by decreasing insulin receptor number and causing a postreceptor defect in insulin action, which has yet to be fully elucidated. High circulating concentrations of progesterone may contribute to pregnancy-related insulin resistance.
In nonpregnant women, there is a constant need to maintain circulating glucose concentrations for use by the brain. During an overnight fast, glucose is released from the liver by both glycogenolysis (breakdown of glycogen stores [75%]) and gluconeogenesis (production of glucose from circulating metabolic precursors [25%]). The precursors for gluconeogenesis include pyruvate, alanine (from muscle), glycerol (from the breakdown of triglycerides in adipose tissue), and lactate (from anaerobic metabolism).
Pregnancy is associated with an increased demand for glucose and alanine, both of which are required by the developing fetus. As such, the fasting state in pregnancy is characterized by a rapid and often severe decrease in maternal serum glucose and alanine concentrations. Associated with these changes is an increase in the circulating levels of free fatty acids (derived from triglyceride breakdown in adipose cells) and ketone bodies ( Fig. 29.3 ; Table 29.1 ). , The hyperketonemia that characterizes late pregnancy is the result of enhanced lipolysis, which is likely due, in turn, to the insulin resistance in adipocytes caused primarily by the placental counterregulatory hormones.
| Measurement (Mean ± SEM) | ||
|---|---|---|
| Nonpregnant State | Late Pregnancy | |
| Glucose (mg/dL) | 79 ± 2.4 | 68 ± 1.5 ∗ |
| Insulin (μ/mL) | 9.8 ± 1.1 | 16.2 ± 2.0 ∗ |
| Glucagon (pg/mL) | 126 ± 6.1 | 130 ± 5.2 |
| Amino acids (μM) | 3.82 ± 0.13 | 3.18 ± 0.11 ∗ |
| Alanine (μM) | 286 ± 15 | 225 ± 9 ∗ |
| Free fatty acids (mg/dL) | 76 ± 7 | 181 ± 10 ∗ |
| Cholesterol (mg/dL) | 163 ± 8.7 | 205 ± 5.7 ∗ |
In pregnancy, the acceleration of lipid catabolism during fasting helps the mother to rely on fat as a major energy source, thereby minimizing protein catabolism (preserving muscle mass) and allowing both glucose and amino acids to be used preferentially by the fetus. These metabolic adaptations have been termed “accelerated starvation” by Freinkel. Although a useful descriptive term, this characterization of pregnancy is largely inaccurate because fat mass is known to increase significantly during pregnancy.
The fetus is a thief! Many of the metabolic and endocrine adaptations associated with pregnancy are designed to maintain a preferential and uninterrupted supply of metabolic fuel from mother to fetus as dictated by the progressively increasing demands of the growing fetus. The placenta is relatively impermeable to fat but readily transports glucose, amino acids, and ketone bodies from the maternal to the fetal circulation.
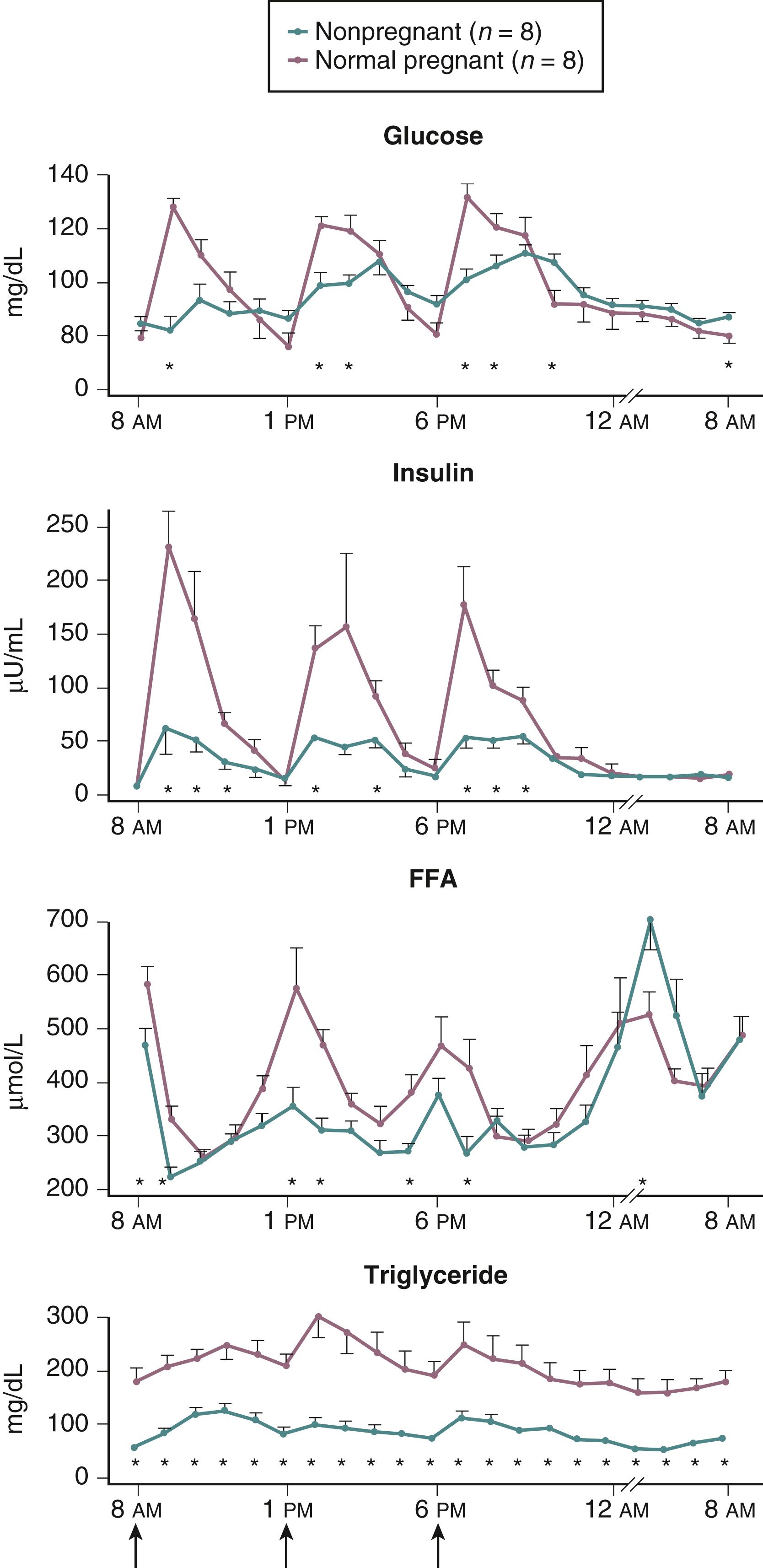
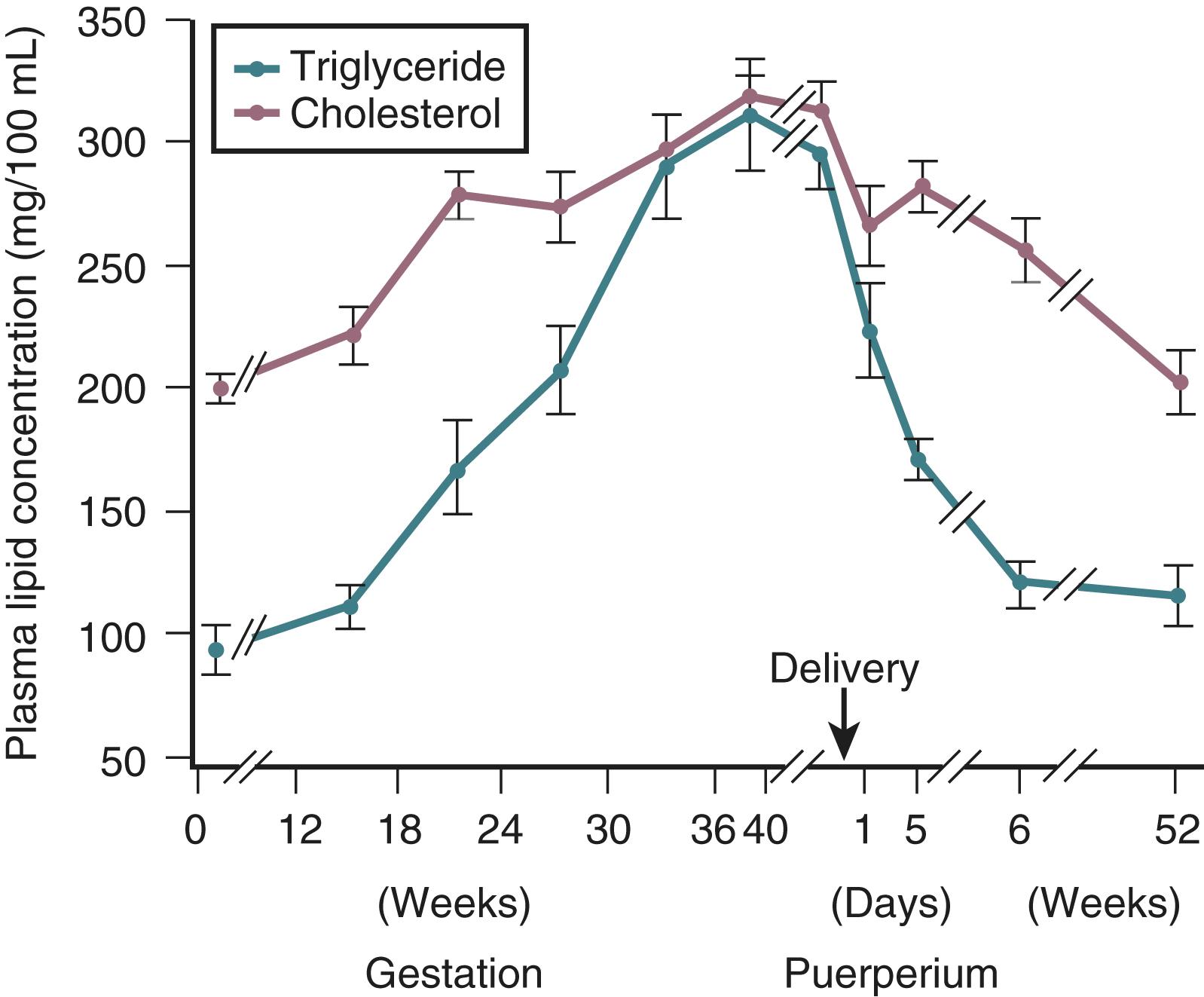
Pregnancy is associated with hyperlipidemia, both in the fasting and the fed states. Total plasma lipid concentrations increase progressively after 24 weeks of gestation. Increases in triglycerides, cholesterol, and free fatty acids are significant ( Figs. 29.5 and 29.6 ; Table 29.1 ). , , , High-density lipoprotein cholesterol levels rise during early pregnancy, and low-density lipoprotein cholesterol concentrations increase in later pregnancy. ,
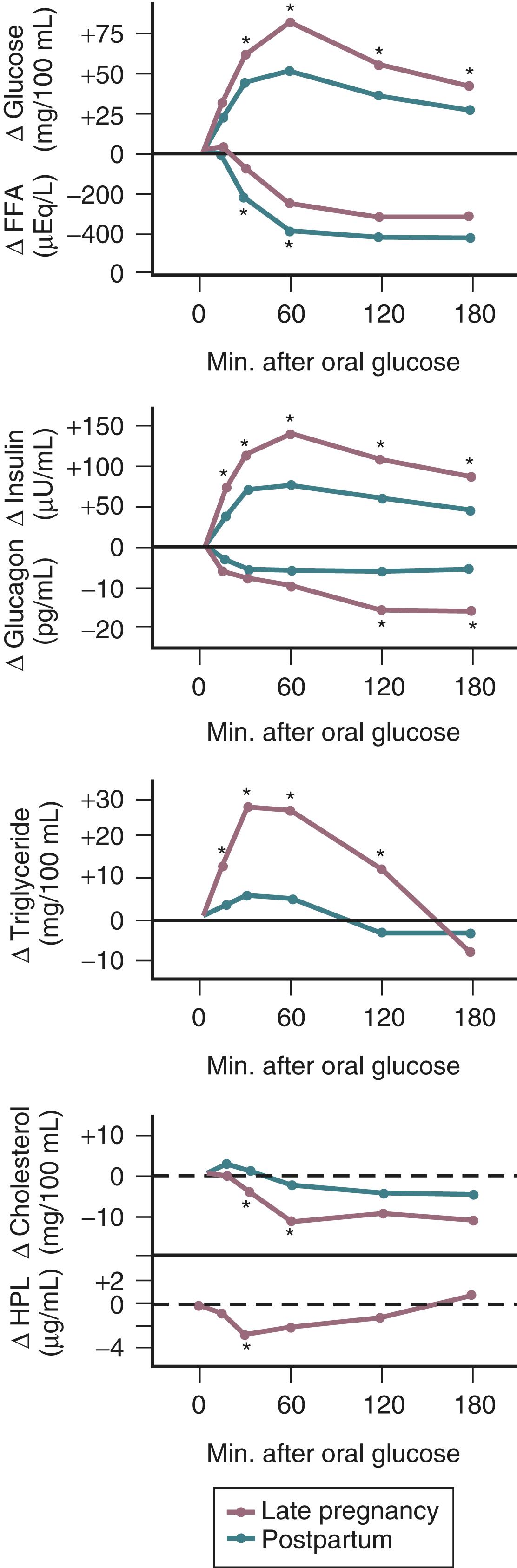
In pregnancy, an oral glucose load is associated with a greater increase in circulating glucose concentration, a smaller decline in free fatty acids, and a larger increase in serum triglycerides than that seen in the nonpregnant state ( see Fig. 29.5 ). , A similar effect has been observed in pregnancy after meals ( Fig. 29.3 ). These adaptations allow the mother to use primarily available triglycerides, glycerol, and free fatty acids for metabolic fuel after meals and to preserve glucose and amino acids for preferential use by the fetus ( Fig. 29.7 ). The cause of these metabolic changes is likely the lipolytic action of the placental counterregulatory hormones (GH, hCS, cortisol, and progesterone), which serve to promote lipolysis during fasting and hypertriglyceridemia in the fed state.
To a point, pancreatic β-cells will respond to insulin resistance with increased insulin secretion. Bergman and colleagues first characterized a predictable hyperbolic relationship between the quantity of insulin produced by β-cells and tissue sensitivity to insulin. The disposition index, or the “hyperbolic correction,” is a measure of insulin secretion corrected for insulin resistance. A left-shifted curve, representing decreased compensatory insulin secretion for a given level of insulin resistance, may be seen in women who develop both gestational diabetes (GDM) and type 2 diabetes mellitus (DM2). Buchanan posits that insulin resistance actually causes the β-cell dysfunction observed in GDM and that chronic insulin resistance leading to β-cell failure may be the mechanism by which women with GDM progress to DM2.
Gestational diabetes mellitus may be difficult to distinguish from prepregnancy diabetes. There is no consensus on whether women with a positive diabetes screen in the first trimester of pregnancy should have a unique designation.
There is also a lack of consensus on how and when to screen for gestational diabetes. Some advocate a 1-step screening process (75 gram, 2-hour oral glucose tolerance test (OGTT)), while others advocate a 2-step screening (50-gram, 1-hour glucose challenge test, followed by a 100-gram, 3-hour OGTT for diagnosis if the initial test is positive).
Gestational diabetes is associated with increased maternal and fetal morbidity, including increased risk of hypertensive disorders of pregnancy, cesarean delivery, fetal macrosomia, stillbirth, and neonatal hypoglycemia.
The balance of evidence suggests that treating gestational diabetes to optimize glycemic control decreases the risk of preeclampsia, fetal macrosomia, and shoulder dystocia. Both oral agents (glyburide, metformin) and insulin are accepted therapies.
Depending on the patients screened and the diagnostic criteria used, GDM complicates between 6% and 20% of all pregnancies in the United States. , GDM is the most common form of diabetes in pregnancy and accounts for approximately 86% of pregnancies complicated by diabetes in the United States. The prevalence of GDM continues to increase over time, likely due to increases in mean maternal age and weight. The American Diabetes Association (ADA) classification of diabetes mellitus is summarized in Box 29.1 . , Gestational diabetes mellitus (GDM) has previously been defined as any degree of carbohydrate intolerance with the onset of pregnancy or first recognized during pregnancy. The American Congress of Obstetricians and Gynecologists (ACOG) still endorses this terminology. Due in part to the increasing prevalence of obesity among young women, as well as low rates of routine screening in nonpregnant women of reproductive age, an increasing proportion of women will have unrecognized type 2 diabetes at the time of screening for gestational diabetes. To address this increased potential for prepregnancy diabetes, in 2010 the International Association of Diabetes and Pregnancy Study Group (IADPSG) recommended changing the classification of diabetes diagnosed during pregnancy to overt or gestational. The American Diabetes Association (ADA) and the World Health Organization (WHO) endorsed this recommendation. , “Overt diabetes” (IADPSG), “diabetes complicating pregnancy” (ADA), or “diabetes mellitus in pregnancy” (WHO) would be diagnosed at the initial prenatal visit (as long as the visit occurs in the first trimester) in women who met any of the following criteria: ( 1) fasting plasma glucose ≥126 mg/dL (7.0 mmol/L) OR ( 2) 2-hour plasma glucose ≥200 mg/dL (11.1 mmol/L) during an OGTT OR 3) hemoglobin A1C ≥6.5% (48mmol/mol) OR ( 3) random plasma glucose ≥200 mg/dL (11.1 mmol/L) in a patient with classic symptoms of hyperglycemia. , Per the ADA, GDM would then be defined as “diabetes diagnosed in the second or third trimester of pregnancy that is not clearly either type 1 or type 2 diabetes.” ACOG does not address whether there should be a unique designation for women who have a positive diabetes screen in the first trimester of pregnancy.
Caused by β-cell dysfunction, usually leading to absolute insulin deficiency
Due to progressive loss of insulin secretion imposed on the background of insulin resistance
Diabetes diagnosed in the second or third trimester of pregnancy that is not clearly overt diabetes
Genetic defects in carbohydrate metabolism (i.e., maturity-onset diabetes of the young [MODY])
Diseases of the exocrine pancreas
Endocrinopathies
Drug- or chemical-induced diabetes
Infections
Uncommon forms of immune-mediated diabetes
Genetic syndromes sometimes associated with diabetes
Patients with GDM are typically asymptomatic. Many experts and organizations, including ACOG, the ADA, the WHO, and the U.S. Preventive Services Task Force (USPSTF), among others, have recommended screening all pregnant women for GDM. , , The World Health Organization has recommended utilizing a 75-gram, 2-hour oral glucose tolerance test (OGTT) for screening and diagnosis. The United Kingdom has also adopted the 2-hour OGTT for screening and diagnosis but does not recommend universal screening. , ACOG and the ADA recommend universal screening at 24–28 weeks’ gestation, with a selective, risk-based approach to screening in the first trimester. , In keeping with the 2013 Eunice Kennedy Shriver National Institute of Child Health and Human Development Consensus Development Conference on diagnosing gestational diabetes, ACOG recommends screening with a 50-gram 1-hour glucose challenge test (GCT), followed by a 100-gram 3-hour OGTT for diagnosis if GCT is positive. , , The most recent IADPSG study group recommends universal screening at 24–28 weeks’ gestation with the one-step 75-gram 2-hour OGTT and screening of high-risk women for pregestational diabetes with fasting or random plasma glucose or hemoglobin A1C at the first prenatal visit. The low-risk category that some recommend excluding from GDM screening includes women under age 25 who have normal body mass index (BMI), who have no first-degree relatives with the condition, and who are not members of ethnic or racial groups with a high prevalence of diabetes (Hispanic, Native American, Asian, or African American).
Screening traditionally has been performed at 24 to 28 weeks of gestation. , For women at risk for undiagnosed type 2 diabetes, early screening for diabetes mellitus in pregnancy (or GDM, depending on which governing body’s nomenclature is being followed) should be performed at the first prenatal visit. ACOG and the ADA define those at risk as women who are overweight or obese and/or have one or more of these risk factors: physical inactivity, first-degree relative with diabetes, prior history of an infant weight 4000 g or more, hypertension, hyperlipidemia, polycystic ovarian syndrome, prior testing indicating prediabetes, and history of cardiovascular disease. If the early screen is negative, the screen should be repeated at 24 to 28 weeks.
At this time, there is no universally accepted standard for screening and diagnosis of diabetes in pregnancy. Screening for diabetes in pregnancy has traditionally been performed as a two-step approach. The first step utilizes the glucose load test (GLT), also known as the glucose challenge test (GCT). First proposed as a screening test for GDM by O’Sullivan et al in 1973, the GLT is a nonfasting 50-g oral glucose challenge followed by a venous plasma glucose measurement at one hour. , The GLT is considered positive if the 1-hour glucose measurement is greater than a previously agreed threshold. 130 mg/dL, 135 mg/dL and 140 mg/dL have been suggested as threshold values. , , Use of a lower cutoff will increase the detection rate of women with GDM but will result in a substantial increase in the false-positive rate ( Table 29.2 ) . There is no absolute GLT cutoff that should be regarded as diagnostic of GDM. , ,
| Proportion of | Sensitivity for | |
|---|---|---|
| Women With a | Gestational | |
| Glucose Cutoff | Positive Test | Diabetes Mellitus |
| ≥140 mg/dL | ||
| (≥7.8 mmol/L) | 14%–18% | ~80% |
| ≥130 mg/dL | ||
| (≥7.2 mmol/L) | 20%–25% | ~90% |
∗ From Kjos SL, Buchanan TA. Gestational diabetes mellitus. N Engl J Med . 1999;341:1749.
The second step of the two-step approach requires a three-hour glucose tolerance test (GTT), which is only performed if the GLT is positive. A fasting glucose measurement of at least 105 mg/dL in the setting of a positive GLT is highly predictive of an abnormal GTT. In pregnancy, the GTT involves an overnight fast and subsequent 100-g oral glucose challenge. Venous plasma glucose is measured fasting at one hour, two hours, and three hours after the glucose load.
Although there is general agreement that two or more abnormal values are required to confirm the diagnosis, , there is little consensus about the glucose values that define the upper range of normal in pregnancy and no threshold that perfectly predicts an adverse pregnancy outcome. Even one elevated value has been associated with an increased incidence of macrosomia and birth injury. A secondary analysis of a treatment trial for mild gestational diabetes found that elevations in fasting glucose and 3-hour GTT levels were associated with adverse pregnancy and neonatal outcomes. The same study found that a fasting glucose of 90 mg/dL or greater and a 1-hour value of 165 mg/dL or greater were associated with an increased risk for adverse neonatal outcomes, while a 1-hour value of 150 mg/dL or greater was associated with an increased risk for large for gestational age neonates. Current diagnostic cutoffs for the 3-hour GTT are depicted in Table 29.3 .
| Plasma Glucose Values mg/dL (mmol/L) | |||
|---|---|---|---|
| National Diabetes | Carpenter and | ||
| Data Group ∗ | Sacks et al. # | Coustan § | |
| Fasting | 105 (5.8) | 96 (5.3) | 95 (5.2) |
| 1-hour | 190 (10.6) | 172 (9.4) | 180 (9.9) |
| 2-hour | 165 (9.2) | 152 (8.3) | 155 (8.6) |
| 3-hour | 145 (8.1) | 131 (7.2) | 140 (7.7) |
∗ National Diabetes Data Group. Classification and diagnosis of diabetes and other categories of glucose intolerance. Diabetes . 1979;28:1039.
# Sacks DA, Abu-Fadil S, Greenspoon JS, Fotheringham N. Do the current standards for glucose tolerance testing in pregnancy represent a valid conversion of O’Sullivan’s original criteria? Am J Obstet Gynecol . 1989;161:638.
§ Carpenter MW, Coustan DR. Criteria for screening tests for gestational diabetes. Am J Obstet Gynecol . 1982;144:768.
In 2010, the one-step approach to diagnosing diabetes in pregnancy was proposed by IADPSG. This approach was subsequently endorsed by the ADA, the WHO, and the National Institute for Health and Care Excellence (NICE) in the United Kingdom, but not by ACOG , , The IADPSG approach advocates diagnosing gestational diabetes in women who meet either of the following criteria: (1) fasting plasma glucose ≥92 mg/dL (5.1 mmol/L), but <126 mg/dL (7.0 mmol/L) at any gestational age, or (2) at least one abnormal result on a 75-gram 2-hour oral glucose tolerance test, which could be administered at 24-28 weeks of gestation. Abnormal values are defined as: (1) fasting plasma glucose ≥92 mg/dL (5.1 mmol/L), but <126 mg/dL (7.0 mmol/L) or (2) one-hour value ≥180 mg/dL (10.0 mmol/L) or (3) two-hour value ≥153 mg/dL (8.5 mmol/L) ( Box 29.2 ). The IADPSG-selected thresholds for the 2-hour OGTT are based on data from the Hyperglycemia and Adverse Pregnancy Outcomes (HAPO) study, a multinational, multicentre prospective observational study of more than 23,000 pregnancies that demonstrated a continuous association between maternal glycemic concentrations and adverse perinatal outcomes. As maternal fasting plasma glucose levels increased from 75 mg/dL, and as the 1- and 2-hour oral GTT values increased, the risk of large-for-gestational-age infants, elevated cord blood C-peptide, neonatal hypoglycemia, and cesarean delivery increased continuously. The IADPSG-selected thresholds thus represent the average glucose values in the HAPO study, in which there were 1.75 times the odds of infant birth weight, cord blood C-peptide (a proxy for fetal hyperinsulinemia), and percent neonatal body fat greater than the 90 th percentile. Women with one or more values above these thresholds had a twofold higher frequency of preeclampsia and large for gestational age infants, and more than a 45% increase in preterm delivery and primary cesarean delivery.
∗ Data and format adapted from Metzger, BE et al. for the International Association of Diabetes and Pregnancy Study Groups Consensus Panel. International association of diabetes and pregnancy study groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care. 2010;33(3):676–682. Guidelines are to be applied to women without known diabetes antedating pregnancy. Postpartum glucose testing should be performed for all women diagnosed with overt diabetes during pregnancy or GDM.
FIRST PRENATAL VISIT
Measure FPG, A1C, or random plasma glucose on all or only high-risk women †
† Decision to perform evaluation for glycemia on all pregnant women at first prenatal visit or only on women with characteristics indicating a high risk for diabetes is to be made on the basis of the background frequency of abnormal glucose metabolism in the population and on local circumstances.
‡ The IADPSG panel concluded there is insufficient evidence to know whether there is a benefit of generalized testing to diagnose and treat GDM before the usual 24–28 weeks’ gestation.
If results indicate overt diabetes (FPG ≥7.0 mmol/L (126 mg/dL), A1C ≥6.5%, random plasma glucose ≥11.1 mmol/L (200 mg/dL) with confirmation):
Treat and follow-up as for preexisting diabetes
If results not diagnostic of overt diabetes and FPG ≥5.1 mmol/L (92 mg/dL) but <7.0 mmol/L (126 mg/dL):
Diagnose as GDM
If results not diagnostic of overt diabetes and FPG <5.1 mmol/L (92 mg/dL):
Test for GDM from 24–28 weeks’ gestation with a 75-gram OGTT ‡
24–28 Weeks’ Gestation: Diagnosis of GDM
2-hour 75-gram OGTT: perform after overnight fast on all women not previously found to have overt diabetes or GDM during testing earlier in this pregnancy:
Diagnose overt diabetes if FPG ≥7.0 mmol/L (126 mg/dL)
Diagnose GDM if one or more values equals or exceeds the following thresholds:
FPG ≥5.1 mmol/L or 92 mg/dL
1-hour plasma glucose ≥10.0 mmol/L or 180 mg/dL
2-hour plasma glucose ≥8.5 mmol/L or 153 mg/dL
OGTT is normal if all values are less than above thresholds
Abbreviations used: FPG : fasting plasma glucose, GDM : gestational diabetes mellitus, OGTT : oral glucose tolerance test
The utilization of IADPSG diagnostic criteria for overt and gestational diabetes would result in 18% of women being diagnosed with diabetes in pregnancy. An analysis of regions that have implemented the one-step approach suggests that this approach increases the prevalence of GDM, with a 1.03 to 3.78-fold increase above the traditional two-step approach. The potential long-term economic impact of implementing the IADPSG guidelines is unknown at this time, but in the short-term, healthcare costs would likely be increased. Including the costs of long-term health intervention with diet and exercise, a cost-effectiveness analysis estimated that for every 100,000 pregnancies, the IADPSG approach would increase costs by over $125,600,000. This analysis concluded that the IADPSG screening recommendations are cost-effective only if postdelivery care reduces diabetes incidence. Based on the 2013 Eunice Kennedy Shriver National Institute of Child Health and Human Development Consensus Conference on Diagnosis of Gestational Diabetes and a subsequent 2015 Cochrane review, ACOG and the expert NIH panel determined that there are no clear data suggesting improvements to neonatal or maternal health outcomes with the one-step approach and that increased prevalence of GDM detected by this method contributes to increased healthcare costs and medicalization of the pregnant state. As a result, most organizations continue to support the two-step approach. ,
Identification of overt diabetes early in pregnancy is important due to the associated increased risk of congenital anomalies, and maternal complications such as nephropathy and retinopathy. In contrast, GDM poses little immediate risk to the mother. Women with GDM are not at risk of diabetic ketoacidosis (DKA), which is primarily a disease of absolute insulin deficiency, but are at increased risk for developing hypertensive disorders of pregnancy, perinatal depression, preterm delivery, birth trauma, and greater weight gain in pregnancy. , , GDM has been associated with a variety of perinatal and neonatal complications, including increased risk of macrosomia, operative delivery, shoulder dystocia, birth trauma, hypoglycemia, hyperbilirubinemia, hypocalcemia, and perinatal mortality. ,
Transplacental glucose transport is a facilitated process that is mediated by the glucose transporter isoform GLUT-1. , Some have hypothesized that maternal hyperglycemia in diabetes increases placental glucose transfer, resulting in fetal hyperglycemia and increased fetal insulin concentration that in turn stimulates fetal growth. However, even in diabetic pregnancies with evidence of strict glycemic control, fetal macrosomia (defined as an estimated fetal weight of at least 4500g ) is not uncommon, which suggests a complex relationship between metabolic derangement and fetal growth in diabetes. Other factors that may contribute to fetal macrosomia include obesity and high circulating levels of amino acids and lipids.
Many of the complications of GDM are due to fetal macrosomia. Increased birth weight is associated with an increased risk of cesarean delivery, operative vaginal delivery, and birth injury to both the mother (vaginal, perineal, and rectal trauma) and fetus (including orthopedic and neurologic injury). , , , , Shoulder dystocia with resultant brachial plexus injury is a serious consequence of fetal macrosomia, and the risk is further increased in the setting of GDM because the macrosomia of diabetes is associated with increased diameters in the upper thorax of the fetus. Other short-term complications of GDM for the neonate include hypoglycemia, hypocalcemia, hypomagnesemia, polycythemia, respiratory distress syndrome, hypertrophic cardiomyopathy, and abnormalities in iron metabolism.
Recent data also suggest that offspring of mothers with both gestational and prepregnancy diabetes may be predisposed to develop obesity, diabetes, and hypertension, via intrauterine programming/epigenetic modification. For example, a study of 9439 children aged 5 to 7 evaluated the prevalence of childhood obesity relative to maternal glycemia in pregnancy. This study found that maternal fasting glucose level of ≥95 mg/dL on a 3-hour 100-gram oral glucose tolerance test, gestational diabetes by Carpenter and Coustan criteria, and a top quartile 50-gram 1-hour glucose challenge test result were all associated with childhood obesity. Treatment of GDM reduced the rates of childhood obesity to rates similar to that of offspring born to women with normal glucose challenge tests, but this effect was only observed among offspring with a birth weight of 4000 grams or less. The authors concluded that maternal hyperglycemia resulted in metabolic imprinting for childhood obesity in offspring. In a study that performed transcriptomic profiling on the placentas of women with GDM, T1DM, and controls, GDM was associated with upregulation of genes involved in lipid and amino acid transport, suggesting that GDM may result in metabolic alterations in the placenta that could predispose infants to diabetes and metabolic syndrome later in life.
Interventions for GDM that definitively decrease the risk of macrosomia and subsequent adverse outcomes are limited. Two multicenter randomized clinical trials have demonstrated that the treatment of mild hyperglycemia in pregnancy reduces neonatal morbidity and macrosomia. , The Australian Carbohydrate Intolerance Study in Pregnant Women Trial (ACHOIS) demonstrated that treating pregnant women with impaired glucose tolerance on a 75-gram OGTT resulted in a significant reduction in a composite perinatal outcome including perinatal death, shoulder dystocia, orthopedic injury, and nerve palsy. In the ACHOIS trial, glycemic control was achieved through a combination of dietary counseling, four times daily home blood glucose monitoring (maintaining fasting glucose levels <99 mg/dL and two-hour postprandial levels <126 mg/dL), and insulin for persistent hyperglycemia. There was a reduction in the diagnosis of macrosomia (21%–10%) but an increase in neonatal ICU admissions (61%–71%). Induction of labor was increased in the intervention groups (from 29% to 39%) with no increase in the cesarean delivery rate, which was stable at 31% to 32%. In 2009, a second randomized controlled trial also demonstrated improved maternal and neonatal outcomes in women with glucose intolerance on a 100-gram OGTT. Landon and colleagues reported that glycemic control was achieved through a combination of dietary counseling, four times daily home blood glucose monitoring (maintaining fasting glucose levels <95 mg/dL and two-hour postprandial levels <120 mg/dL), and insulin for persistent hyperglycemia did not result in a significant reduction in combined neonatal morbidity and mortality—a composite outcome including stillbirth, perinatal death, neonatal hyperbilirubinemia, hypoglycemia, hyperinsulinemia, and birth trauma. Treatment of glucose intolerance did, however, result in significant reductions in mean neonatal birth weight and fat mass, reduced frequency of large for gestational age neonates (14.5%–7%), birth weight greater than 4000 grams (14%–6%), shoulder dystocia (4%–1.5%) cesarean delivery (34%–27%), and pregnancy-induced hypertension (14%–9%), compared to usual care. A 2013 systematic review and meta-analysis found that treatment of GDM with dietary interventions or insulin administration (if blood glucose targets are not achieved with diet alone) resulted in significant reductions in preeclampsia (RR 0.62, 95% CI 0.42–0.89); shoulder dystocia (RR 0.42, 95% CI 0.23–0.77); and neonatal birth weight >4000 g (RR 0.50, 95% CI 0.35–0.71). The only potential harm recognized from treatment of GDM in this meta-analysis was an increased number of prenatal visits.
The goal of antepartum management is to prevent fetal macrosomia and its resultant complications by maintaining maternal blood glucose at desirable levels throughout gestation (fasting, below 95 mg/dL; one hour postprandial, below 140 mg/dL or two hours postprandial, below 120 mg/dL). , Initial recommendations should include a diabetic diet consisting of 30 to 35 kcal/kg of ideal body weight given as 40 to 50% carbohydrate, 20% protein, and 30 to 40% fat to avoid protein catabolism. Daily home glucose monitoring and weekly antepartum visits to monitor glycemic control should also be instituted. If diet alone does not maintain blood glucose at desirable levels, pharmacologic therapy may be recommended. If initial fasting glucose levels are consistently greater than 95 mg/dL and/or postprandial values are consistently above 140 mg/dL (1 hour after starting a meal) or 120 mg/dL (2 hours after starting a meal), insulin therapy can be started immediately with every effort made to avoid iatrogenic hypoglycemia. , The Fifth International Workshop on Gestational Diabetes recommended exercise as an adjunct to diet for the treatment of GDM, although data are conflicting on the beneficial effects of exercise on glycemic control. Some randomized trials have failed to demonstrate a beneficial effect of exercise on glycemic control in women with GDM, , but other studies have demonstrated that exercise is associated with a reduction in the need for insulin in women with GDM, and a reduction in macrosomia and cesarean delivery rates. A 2017 review including 15 randomized controlled trials demonstrated that women randomized to lifestyle interventions (diet, exercise, education, self-monitoring of blood glucose) as compared to usual care had a lower incidence of postnatal depression and a higher likelihood of achieving postpartum weight goals. Their neonates were less likely to be born large-for-gestational-age and had lower rates of macrosomia and neonatal fat mass. There were no differences between groups in rates of childhood elevated BMI, perinatal death, composite serious neonatal morbidity, neonatal hypoglycemia, hypertensive disorders of pregnancy, cesarean delivery, development of type 2 diabetes within 10 years, perineal trauma, or induction of labor. The ADA and ACOG recommend including moderate exercise in the treatment of women with GDM and no medical or obstetrical contraindications to physical activity. ,
ACOG and the ADA have recently endorsed the use of oral antihyperglycemic agents in pregnancy, although the United States FDA has not specifically approved these medications for the treatment of GDM. The ADA and ACOG specifically recommend the use of insulin or metformin over glyburide, due to the higher rates of neonatal hypoglycemia and macrosomia reported with glyburide (see below). ,
In the United States, oral hypoglycemic agents (sulfonylureas in particular) have not traditionally been recommended as first-line agents for use during pregnancy because of the possibility of fetal teratogenesis, macrosomia, and prolonged neonatal hypoglycemia. , This class of drugs works by stimulating pancreatic β-cells to synthesize and release insulin. Because the adverse fetal consequences of GDM are likely related to fetal hyperinsulinemia, any agent that could cross the placenta and increase fetal insulin production should be used with caution in pregnancy. First-generation sulfonylureas have been shown to cross the placenta and, as such, are contraindicated in pregnancy. There are conflicting data regarding the transplacental passage of second-generation sulfonylurea agents (glyburide and glipizide). Older human studies demonstrated minimal fetal exposure, , likely secondary to high protein binding and active transport of glyburide from the fetal to the maternal circulation. One study reported umbilical cord blood concentrations of glyburide as high as 70% of maternal serum concentrations, and another found significant variability in the transplacental transfer of glyburide among patients, with 37% of cord blood samples containing higher glyburide concentrations than maternal blood. No data have been published on the long-term effects of maternal glyburide use on offspring, and patients prescribed glyburide for treatment of GDM should be informed of uncertainties with respect to extent of transplacental passage and long-term effects on offspring.
Congenital malformations associated with the use of oral sulfonylurea drugs in pregnancy have been described, but most of these reports failed to take into account maternal glycemic control. More recent studies have shown that the risk of malformations correlates strongly with the degree of glycemic control at the time of conception and is unrelated to the type of antidiabetic therapy. Moreover, such reports refer to oral hypoglycemic treatment in early pregnancy, whereas treatment for GDM only begins after the period of fetal organogenesis, thereby eliminating any concern regarding malformations due to treatment alone.
Between 1974 and 1983, Coetzee and Jackson treated 423 women with a new diagnosis of diabetes in pregnancy with oral hypoglycemic agents and found no cases of serious neonatal hypoglycemia and no increase in perinatal mortality. A clinical trial by Langer et al randomized 404 women with singleton pregnancies and GDM requiring treatment with either glyburide or insulin therapy. Results showed no difference in glycemic control or neonatal outcome (including congenital malformations, macrosomia, neonatal hypoglycemia, or admission to neonatal intensive care). Eight women (4%) in the glyburide group required insulin therapy. Jacobsen et al performed a retrospective analysis comparing 236 women taking glyburide to 268 women taking insulin for control of gestational diabetes unresponsive to diet therapy. Women in the insulin group had a higher mean BMI and higher mean fasting value on GTT, suggesting these women may have had a greater predisposition to poor glycemic control. The study reported no significant differences between groups in birth weight, macrosomia, or cesarean delivery. More women in the glyburide group achieved mean fasting and postprandial goals (86% versus 63%), and their neonates were less likely to be admitted to the NICU compared to women taking insulin (15% versus 24%). Women treated with glyburide, however, had a higher incidence of preeclampsia (12% versus 6%) and their neonates were more likely to receive phototherapy (9% versus 5%). From the standpoint of maternal safety, hypoglycemia is the most commonly reported maternal side effect. Langer et al reported that 2% of patients taking glyburide had blood glucose measurements <40 mg/dL, compared with 20% of patients taking insulin. Other studies reported no significant difference in rates of maternal hypoglycemia between women taking glyburide compared to insulin. , A 2015 systematic review and meta-analysis of randomized trials comparing glyburide to insulin for treatment of GDM found that women assigned to glyburide had a higher risk of macrosomia (RR 2.62, 95% CI 1.35–5.08), higher mean birth weight in offspring (mean increase of 109 g, 95% CI 36-181 g), and a higher rate of neonatal hypoglycemia (RR 2.04, 95% CI 1.3–3.2). While the limited data currently available do not permit firm conclusions to be drawn about the efficacy and safety of oral hypoglycemic drugs in pregnancy, these agents are being used more frequently by obstetric care providers.
The largest randomized clinical trial to date investigating the efficacy of metformin compared to insulin for treatment of gestational diabetes is the Metformin in Gestational Diabetes Trial (MiG). In this randomized, open-label trial, 363 women were randomized to metformin and 370 to insulin, with 46% of women on metformin requiring supplemental insulin. There was no significant difference in perinatal complications between treatment groups. The primary composite outcome of neonatal hypoglycemia, respiratory distress, need for phototherapy, birth trauma, 5-minute Apgar score less than 7, or prematurity was 32% in both the metformin and the insulin group. Women preferred metformin to insulin therapy (77% versus 27%), and there were no serious adverse events associated with the use of metformin. A smaller open-label study randomized 100 women with GDM to therapy with insulin or metformin, finding no significant differences in the incidence of large for gestational age, mean birth weight, or neonatal morbidity. Thirty-two percent of women randomized to metformin required supplemental insulin; these women tended to have higher BMIs, higher fasting blood glucose levels, and required medical therapy for GDM earlier than those women who achieved adequate glycemic control with metformin alone. The 2015 systematic review and meta-analysis comparing glyburide, insulin, and metformin for the treatment of GDM found that compared with the use of insulin, metformin resulted in less gestational weight gain but lower gestational age at delivery and higher risk of preterm birth. There were no statistically significant differences in mean birth weight or macrosomia between metformin and insulin users, but there was a nearly significant trend toward lower neonatal hypoglycemia in metformin users (pooled risk ratio 0.78, 95% CI 0.6–1.01). Thus, metformin may provide a reasonable alternative to insulin in the treatment of gestational diabetes, particularly in lean or moderately overweight women who develop GDM later in gestation.
The use of metformin has several advantages over glyburide, including less macrosomia (RR 0.33 [0.13–0.81]), lower mean birth weight (mean difference −209 grams, [−314 to −104 g]), and lower gestational weight gain (mean difference −2.06 kg [−3.98 to −0.14 kg]). Compared to women taking glyburide (4%–16%), women using metformin are more likely (35%–50%) to require supplemental insulin to achieve adequate glycemic control. , ,
Metformin is known to cross the human placenta, which has led to some trepidation regarding its safety profile in pregnancy. Metformin is a biguanide agent that acts by reducing peripheral insulin resistance and inhibiting gluconeogenesis. Since insulin acts as a potent growth factor, there is a theoretical concern that metformin passage across the placenta may lead to excessive fetal growth. Despite these concerns, metformin is commonly used by reproductive endocrinologists to help achieve pregnancy in women with polycystic ovarian syndrome (PCOS). In vitro models , and in vivo studies have shown that metformin crosses from the maternal to the fetal compartment. In one study, umbilical cord blood levels of metformin were twice as high as maternal venous levels.
There are very few data on whether fetal exposure to an insulin-sensitizing agent like metformin is beneficial or harmful. Multiple small retrospective studies have failed to show any significant adverse outcome with first trimester metformin exposure. , , A follow-up study of offspring of the Metformin in Gestational diabetes trial (MiG TOFU) reported no difference between metformin-exposed and nonexposed offspring in neurodevelopmental outcomes, total fat, or central adiposity at two years but did find an increase in subcutaneous fat deposition. Experts disagree on whether this may represent a healthier fat distribution in exposed offspring (i.e., if offspring exposed to metformin develop less visceral fat, they would theoretically be more insulin-sensitive), , and longer-term studies are needed. Until longer-term follow-up data are available regarding the impact on offspring metabolic profile and neurodevelopment after in utero exposure to metformin, patients should be counseled regarding the uncertainty about the effects of transplacental passage. As a result of these data, ACOG and the ADA recommend insulin as the preferred treatment for GDM, recognizing that there are clinical situations in which oral agents are necessary. In those situations, patients should be counseled regarding the limited long-term safety data available for oral agents.
The risk of stillbirth is increased in women with poorly-controlled GDM, , , , although it is not clear whether this is true also of pregnancies with mild disease. , For this reason, many centers recommend weekly fetal testing starting at 32 weeks and early delivery (typically at 38 to 40 weeks) for women with GDM who require oral or insulin therapy and those with pregnancy complications (macrosomia, polyhydramnios, or hypertension). Whether weekly fetal testing and early delivery are necessary in pregnancies complicated only by diet-controlled GDM is not clear. Sonographic estimation of fetal weight should be considered at 36 to 38 weeks’ gestation. The use of prophylactic elective cesarean delivery to reduce the risk of maternal and fetal birth injury in the setting of fetal macrosomia remains controversial, and ACOG recommends based on limited available data that women with GDM should be counseled regarding the risks and benefits of scheduled Cesarean delivery to prevent birth trauma when the estimated fetal weight is >4500 g. , It is clear, however, that induction of labor for so-called “impending macrosomia” does not decrease the risk of cesarean delivery or intrapartum complications. , In labor, maternal glucose levels in pregnancies complicated by GDM should be maintained at 100 to 120 mg/dL to minimize the risk of fetal hypoxic injury. For the same reason, neonatal blood glucose levels should be measured within one hour of birth, and early feeding should be encouraged. Delivery of the fetus and placenta effectively removes the source of the antiinsulin hormones that cause GDM. As such, no further management is required in the immediate postpartum period.
GDM frequently indicates underlying insulin resistance. Fifty percent of women with GDM will experience GDM in subsequent pregnancies, and 30% to 65% will develop type 2 diabetes later in life. , All women with GDM should, therefore, have a standard (nonpregnant) 75-g GTT approximately six weeks postpartum and should consider preventative and early diagnostic strategies such as weight reduction, increased exercise, and regular screening for diabetes every 1-3 years. Those with fasting plasma glucose greater than 125mg/dL or 2-hour glucose greater than >100 mg/dL should be referred for diabetes management. Those with borderline glucose test results (fasting glucose 100-125 mg/dL or 2-hr 140-199 mg/dL) should be referred for weight loss, nutrition, and physical activity counseling and screened more frequently for glycemic status.
Pregestational diabetes is associated with significant maternal and perinatal morbidity and mortality.
There is a significantly increased risk of congenital anomalies, particularly cardiac defects, neural tube defects, and renal agenesis. Fetal echo is indicated in those with poor prepregnancy glucose control.
Insulin, rather than oral agents, has been the mainstay of therapy in patients with pregestational diabetes
Pregestational diabetes, which affects approximately 1% of women of childbearing age, can be due either to absolute insulin deficiency (type 1, insulin-dependent diabetes mellitus [IDDM]) or to increased peripheral resistance to insulin action (type 2, noninsulin-dependent diabetes mellitus [NIDDM]), as shown in Box 29.1 . The fasting glucose cutoff for diagnosing pregestational diabetes was reduced from 140 mg/dL to 126 mg/dL in 1997. On the basis of this change, there are more than 29 million people with diabetes in the United States alone. The White classification of diabetes in pregnancy ( Table 29.4 ) was developed by Dr. Priscilla White at the Joslin Diabetic Center in Boston, Massachusetts, in an attempt to correlate severity of diabetes with pregnancy outcome. Although this classification is commonly used, any direct correlation between the White class and prognosis remains unclear. Features known to be associated with poor pregnancy outcomes include DKA, poor compliance, hypertension, pyelonephritis, and vasculopathy.
| White Class | Age of Onset (Year) | Duration (Years) | Vascular Disease | Therapy |
|---|---|---|---|---|
| A | Only in pregnancy | Only in pregnancy | No | A1-diet controlled |
| A2-insulin requiring | ||||
| B | >20 or | <10 | No | Insulin |
| C | 10–19 or | 10–19 | No | Insulin |
| D | <10 or | >20 | Benign retinopathy hypertension | Insulin |
| F | Any | Any | Nephropathy | Insulin |
| R | Any | Any | Proliferative retinopathy | Insulin |
| H | Any | Any | Atherosclerotic heart disease | Insulin |
| T | Any | Any | Renal transplant | Insulin |
In contrast to GDM, pregestational diabetes is associated with significant maternal and perinatal mortality and morbidity ( Box 29.3 ). , One of the more important complications is the increased risk of congenital malformations associated with hyperglycemia at the time of fertilization and embryo development. The incidence of congenital anomalies and spontaneous abortions in such patients correlates directly with the degree of glycemic control at conception, as measured by circulating maternal glycosylated hemoglobin levels. Overall, approximately 30% to 50% of the perinatal mortality in diabetic pregnancy is due to fetal malformations. Structural anomalies commonly seen in association with diabetes include cardiac defects (ventricular septal defects, transposition of the great vessels), renal agenesis, and neural tube defects (anencephaly, open spina bifida). Some congenital defects—specifically sacral agenesis and caudal regression syndrome—are up to 400 times more common in the offspring of women with diabetes than in women with normal glucose metabolism and, as such, are considered pathognomonic. The overall prevalence of these anomalies, however, is low.
Spontaneous abortion
Diabetic ketoacidosis
Hypertension
Preeclampsia/eclampsia
Preterm birth
Cesarean delivery
Severe perineal injury
Infectious morbidity (chorioamnionitis, endometritis, wound infection)
Congenital anomalies
Macrosomia
Intrauterine growth restriction
Late fetal demise
Nonreassuring fetal testing (previously referred to as “fetal distress”)
Birth trauma (e.g., hypoxic ischemic cerebral injury; skull, clavicular, and long bone fractures; shoulder dystocia and brachial plexus injury)
Hypoglycemia
Neonatal sepsis
Delayed organ maturation (respiratory distress syndrome, hyperbilirubinemia)
The factors responsible for diabetic embryopathy are not well defined, but glucose , and ketone bodies such as β-hydroxybutyrate , have both been implicated. Oxidative stress , and apoptosis dysregulation , have also been identified as potential mechanisms for diabetic embryopathy in animal models. Several prospective randomized studies have shown that strict glycemic control around the time of conception is effective in reducing the risk of congenital malformations in women with established diabetes. , , In one trial, intensive preconception management of diabetic women with vascular disease reduced the malformation rate from 19% to 8.5%. Unfortunately, most women with diabetes do not seek care prior to conception. , Maternal serum α-fetoprotein estimation at 15 to 20 weeks’ gestation and a detailed sonographic fetal anatomic survey (with or without a fetal echocardiogram) at 18 to 22 weeks can be useful in screening for fetal malformations.
Intensive antepartum management should be initiated as early as possible and continued throughout gestation with a view to maintaining maternal blood glucose at desirable levels (fasting, below 95 mg/dL; premeal, below 100 mg/dL; one hour postprandial, below 140 mg/dL or two hours postprandial, below 120 mg/dL; during the night, not below 60 mg/dL; with an overall goal of mean capillary glucose levels of 100 mg/dL, corresponding to a glycated A1C ≤6%). Although the frequency of self-glucose monitoring recommended in pregnancy varies by organization, glucose should be monitored a minimum of four times daily (fasting, and one or two hours postprandial), and could be monitored as many as nine times daily (fasting, premeal, one or two hours postprandial, before bedtime, and at 3 am if nocturnal hypoglycemia is suspected). ACOG and the ADA provide recommendations for not only postprandial but premeal blood glucose levels. , , , If compliance with frequent fingersticks is in question, postprandial blood glucose levels have been shown to correlate more closely with adverse pregnancy and neonatal outcomes than do fasting or premeal blood glucose levels. , , Initial recommendations for management of pregestational diabetes include a strict diabetic diet, regular exercise, daily home glucose monitoring, insulin treatment, and weekly antepartum visits to monitor glycemic control. Such an approach has been shown to decrease perinatal mortality from a baseline of 20% to 30% to approximately 3% to 5%. , , , ,
Continuous glucose monitoring with the use of a wearable device to provide real-time glucose readings has been proposed as a solution for improving glycemic control for women with pregestational diabetes in pregnancy. In the 2017 CONCEPTT Trial, 215 pregnant women with type 1 diabetes were randomized in the first trimester to use a continuous glucose monitor (CGM) with standard fingerstick monitoring versus standard fingerstick monitoring alone; those in the CGM group had a greater reduction in A1c throughout pregnancy, a greater reduction in hyperglycemia without an increase in hypoglycemia, lower rates of large-for-gestational-age neonates, neonatal hypoglycemia, and NICU admission, as well as shorter duration of neonatal hospital admission. There were no differences in rates of hypertensive disorders of pregnancy or preterm birth. The authors conclude that the use of a CGM in pregnancy is associated with improved maternal glycemic control and neonatal outcomes and should be offered to all pregnant women with T1DM. In the 2018 GlucoMOMS randomized controlled trial of 304 women in the Netherlands with type 1, type 2, or gestational diabetes on insulin, the use of a CGM led to lower rates of preeclampsia but no difference in HbA1c levels or risk of macrosomia, though there was notably a 44% drop out rate in the group assigned to use of CGM. Two smaller randomized trials of women with preexisting diabetes showed similar to slightly lower HbA1c in the group using CGM, with one study demonstrating a reduced risk of macrosomia in the CGM group and the second showing similar rates of macrosomia and large-for-gestational-age neonates. ,
Insulin, rather than oral agents, has been the mainstay of therapy in patients with pregestational diabetes. To date, there are no randomized clinical trials to establish the efficacy of oral agents in the management of pregestational diabetes in pregnancy. Insulin should be administered subcutaneously at 0.7 to as high as 2.0 units/kg (present pregnancy weight, morbidly obese women may require doses as high as 1.5–2.0 units/kg) per day in divided doses. Traditional recommendations have been to administer two-thirds of the total daily dose in the morning (60% NPH, 40% regular/rapid acting) and one-third in the evening (50% NPH, 50% regular/rapid acting). Rapid-acting insulin, namely lispro or aspart, achieves better glycemic control with fewer hypoglycemic episodes compared to regular insulin. Lispro or aspart has the additional benefit of convenience, as these rapid-acting insulins can be administered immediately premeal, while regular insulin has to be administered 20 to 30 minutes before a meal. Alternative regimens may include dividing the total daily insulin requirement into 50% basal and 50% prandial, with either half or two-thirds of the basal administered as NPH in the morning, and the other half or one-third of basal insulin administered as NPH before bedtime. The remaining 50% of the total daily insulin requirement may be divided into thirds and administered as lispro or aspart before meals. Insulin doses should be adjusted by approximately 10% to 20% up or down in response to the results of capillary blood glucose monitoring. Care should be taken to avoid iatrogenic hypoglycemia due to excessive insulin administration.
In women with pregestational diabetes, assessment of thyroid function is recommended (6% have coexisting thyroid disease), and baseline liver and renal function tests (including urine protein quantification and creatinine clearance determination) should be performed at the first prenatal visit. An ophthalmologic examination should also be carried out every trimester in those with known retinopathy or other evidence of small vessel disease, as rapid achievement of euglycemia can exacerbate preexisting retinopathy. Glycosylated hemoglobin levels should be determined at a minimum every trimester, and as frequently as every four to eight weeks throughout gestation. , , , At any one time, approximately 5% of maternal hemoglobin is glycosylated, known as hemoglobin Al (HbA1). HbAlc refers to the 80% to 85% of HbA1 that is irreversibly glycosylated and is therefore a more accurate measure of glycemic control. Because red blood cells have a life span of around 120 days, HbAlc measurements reflect the degree of glycemic control over the past three months. However, given physiologic increases in red blood cell turnover in pregnancy, HbA1c levels are reduced in pregnancy. A1c levels <6.0% to 6.5% in early gestation have been associated with the lowest risks of poor fetal outcomes, and levels <6.0% in the second and third trimesters are associated with lower rates of preterm delivery, preeclampsia, and large-for-gestational-age neonates. Due to these data, the ADA recommends a target HbA1c <6.0% throughout pregnancy, if it can be achieved without significant hypoglycemia.
Hypertension, prematurity, and late fetal demise are the most common complications of pregnancy in diabetic women. , , , Approximately 30% of women with diabetes will develop hypertension in the third trimester. Pregnancy-induced hypertension often results in labor induction and is a major contributor to premature delivery in women with preexisting diabetes. Sustained maternal hyperglycemia results in fetal hyperglycemia that leads, in turn, to fetal hyperinsulinemia and increased oxygen demand. As such, fetuses of diabetic mothers are at increased risk of antepartum hypoxic-ischemic cerebral injury and late fetal demise. , Another possible mechanism for fetal hypoxia in diabetic pregnancy is maternal vasculopathy and hyperglycemia leading to uteroplacental perfusion. Given the increased risk for fetal growth restriction, hypoxia, and fetal demise, in addition to serial third-trimester evaluation of fetal growth, weekly antepartum fetal testing (fetal cardiotocography) is usually recommended starting at 32 weeks’ gestation. After 36 weeks, testing is usually performed twice weekly. If the fetal heart rate tracing is abnormal, further testing (either a biophysical profile or contraction stress test) is mandatory.
A major issue in the care of the pregnant woman with diabetes is the proper timing of delivery. Given the risk of intrauterine fetal demise, ACOG recommends delivery for women with preexisting diabetes who do not have vascular complications and who have well-controlled blood glucose levels in the 39 th week of gestation; those with poorly controlled blood sugar or with vascular complications should be recommended for delivery between 36 and 39 weeks of gestation, or in some cases earlier. If glycemic control is good and there is no evidence of maternal vascular disease, spontaneous labor at term should be awaited. Women with poorly controlled diabetes or with complications (known vascular disease, worsening/new onset hypertension, FGR, oligohydramnios), on the other hand, should be delivered between 37 and 39 weeks. , Delivery as early as 34 weeks may be considered on an individual basis in patients with poor glycemic control. , Early delivery in pregnancies complicated by pregestational diabetes is associated with an increased risk of fetal respiratory distress syndrome, and consideration should be given to the validation of fetal lung maturity prior to elective induction. Fetal lung maturation is inhibited by insulin and testosterone and enhanced by endogenous cortisol, thyroxine, prolactin, and estradiol-17β. In infants of diabetic mothers, hyperinsulinemia and hyperandrogenemia are common findings and may contribute to the delay in lung maturation observed in diabetic pregnancies. The increased testosterone observed in male infants of diabetic mothers may be due to elevated concentrations of human chorionic gonadotropin (hCG), which stimulates testosterone synthesis in fetal Leydig cells.
As many as 25% of infants of women with diabetes have macrosomia. , If the estimated fetal weight is at least 4500 g, many authorities would recommend elective cesarean delivery at or beyond 39 weeks to minimize the risk of birth trauma, primarily shoulder dystocia and resultant brachial plexus injury. Elective cesarean delivery in women with pregestational diabetes should be scheduled early in the morning, and the patient’s morning prandial insulin dose should be withheld. The usual night-time dose of intermediate-acting (NPH) and rapid-acting insulin should be maintained prior to morning cesarean delivery, but if the patient uses long-acting insulin at night such as detemir or glargine, 50% of the usual dose should be given the night before delivery.
In early labor, if oral intake is permitted, even consumption of 50% of the typical daily calories consumed will usually be sufficient to meet metabolic demands. During active labor, metabolic demand increases. Intravenous glucose should therefore be administered (typically 5% dextrose in half-normal saline at a rate of 75 to 100 mL per hour) to all women with pregestational diabetes in active labor (or to those in latent labor when oral intake is prohibited), and blood glucose levels should be checked every one to two hours. Regular insulin should be given as needed either by intravenous infusion (starting at 0.5 to 1 unit per hour) or subcutaneous injection to maintain maternal glucose levels at >70 and <120 mg/dL (goal range 100-120 mg/dL). Strict maternal glycemic control in labor is critical to preventing fetal hyperglycemia and hyperinsulinemia, both of which increase fetal oxygen demand and thereby predispose to fetal cerebral hypoxic-ischemic injury.
During the first 48 hours postpartum, women may have a “honeymoon period” during which their insulin requirement is decreased. Moreover, the need for strict glycemic control is reduced, and circulating glucose levels of 150 to 200 mg/dL can be comfortably tolerated during this period pending discharge from the hospital and regulation of glucose levels in the home environment. Once a woman is able to eat, she can return to her prepregnancy insulin regimen. Women who desire to breastfeed should be supported in doing so. Breastfeeding may have long-term benefits in terms of weight management and glucose control for postpartum women with diabetes and their children. Women should be counseled that lactation may lead to hypoglycemia and insulin regimens may need to be adjusted accordingly.
Obesity is associated with an increased risk of adverse obstetrical outcomes, including miscarriage, congenital malformations, stillbirth, preeclampsia, gestational diabetes, cesarean delivery, and VTE, among others.
Limiting gestational weight gain and prepregnancy weight loss (sometimes through bariatric surgery) may mitigate maternal and fetal risks such as diabetes, hypertensive disorders of pregnancy, and macrosomia. However, maternal bariatric surgery has also been associated with an increased risk for small-for-gestational-age neonates .
The neuroendocrine milieu of maternal obesity may contribute to the risk for metabolic malprogramming of the fetus, and subsequent increased risk of obesity and metabolic syndrome in offspring of obese women.
Obesity is one of the greatest public health challenges in the United States and throughout the world. The prevalence of obesity has steadily risen since the 1980s. In the United States, approximately 40% of women of reproductive age have obesity, , representing a 70% rise in prepregnancy obesity over the last decade. Obesity prevalence has also increased in Europe among women of reproductive age, although overall rates are lower (20% to 25%).
The preferred method of weight assessment is the body mass index (BMI). BMI is calculated as the body weight in kilograms divided by the square of the height in meters. Normal weight is defined as a BMI between 18.5 and 24.9 kg/m 2 . Overweight refers to a BMI between 25 and 29.9 kg/m 2 . Obesity is a BMI greater than or equal to 30 kg/m 2 . Obesity is further divided into Class I (30-34.9 kg/m 2 ), Class II (35-39.9 kg/m 2 ), and Class III (BMI greater than 40 kg/m 2 ). The advantage of using BMI for defining obesity is that no adjustments need to be made for gender or height, and no tables are required for determining the normal range.
Obesity is a complex neuroendocrine and metabolic disorder that has been implicated in a large number of fetal and maternal complications, including spontaneous abortion, congenital malformations, stillbirth, preeclampsia, GDM, fetal macrosomia, cesarean delivery, venous thromboembolic disease, surgical complications, and urinary tract infections. Maternal obesity has also been associated with the risk of childhood obesity, cardiovascular disease, metabolic disorders, and allergic and atopic disease in offspring.
Congenital malformations classically associated with obesity include neural tube defects, ventral wall defects, and abnormalities of the great vessels. There is some evidence suggesting an increased risk for other anomalies in offspring of women with obesity, including hypospadias, isolated hydrocephalus, and orofacial clefts. In one study, women weighing more than 110 kg had a fourfold increased risk of having a fetus with a neural tube defect as compared with a control population weighing 50 to 59 kg. For women weighing 80 to 89 kg, the risk was increased 1.9-fold. Interestingly, folic acid supplementation (0.4 mg daily) did not appear to reduce the risk of neural tube defects in this cohort. These data are consistent with other studies showing that a BMI greater than 29 kg/m 2 is associated with a 1.9-fold increase in the risk for neural tube defects. Furthermore, the ability to detect congenital anomalies using ultrasound decreases as maternal BMI increases. The mechanism by which obesity causes congenital anomalies is not known. It is conceivable that, in women with obesity, subtle abnormalities in glucose metabolism contribute to the increased risk of congenital malformations, similar to that of pregestational diabetes.
Perinatal mortality is increased with progressive obesity. In a cohort of 167,750 Swedish women, Cnattingius and colleagues demonstrated a 1.7-fold increased risk of late fetal death for overweight women (BMI 25-29.9 kg/m 2 ) and a 2.7-fold increase for obese women (BMI >30 kg/m 2 ) when compared to women with a prepregnancy BMI <20 kg/m 2 . Obesity has consistently been associated with hypertensive disorders of pregnancy. , For example, one large prospective multicenter cohort study of more than 20,000 women demonstrated that obese women with a BMI between 30-34.9 kg/m 2 had a 2.5- and 1.6-fold relative risk for gestational nonproteinuric hypertension and preeclampsia, respectively. For obese women with a BMI >35 kg/m 2 , a similar association was found with a relative risk of 3.0- and 3.3-fold, respectively. In a 2003 systematic review of 13 studies including over 1.4 million women, O’Brien et al calculated a 2.0-fold increased risk of developing preeclampsia with every 5-7 kg/m 2 increase in BMI.
Other well-documented risks of obesity include an increased incidence of GDM and fetal macrosomia, birth injury, obstructive sleep apnea (OSA), maternal perineal trauma, and cesarean delivery. , In a study of 20,130 births, a BMI greater than 39 kg/m 2 was associated with a 46% cesarean delivery rate compared with 20% in a control group of women with a BMI less than 29 kg/m 2 , The increased cesarean delivery rate is also associated with increased surgical morbidity in obese women, including anesthetic complications, wound separation and infection, and venous thromboembolic events. Interestingly, maternal obesity appears to be protective against spontaneous preterm birth. The risk of spontaneous preterm birth decreases with increasing maternal BMI, perhaps due to decreased uterine activity in these women compared to normal BMI controls.
Obstetric management of the obese patient should include calculation of BMI, careful attention to blood pressure, a nutrition consultation, institution of a daily exercise program, and early screening for GDM. Randomized controlled trials seeking to establish the optimum gestational weight gain in obese women are ongoing. , Limiting total pregnancy weight gain to no more than 15 to 25 pounds appears to decrease the risk of fetal macrosomia without increasing the risk of low birth weight or FGR. A careful sonographic anatomy survey (with or without fetal echocardiogram) at 18 to 22 weeks’ gestation is indicated in obese women given the increased risk of fetal structural anomalies, although body habitus may result in suboptimal imaging. Serial growth scans should also be considered given the limitations of other methods of fetal growth assessment. Anesthesia consultation in the third trimester should also be considered prior to the onset of labor. If cesarean section is required, every effort should be made to reduce the risk of wound separation and infection, including prophylactic antibiotics, and closure of the subcutaneous layer.
Obesity has become one of the leading endocrine causes of morbidity and mortality in women of reproductive age. Bariatric surgery, which includes a number of procedures to reduce gastric capacity or bypass the stomach, has been shown to promote weight loss and reduce long-term mortality and morbidity in patients with obesity. , Its use as a weight loss tool has increased in popularity, and the resultant weight loss often leads to improved fertility. The safety of pregnancy after these procedures is unclear. Early case reports suggested an increased risk of adverse pregnancy outcomes. However, more recent studies, including a meta-analysis, have suggested a number of potential benefits, including reduced risk of diabetes, hypertensive disorders of pregnancy, and macrosomia. A large population-based cohort study including 670 Swedish women who underwent bariatric surgery and up to 5 matched control pregnancies for each case, reported that bariatric surgery prior to pregnancy was associated with reduced risk of gestational diabetes (OR 0.25, 95% CI 0.13–0.47) and large-for-gestational-age infants (OR 0.33, 95% CI 0.24–0.44), with no significant change in the risk of preterm birth. More concerning, however, was the higher risk of small-for-gestational-age infants in the women who underwent surgery (OR 2.2, 95% CI 1.64–2.95) and the nearly-significant increased risk for stillbirth or neonatal death in women who underwent surgery (OR 2.39, 95% CI 0.98–5.85). The link between prior bariatric surgery and possible increased neonatal mortality merits further study. Women who have undergone bariatric surgery are at risk for malabsorption and vitamin deficiencies, so levels should be followed during pregnancy and all these patients should receive vitamin and mineral supplementation, especially iron, folate, and vitamin B12.
Leptin is a hormone secreted primarily by adipocytes and acts to suppress appetite while increasing energy expenditure, thereby regulating body weight. Mice lacking the leptin gene (ob/ob mice) are obese and anovulatory. Administration of human leptin to ob/ob mice increases energy expenditure, reduces weight and fat mass, and restores ovulation and fertility. , In humans, absolute leptin deficiency is rare, and supplemental recombinant leptin has not been shown to promote weight loss. During pregnancy, leptin is produced by maternal and fetal adipocytes as well as the syncytiotrophoblast. Circulating leptin levels rise rapidly in the first trimester, maintain high levels throughout pregnancy, and drop precipitously after delivery, suggesting a major contribution from the placenta. , Leptin levels in the maternal circulation correlate with maternal body mass but not with levels in umbilical cord blood at birth or birth weight. However, leptin levels in umbilical cord blood do correlate directly with both birth weight , and fetal adiposity. The significance of the increased leptin levels in the maternal circulation during pregnancy is unclear, although it has been suggested that leptin may act to mobilize maternal fat stores and increase the availability of substrates to the fetus. In pregnant women with diabetes, alterations in maternal and fetal leptin levels have not been particularly informative, though one recent case-control study demonstrated higher baseline leptin levels with less increase in leptin throughout pregnancy in pregnant women with GDM compared to controls. , In pregnancies complicated by preeclampsia, umbilical cord leptin levels are decreased and reflect the reduction in fetal growth and fat stores. Interestingly, maternal leptin levels are increased in preeclampsia and appear to correlate with the severity of the disease. , The significance of this observation remains unclear. Although the regulation and mechanisms of action of leptin are not fully understood, it is possible that manipulation of the leptin-leptin receptor system may ultimately prove to be a safe and effective treatment for adult obesity and/or fetal macrosomia.
The developmental origins of health and disease hypothesis posit that the in utero environment induces fetal adaptive responses to facilitate offspring health and survival. The metabolic and hormonal changes that result from these responses lead to altered homeostatic set points, which ultimately may prove maladaptive in other environments. Fetal adaptation is likely mediated by epigenetic phenomena, including DNA methylation and histone modification. There is growing evidence that maternal obesity/overnutrition may induce fetal adaptive responses that predispose to obesity, diabetes, metabolic derangements, and increased cardiovascular risk later in life. Maternal hyperglycemia, an inflammatory intrauterine and ovarian milieu, and leptin dysregulation all may contribute to the increased cardiometabolic risk for offspring born to obese gravidas. , Sheep and rat models suggest that in utero overnutrition may predispose to childhood and adult obesity via increased leptin exposure, with subsequent inhibited leptin receptor development in the hypothalamus, and thus decreased sensitivity to leptin-mediated appetite suppression. Indeed, increased leptin and insulin concentrations have been described in human offspring of obese mothers, suggesting that fetal programming of appetite and satiety pathways may be an important determinant of adult disease states. ,
Pregnancy is associated with multiple physiological changes in the hypothalamic-pituitary axis, including: (1) increased pituitary weight and volume; (2) increased proportion of lactotrophs in the adenohypophysis; (3) increased circulating levels of prolactin; (4) increased ACTH; (5) increased total and free cortisol; (6) increased maternal serum growth hormone; and (7) mild thyroid glandular hypertrophy.
Pituitary tumors are classified as either microadenomas (<10 mm in diameter) or macroadenomas (>10 mm) .
Microadenomas typically have a benign course in pregnancy, while macroadenomas are more often associated with extrasellar extension, local invasion, or compression of the optic chiasm.
Prolactinoma is the most common pituitary tumor in pregnancy. First-line therapy is typically medical and includes dopamine agonists bromocriptine and cabergoline.
The pituitary gland is composed of three parts: the anterior lobe (adenohypophysis), the intermediate lobe (prominent in the fetus but attenuated in the adult), and a posterior lobe (neurohypophysis). During pregnancy, the structure and function of the pituitary gland are significantly altered. In the nonpregnant state, the pituitary gland weighs between 0.5 and 1.0 g. One autopsy study of 118 pregnant women demonstrated a 30% increase in the weight of the pituitary gland compared with nonpregnant controls (1070 mg versus 820 mg, respectively). This increase in weight is associated also with an increase in volume ( Table 29.5 ) , and a change in shape. In pregnancy, the pituitary gland develops a convex, dome-shaped superior surface, which may impinge on the optic chiasm and account, in part, for the bitemporal hemianopia observed in some apparently healthy pregnant women. , Pregnancy is not associated with an increased incidence of pituitary adenoma.
| Gestational Age (Weeks) | Subject Number | Pituitary Volume (mm 3 ) Mean ± SEM |
|---|---|---|
| Nonpregnant | 20 | 300 ± 60 |
| 9 | 10 | 437 ± 90 |
| 21 | 11 | 534 ± 124 |
| 37 | 11 | 708 ± 123 |
∗ Data from Gonzalez JG, Elizondo G, Saldivar D, et al. Pituitary gland growth during normal pregnancy: an in vivo study using magnetic resonance imaging. Am J Med . 1988;85:217.
On the basis of the hormones they produce, the adenohypophysis contains at least five different cell types: lactotropes (that primarily secrete prolactin), corticotropes (adrenocorticotropic hormone [ACTH]), somatotropes (GH), gonadotropes (luteinizing hormone [LH] and follicle-stimulating hormone [FSH]), and thyrotropes (thyroid-stimulating hormone [TSH]). The cellular composition of the adenohypophysis, however, changes throughout pregnancy. This is especially true of the lactotrope cell population. Immunohistochemical studies have shown that, in the nonpregnant state, approximately 20% of cells in the adenohypophysis are lactotropes. , This number increases in pregnancy such that, by the third trimester, approximately 60% are lactotropes. Moreover, the increase in lactotropes is most pronounced in the lateral portions of the adenohypophysis. By one month postpartum, the number of lactotropes in the anterior pituitary of nonlactating women is decreased. However, postpartum resolution of lactotrope hyperplasia is incomplete, and nonpregnant multiparas have on average more lactotrope cells than do nulligravid women.
In contrast to lactotropes, the numbers of somatotropes, gonadotropes, and α-subunit-secreting cells in the adenohypophysis decrease in pregnancy and the number of thyrotropes do not change. These changes in cellular composition are associated with changes in circulating hormone levels.
Levels of prolactin in the maternal circulation increase throughout pregnancy, reaching concentrations of approximately 140 ng/mL at term ( Fig. 29.8 ). Although the maternal decidua is a major site of prolactin production during pregnancy, prolactin in the maternal circulation originates primarily from the maternal pituitary with small contributions from the maternal decidua and fetal pituitary. The observation that circulating levels of prolactin in pregnant women with preexisting hypopituitarism remain low throughout pregnancy supports the hypothesis that very little decidual prolactin enters the maternal circulation. , Decidual production of prolactin leads to elevated levels in the amniotic fluid, which peaks at approximately 6000 ng/mL at the end of the second trimester.
The hyperprolactinemia of pregnancy is likely due to increased circulating levels of estradiol-17β. Aside from this increase in basal levels, prolactin secretion by the maternal pituitary is stimulated by thyrotropin-releasing hormone (TRH), arginine, meals, and sleep in a manner similar to that seen in nonpregnant women. After delivery, maternal prolactin concentrations in nonlactating women decrease to prepregnancy levels within three months. In lactating women, basal circulating prolactin levels decrease slowly to nonpregnant levels over a period of several months with intermittent episodes of hyperprolactinemia in conjunction with nursing.
Pregnancy is also associated with a shift in prolactin isoforms. In the nonpregnant state, the N-linked glycosylated isoform of prolactin (G-PRL) predominates in the circulation. As pregnancy progresses, increasing amounts of nonglycosylated prolactin appear in the circulation. In the third trimester, the concentration of circulating nonglycosylated prolactin exceeds that of GPRL. Nonglycosylated prolactin appears to be more biologically active than G-PRL. The precise function of the elevated circulating levels of prolactin in pregnancy is not clear, but it appears to be important in preparing breast tissue for lactation by stimulating glandular epithelial cell mitosis and increasing production of lactose, lipids, and certain proteins. The role of prolactin in amniotic fluid is not known.
Pregnancy produces changes in prolactin secretion that persist long after delivery. Musey and colleagues reported that the basal serum prolactin level and the prolactin response to perphenazine stimulation were lower after pregnancy than before pregnancy. They also found that the serum prolactin concentration was significantly lower in the parous women (mean, 4.8 ng/mL) than in the nulliparous women (8.9 ng/mL). These and other studies , suggest that pregnancy permanently suppresses the secretion of prolactin by the maternal pituitary.
ACTH levels in the maternal circulation increase from approximately 10 pg/mL in the nonpregnant state to 50 pg/mL at term, and increase further to approximately 300 pg/mL in labor ( Fig. 29.9 ). Although the placenta can produce ACTH, the majority of circulating ACTH appears to come from the maternal pituitary. Placental production of corticotropin-releasing hormone (CRH) may be a major cause of the elevated levels of ACTH in the maternal circulation. In nonpregnant women, serum CRH levels range from approximately 10 to 100 pg/mL. In the third trimester of pregnancy, these concentrations increase to 500 to 3000 pg/mL but then decrease precipitously after delivery. ,
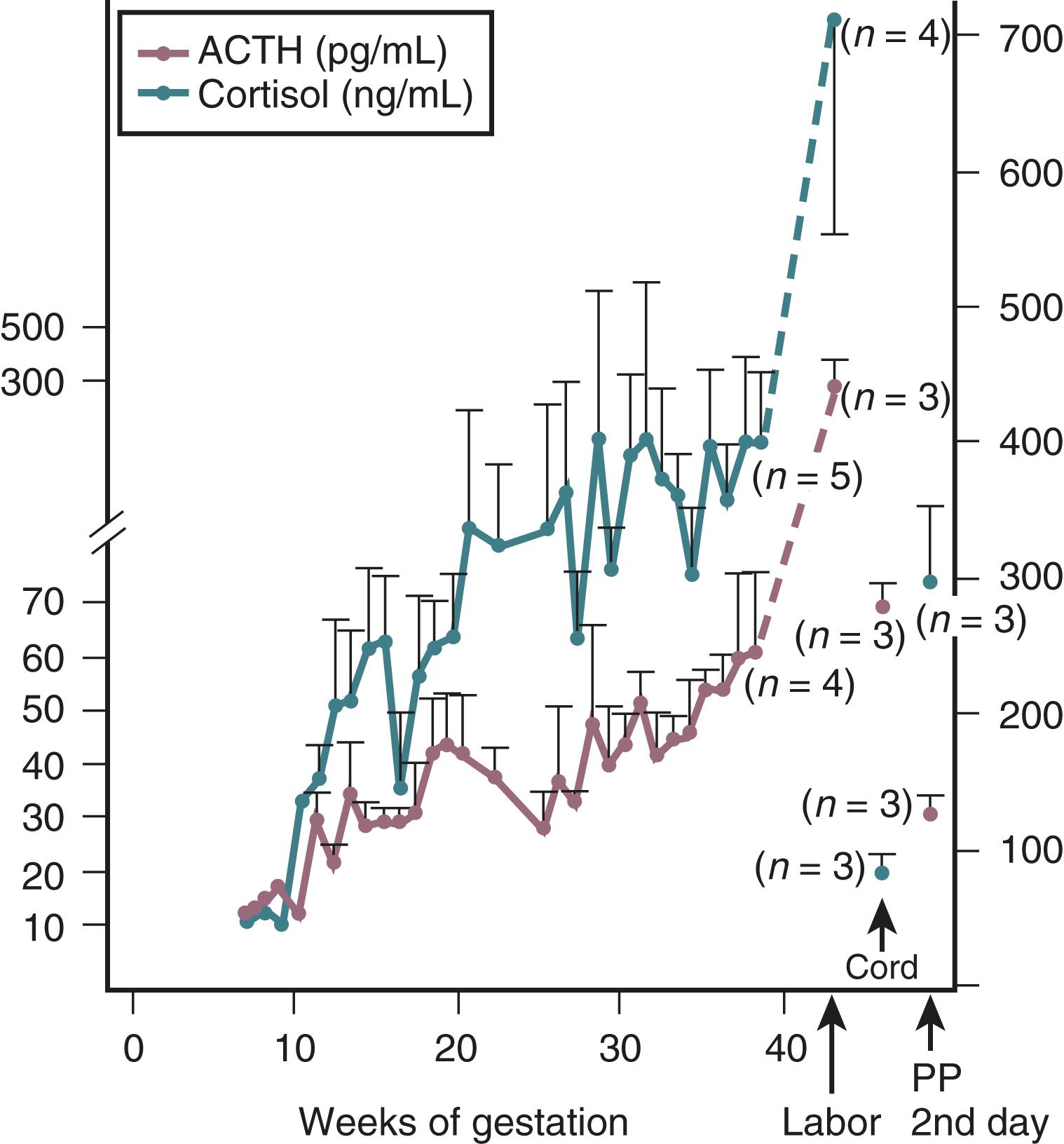
In addition to increasing pituitary ACTH secretion, chronically elevated levels of CRH in the maternal circulation reduce the ability of exogenous glucocorticoids to suppress the maternal ACTH–cortisol axis, enhance the ability of vasopressin to induce an ACTH response, and diminish the effect of exogenous CRH. , CRH binding protein (CRH-BP) inactivates CRH, thereby preventing its action on the maternal or fetal pituitary. CRH-BP levels in the maternal circulation decrease during the last few weeks of pregnancy, resulting in an increase in free (biologically active) CRH.
Although the maternal adrenal glands do not change in size, pregnancy is associated with significant changes in the circulating concentrations of adrenal hormones. For example, serum cortisol levels increase substantially in pregnancy. The majority of circulating cortisol is bound to cortisol-binding globulin (CBG), which is produced by the liver. Circulating levels of CBG increase during pregnancy in response to elevated levels of estrogen, and CBG may retard the clearance of these hormones. It is not surprising, therefore, that total cortisol levels increase in pregnancy. However, levels of free cortisol in the circulation , —as well as in saliva and urine , , —are also increased, which is likely due to the increased levels of ACTH in the maternal circulation. Maternal hypercortisolemia is also observed in complete molar pregnancy, suggesting that the increased cortisol is not derived from a fetal source. Other changes in adrenal hormone levels that occur in association with pregnancy include an increase in circulating levels of aldosterone and adrenal androgens, primarily androstenedione and testosterone.
Maternal serum GH levels begin to increase at around 10 weeks’ gestation, plateau at approximately 28 weeks, and can remain elevated for several months postpartum. The majority of this GH is derived from the placenta (GHV), with a marked reduction in basal somatotropin (GH–N) production by the maternal pituitary. Moreover, the release of GH-N in response to either insulin-induced hypoglycemia ( Fig. 29.10 ) or arginine stimulation is markedly attenuated, suggesting that maternal pituitary GH secretory reserve is diminished in pregnancy.
In general, the concentration of TSH (thyrotropin) in the maternal circulation remains within the normal range during pregnancy. Normative data from pregnant women suggest the upper reference range for TSH in pregnancy may be 2.5 to 3.0 mIU/L rather than 4.0 mIU/L, which is the upper limit of normal in healthy, nonpregnant individuals. , At 9 to 13 weeks of gestation, there is a modest decline in circulating TSH levels ( Fig. 29.11 ). , This coincides with the peak placental production of hCG, and some authorities have suggested that the decrease in TSH may be due to the weak thyrotropic properties of hCG. An alternative hypothesis is that the placenta may secrete a hormone with TRH or TSH-like properties, but this hypothesis is not supported by most data.
Enlargement of the thyroid gland is a common finding during pregnancy. This growth is not due to any deficiency in the hypothalamic-pituitary-thyroid axis but rather a result of relative iodide deficiency and increased demand for thyroid hormone secondary to elevated levels of thyroid-binding globulin (TBG). Increased TBG concentrations result in the need for increased production of thyroxine (T 4 ) and triiodothyronine (T 3 ) to maintain adequate concentrations of free hormone. The thyroid responds to the need for increased production with increased vascularity, cellular hyperplasia, and ultimately glandular hypertrophy. Indeed, the TSH response to exogenous TRH stimulation remains normal throughout pregnancy. , An appreciation of the physiologic changes in thyroid hormone levels is important to accurately assess thyroid status in pregnancy. Total T 4 and T 3 levels are elevated due to TBG excess and therefore have not typically been utilized to evaluate thyroid status during pregnancy. The most appropriate test to detect thyroid dysfunction during pregnancy is the TSH assay. If this is abnormal, classically free T 4 and free T 3 levels have been measured. However, more recent uncertainty about the accuracy of free T 4 immunoassays in pregnancy has led some to reconsider the use of adjusted total T 4 in pregnancy in select situations (see section on “Maternal Thyroid Function in Pregnancy”). ,
Maternal serum LH and FSH levels are decreased by six to seven weeks of pregnancy and are below the limits of detection of many radioimmunoassays by the second trimester. The marked decrease in gonadotropin immunoreactivity in the pituitary glands of pregnant women , coupled with the blunted LH and FSH response to exogenous gonadotropin-releasing hormone (GnRH) stimulation ( Fig. 29.12 ) suggests that this effect is localized primarily to the pituitary. The suppression of pituitary LH and FSH synthesis and secretion likely results from elevated circulating levels of sex steroids (estradiol-17β, progesterone) and regulatory peptides (such as inhibin) during pregnancy.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here