Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The authors acknowledge substantial use of material from this chapter in the last edition.
The American Heart Association (AHA) and the European Society of Cardiology, as well as other national societies, provide updated scientific statements on many of the topics discussed in this chapter. Readers are encouraged to review these consensus statements and seek updated documents.
Infective endocarditis is an uncommon infection of the cardiac endothelium. In this disease process, pathogens become enmeshed in fibrin and platelets to form vegetations, which are attached to heart valves, mural endocardial surfaces, or the endocardium that forms over prosthetic material such as prosthetic valves, vascular conduits, pacemaker leads, or catheters. The historical risk factors of rheumatic heart disease and unrepaired congenital heart disease remain relevant, particularly in developing countries, but in developed countries, endocarditis in children and young adults occurs more often after surgery for congenital heart disease, as a complication of intravenous drug use, related to the use of an indwelling central venous catheter (CVC), or as a healthcare-associated infection (see Chapter 100 ).
Endocarditis was uniformly fatal in the preantibiotic era, but the rate of cure in referral centers is as high as 90% with appropriate management. , Mortality rates of 3.5%–6.8% for endocarditis were reported in two studies from the US using administrative datasets of hospitalized children. However, the crude mortality rate of endocarditis when including endocarditis diagnosed postmortem may be as high as 21%–24%. ,
The pathogens causing endocarditis in children from representative series are shown in Table 37.1 . Streptococci, especially viridans streptococci (e.g., Streptococcus sanguis , S. mitis , S. salivarius , S. mutans , and S. oralis ) are frequent causes of endocarditis. These pathogens generally are associated with infection among patients with structural risk factors, such as those with rheumatic heart disease or unrepaired congenital heart disease, as well as with late postoperative endocarditis. Staphylococci are more common than streptococci in some series (particularly among patients without predisposing risk factors), and, taken together, staphylococci and streptococci appear to cause approximately one-half of cases in children. , , Coagulase-negative staphylococci (CoNS) can cause endocarditis after cardiac surgery, and S. aureus , including community-acquired methicillin-resistant S. aureus (CA-MRSA), causes endocarditis in normal hearts as well. Candida and Aspergillus spp. increasingly are common causes of healthcare-associated endocarditis, particularly among patients in the neonatal intensive care unit (NICU), with a CVC, or after prosthetic valve surgery. , , Gram-negative bacilli and fastidious gram-negative oropharyngeal flora, the so-called HACEK organisms, an acronym for H aemophilus spp., A ggregatibacter spp., C ardiobacterium hominis , E ikenella corrodens , and K ingella kingae , are uncommon causes of endocarditis in children. Among the HACEK group, K. kingae and A. parainfluenzae are the most common causes of endocarditis in children. Mycobacteria are another uncommon cause of endocarditis and have been associated with endocarditis affecting prosthetic material. In the preantibiotic era, Streptococcus pneumoniae caused 15% of cases of endocarditis. Today, pneumococcal endocarditis is rare but is associated with a high rate of mortality.
| Period of Data Collection | 2000-2017 | 2000-2003 | 2000-2010 | 2009-2012 |
|---|---|---|---|---|
| Type of data | Case series | Administrative | Administrative | Administrative |
| Number of cases | 53 a | 632 b | 2680 b | 242 b |
| Streptococci | 122 (50%) | |||
| Viridans streptococci | 17 (32%) | 124 (20%) | 697 (26%) | — |
| Streptococcus pneumoniae | — | ≤10 (1%) | — | — |
| Other streptococci c | 1 (2%) | 29 (5%) | 362 (14%) | — |
| Enterococci | 3 (6%) | ≤10 (0%) | — | — |
| Staphylococci | 75 (31%) | |||
| Staphylococcus aureus | 13 (25%) | 362 (57%) | 981 (37%) | — |
| Coagulase-negative staphylococci | 11 (21%) | 91 (14%) | 173 (6%) | — |
| Gram-negative bacilli | 1 (2%) | ≤22 (3%) | 169 (6%) | 17 (7%) |
| Fungi | 4 (8%) | — | — | 7 (3%) |
| HACEK/other organisms | 2 (4%) | — | — | — |
| Polymicrobial cases | — | — | 298 (11%) | 21 (9%) |
| Culture-negative cases | 4 (8%) | — | — | — |
a Percentages sum to >100% due to polymicrobial infections.
b Includes only cases with pathogens reported due to potential misclassification of culture-positive cases as culture-negative cases in the absence of diagnostic codes for an organism.
c Including group A and B Streptococcus , Abiotrophia , and Granulicatella .
Culture-negative endocarditis seems to be less common in children than in adults. Approximately 5%–15% of children with endocarditis have sterile blood cultures. , , Previous treatment with antibiotics is a common cause of negative blood cultures, but fastidious HACEK organisms and other rare fastidious pathogens such as Bartonella , Brucella , Mycoplasma , Legionella , and Coxiella burnetii also can be causative. Non-HACEK pathogens are better detected by serologic or polymerase chain reaction (PCR) testing. Endocarditis in children is rarely caused by Chlamydia psittaci and Tropheryma whipplei .
The true incidence of endocarditis in children has been difficult to ascertain because the literature primarily derives data from referral centers. Between 1930 and 1972, 1 per 2000–5000 pediatric hospital admissions were due to endocarditis, and during the 1960s to 1980s, incidence was 1 per 500–1000 hospitalizations. , , An administrative study of multiple pediatric centers between 2003 and 2010 reported that endocarditis was associated with 0.05–0.12 per 1000 pediatric hospital admissions. On the other hand, the risk of endocarditis between birth and the age of 18 years was found to be 6.1 per 1000 children with congenital heart disease (or 4.1 cases in 10,000 person-years) in a recent population-based cohort from Canada. The overall population-based incidence of hospitalization for endocarditis in the US is approximately 10 per 1,000,000 children annually.
As shown in Table 37.2 , congenital heart disease currently is the most common predisposing condition in the US. In a cohort of children with congenital heart disease from Taiwan, the incidence was 11 cases per 10,000 person-years, and a similar rate was found in adults in the Netherlands. , Although surgical correction of ventricular septal defect or patent ductus arteriosus substantially reduces the risk for endocarditis, other interventions such as palliative shunts, valve replacement, and placement of cardiovascular implantable electronic devices can increase the risk for endocarditis due to the sustained presence of foreign material in the bloodstream. Additionally, longitudinal data in adults with unrepaired simple ventricular septal defect suggests a substantial increase in endocarditis risk in these patients compared with the general population. Adults with a bicuspid aortic valve also appear to have a higher rate of endocarditis than those with a tricuspid aortic valve, reinforcing the importance of underlying congenital heart disease. Advances in surgical techniques and supportive care now permit earlier repairs of certain lesions (e.g., tetralogy of Fallot) and neonatal palliation of previously inoperable lesions. , These changes are associated with increased endocarditis, including early postoperative endocarditis, occurring in younger patients, with a median age of ≤2 years in some series. , , , , However, endocarditis can occur years after repair of congenital heart defects. Of note, 18%–53% of patients with endocarditis in developed countries have no previously known cardiac defect. , , , , , , , , ,
| Reference | Series 1 | Series 2 | Series 3 | Series 4 |
|---|---|---|---|---|
| Years of study | 1930–1972 | 1977–1992 | 1992–2004 | 2004-2010 |
| Number reported | 266 | 62 | 85 | 1033 |
| Cardiac defect, n (%) | 663 (64%) | |||
| Congenital cardiac defect, n (%) | 208 (78%) | 40 (64%) | 68 (80%) | NAw |
| Acyanotic, n (%) | NA | 18 (45%) | 48 (71%) | 517 (78%) |
| Cyanotic, n | NA | 22 | 20 | 146 |
| Rheumatic heart disease | 37 (14%) | 3 (5%) | 1 (1%) | NA |
| No cardiac defect | 21 (8%) | 19 (31%) | 13 (18%) | 370 (36%) |
Neonatal endocarditis usually arises as a complication of extreme prematurity, major surgery, or prolonged use of an indwelling CVC. , Fewer than 30% of neonates with endocarditis have congenital heart disease, and most endocarditis is right sided. , Older children without cardiac defects also can develop right-sided endocarditis, including adolescents with intravenous drug use, those with a pacemaker, or critically ill patients with a CVC. , ,
A significant increase in the hospitalization rates for endocarditis in the US adult population was noted from 2000 to 2011 with an increase of 11–15 per 100,000 population. The proposed explanations include an aging population, increases in individuals with high-risk conditions such as diabetes and requiring hemodialysis, and increased survival of adults with congenital heart disease and prosthetic implants. A few studies have examined changes in the incidence of endocarditis after the 2007 publication of the AHA guidelines that reduced the number of groups with underlying heart disease for whom routine antibiotic prophylaxis during dental procedures was recommended (also see Prevention, below). No substantial difference in the incidence of endocarditis or hospitalizations for endocarditis was seen between the pre- and post-guideline periods, , , , and dental procedures did not appear to be a risk factor for endocarditis in a cohort followed from 1997 to 2010 in Taiwan, irrespective of antibiotic prophylaxis use. However, a similar analysis performed in the UK after the change in prophylaxis guidelines in 2008 noted an increase in endocarditis cases compared with historical trends.
The most important factor in the pathogenesis of endocarditis is disruption of vascular endothelium, typically caused by turbulent blood flow or foreign body (e.g., a CVC) in contact with the endocardium, or both. Turbulence generally results from a communication between an area of high pressure and an area of low pressure, such as flow through a ventricular septal defect or regurgitation through an incompetent valve. , Erosion of endothelium occurs, facilitating deposition of platelet-fibrin thrombi on the mural surface or on an abnormal valve on the low-pressure side.
Important pathogen factors include an organism’s ability to produce bloodstream infection (BSI) (bacteremia or fungemia), as well as the presence of microbial adhesins. The peptidoglycan surface of oral streptococci facilitates adherence to endothelial cells . Similarly, S. aureus binds to collagen, fibronectin, laminin, vitronectin, and fibrinogen. , Microbial surface components that recognize adhesive matrix molecules likely are virulence factors of S. aureus . Patients with S. aureus endocarditis have higher serum levels of adhesin molecules than patients with S. aureus BSI without endocarditis. The bacterial adhesins of CoNS are less well understood but may be capsular polysaccharides.
The rate of bacteremia associated with common events and medical procedures is shown in Table 37.3 . Although dental procedures, such as drainage of an abscess, gingival surgery, and tooth extraction, are particularly likely to cause bacteremia, transient bacteremia after common daily activities such as tooth brushing is far more frequent. Thus, an antecedent event clearly responsible for endocarditis is only rarely identified.
| Frequency of Bacteremia per Episode | ||
|---|---|---|
| Procedure | Mean (%) | Range (%) |
| Tooth extraction | 60 | 18–85 |
| Periodontal surgery | 88 | 60–90 |
| Tonsillectomy | 35 | 33–38 |
| Rigid bronchoscopy | 15 | |
| Tracheal intubation | <10 | 0–16 |
| Urinary tract catheter insertion or removal | 13 | 0–26 |
| Upper endoscopy | 4 | 0–8 |
| Barium enema | 10 | 5–11 |
| Colonoscopy | 5 | 0–5 |
| Cardiac catheterization | 2 | 0–5 |
Right-sided endocarditis can be due to a CVC extending into the structurally normal heart. In an experimental model in rabbits, endocarditis only develops when bacteremia is preceded by placement of an intravenous polyethylene catheter across the tricuspid valve into the right ventricle. The catheter acts as a foreign body, abrading endocardial and valvular surfaces and resulting in sterile vegetations that serve as a nidus for infection.
The pathogenesis of endocarditis occurring ≤6 months (early) and >6 months after surgery (late) differ. In the early postoperative period, denuded endothelium and exposed sutures are adjacent to prosthetic material; thrombi can form at these sites with fibrin deposition. Endocarditis can result if bacteremia or fungemia follows, potentially as a result of contamination of intravenous solutions, the cardiac bypass pump, a surgical site, CVC, exposed pacemaker wires, or exposed intracardiac catheters. In addition, bacterial or fungal contamination of the operative site can be caused by infected homografts or the colonized hands of healthcare personnel. Late postoperative endocarditis occurs after re-endothelialization of cardiac and vascular surfaces, with pathophysiology more akin to native valve endocarditis. Pacemaker wires do not become re-endothelialized. Residual intracardiac lesions at the site of prior surgical repair leads to inadequate re-endothelialization and an ongoing higher risk of endocarditis. Emerging literature suggests that there may be differential risks of infective endocarditis depending on the surgical approach, with increased incidence of infective endocarditis when pulmonary valves have been replaced using a transcatheter approach rather than an open surgical approach.
Placement of cardiovascular implantable electronic devices (pacemaker or cardioverter-defibrillator) in children and adolescents is increasing. , Contamination during placement and infection of the pulse-generator pocket can result after device placement. Endocarditis is relatively rare and accounts for approximately 10% of implantable device-associated infections, although younger patients may have more frequent lead-wire infections than older patients. , Complete removal of the device is recommended when a deep pocket infection occurs because of the high risk for relapse and for contaminating the intravascular portion of the device. ,
Ventricular assist devices (VADs) are increasingly being used to support patients awaiting heart transplantation in the pediatric population. Although data are limited, the rate of infectious complications is high, occurring in 18%–59% of patients, and complications include surgical site infections, BSIs, and endocarditis presenting as relapsing BSI. Management of VAD-related infections is highly complex owing to the critical need for the device, presence of intravascular and intracardiac foreign material, and high risk for relapse.
Vegetations can be single or multiple and vary in size from 1–2 mm to several centimeters ( Figs. 37.1 and 37.2 ). Endocarditis can destroy the underlying valve or perforate valve leaflets; rupture the chordae tendineae, papillary muscle, or interventricular septum; or lead to abscess of the valve ring. Healing can result in fibrosis and calcification. Myocardial abscesses and pericarditis also can be present. Vegetations consist of fibrin, platelets, and bacteria. Neutrophils and red blood cells also can be seen ( Fig. 37.3 ).
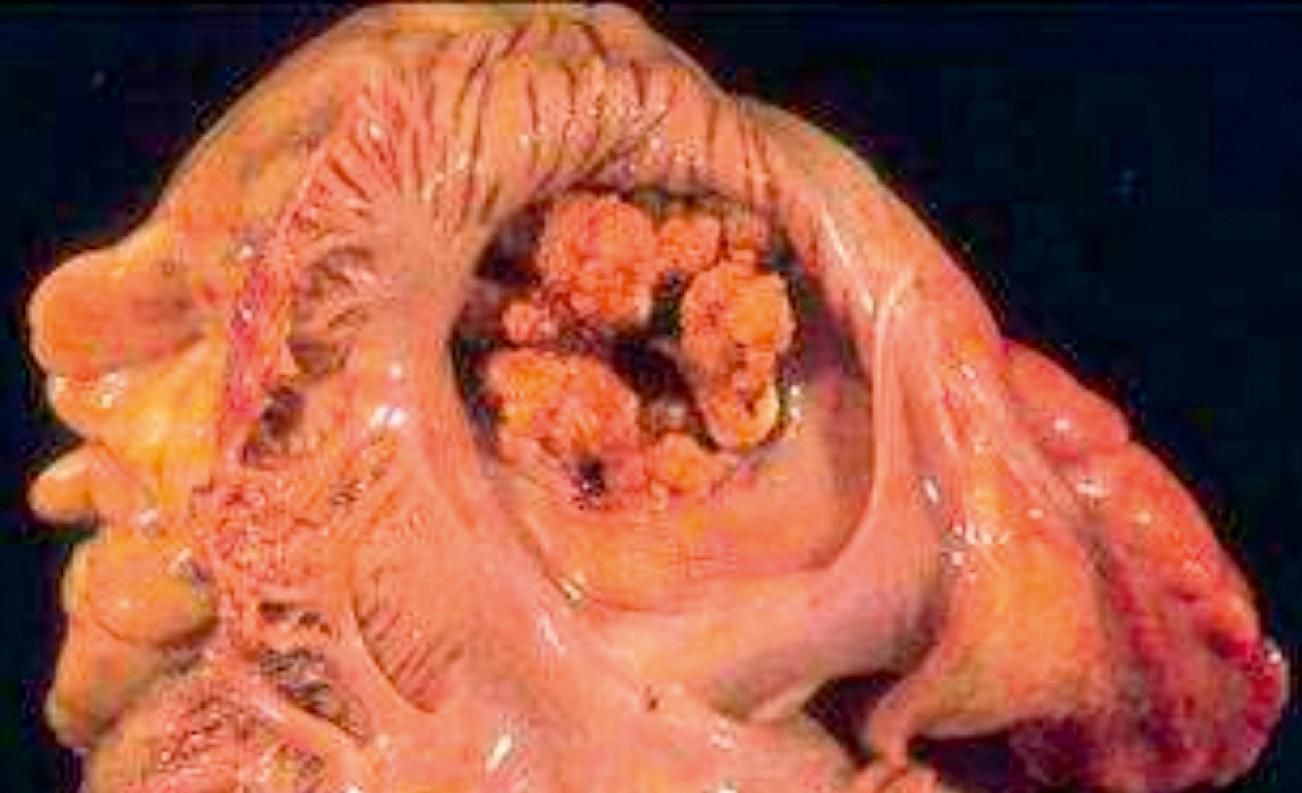
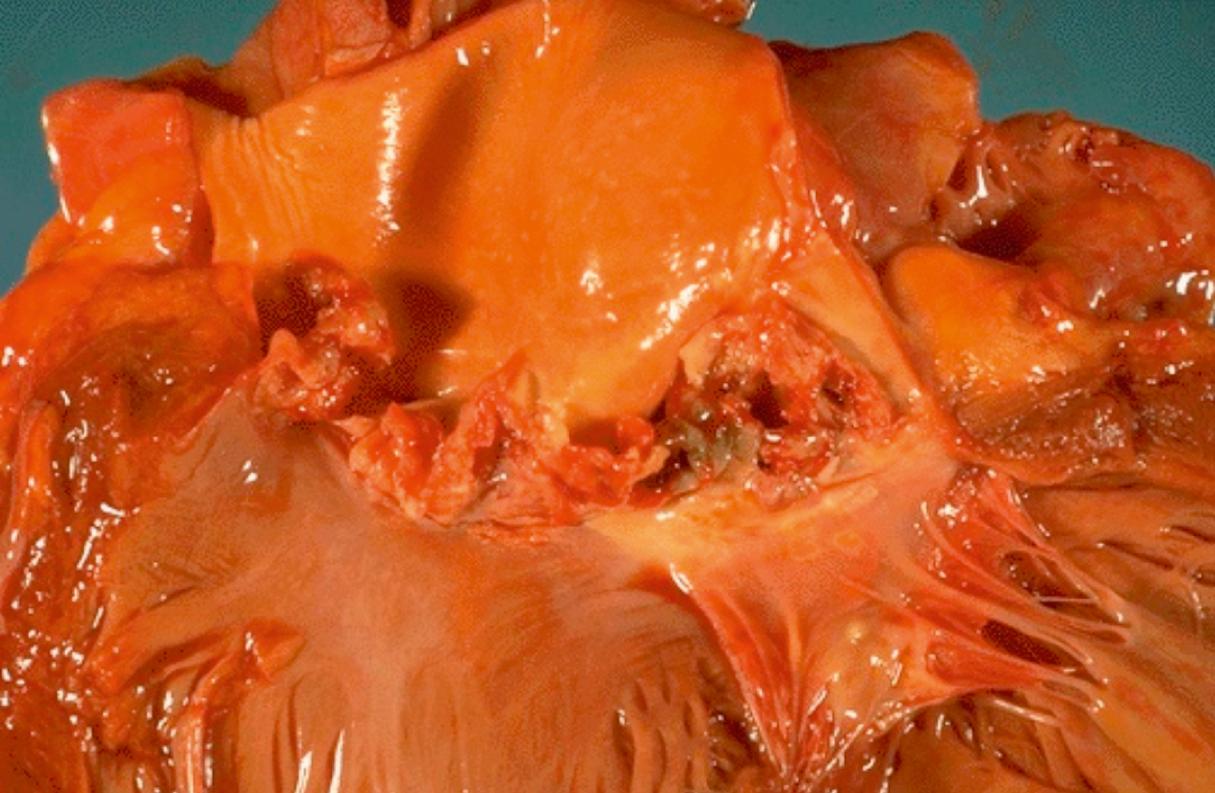
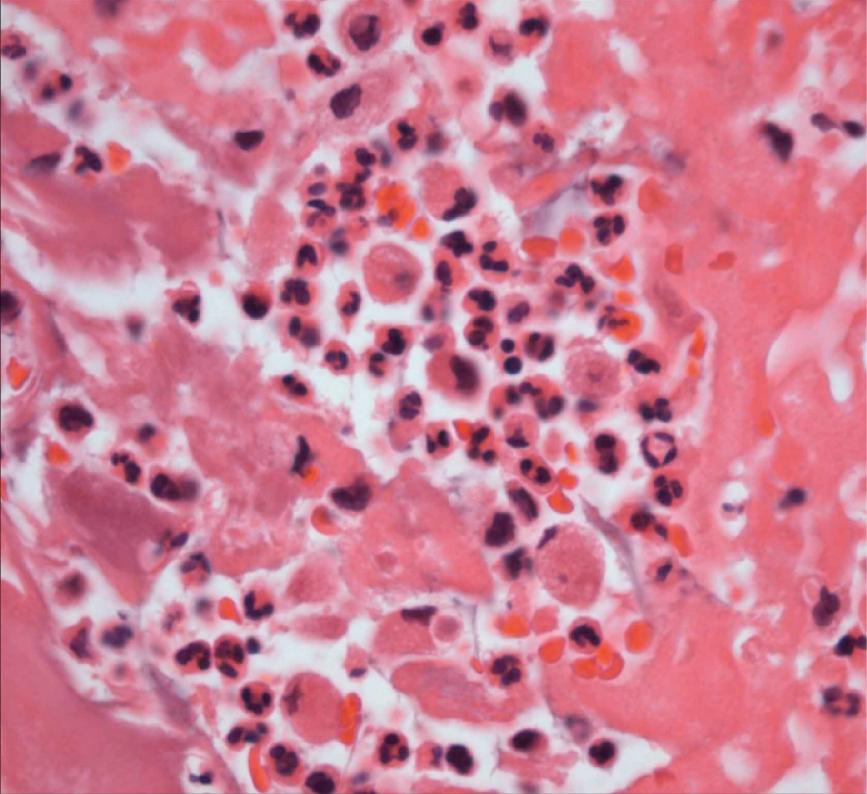
Pathologic processes after emboli to distal organs consist of abscesses, infarction, or both. In the kidney, glomerulonephritis can also occur, characterized by subepithelial deposits containing immunoglobulin G (IgG), IgM, IgA, or complement. Mycotic aneurysms are detected most commonly in the central nervous system and arise from direct bacterial invasion, embolic occlusion, or immune complex deposition within the arterial wall. Intracerebral or subarachnoid hemorrhage also can result from emboli, while emboli to the lung can result in pneumonia, lung abscess, empyema, or effusion.
Classically, the clinical manifestations of endocarditis were categorized as either subacute, generally manifesting with a prodrome of moderate illness and nonspecific symptoms for several weeks, or acute, with a short prodrome and a sepsis-like presentation. However, individual cases can have mixed features, and the presentation does not always predict the infecting microorganism.
Common symptoms of endocarditis in children are shown in Table 37.4 . The subtle and nonspecific nature of symptoms can delay diagnosis for weeks. , Endocarditis should be suspected in patients with (1) congenital heart disease or other risk factors for endocarditis with unexplained fever and fatigue (or anemia) that can remit temporarily when oral antibiotics are prescribed; (2) congenital heart disease with new onset of congestive heart failure or conduction abnormalities, especially if accompanied by fever; (3) abrupt onset of septicemia or appearance of vascular lesions in soft tissues or mucous membranes; and (4) a structurally normal heart with blood cultures persistently positive after optimal treatment is instituted or after removal of a CV.
| Symptom | Frequency (%) |
|---|---|
| Fever | 75–100 |
| Malaise | 50–75 |
| Anorexia/weight loss | 25–50 |
| Heart failure | 25–50 |
| Arthralgia | 17–50 |
| Chest pain | 0–25 |
| Neurologic symptoms (focal neurologic deficit, aseptic meningitis) | 0–25 |
| Gastrointestinal symptoms | 0–50 |
| Sign | Frequency (%) |
|---|---|
| Fever | 75–100 |
| Splenomegaly | 50–75 |
| Petechiae | 21–50 |
| Embolic phenomenon | 25–50 |
| New or changed murmur | 21–50 |
| Clubbing | 0–10 |
| Osler nodes | 0–10 |
| Roth spots | 0–10 |
| Janeway lesions | 0–10 |
| Splinter hemorrhages | 0–10 |
| Conjunctival hemorrhages | 0–10 |
| Abnormal Laboratory Finding | Frequency (%) |
|---|---|
| Positive blood culture | 75–100 |
| Elevated erythrocyte sedimentation rate | 75–100 |
| Anemia | 75–90 |
| Presence of rheumatoid factor | 25–50 |
| Hematuria | 25–50 |
| Low serum complement | 5–40 |
Vegetations, especially those on valve leaflets, can break off into the circulation. Significant morbidity can result from infectious emboli to the lungs (right-sided endocarditis) or to the central nervous system, kidney, spleen, and extremities (left-sided endocarditis). Embolic events can be the predominant clinical feature in some cases. , Patients with fever and unexplained neurologic symptoms or with apparent pulmonary emboli, particularly if multifocal, should be evaluated promptly for endocarditis.
Table 37.4 shows the frequency of physical findings in endocarditis. It may be difficult to discern subtle changes in an existing murmur or detection of a new murmur in a febrile child with tachycardia. In patients with rapidly progressing infection, physical examination may not reveal the classic signs of endocarditis, and thus endocarditis is suspected because of underlying heart disease or persistently positive blood cultures or embolic events, including splinter hemorrhages (i.e., dark linear streaks in the nail beds). Signs due to deposition of immune complexes composed of bacterial antigens, antibodies, and complement in peripheral tissue are rare in children. These include Osler nodes, which are small (2−15 mm), painful, purplish lesions in the pulp of the fingers or toes that persist for hours or days; Janeway lesions, that is, painless hemorrhagic macular plaques on the palms or soles due to emboli that persist for days; or Roth spots, which are small hemorrhagic retinal lesions with a pale center, usually near the optic disk.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here