Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Blastomycosis (North American blastomycosis) is an endemic mycosis that primarily causes infection of the lungs and skin and, less commonly, infection of the osteoarticular and genitourinary systems ( Table 308-1 ).
| CHARACTERISTIC | DESCRIPTON |
|---|---|
| Causative fungi | Blastomyces dermatitidis and other Blastomyces spp. |
| Primary geographic distribution | South central and north central regions of the United States and Canadian provinces surrounding the Great Lakes, Africa, and Middle East |
| Primary route of acquisition | Respiratory (inhalation of conidia) |
| Principal site of disease | Lungs and skin are most common sites. Disseminated disease to bone, central nervous system, liver, spleen, and lymph nodes is uncommon but serious. Pulmonary disease with acute respiratory distress syndrome may occur |
| Opportunistic infection in compromised hosts | Diffuse pneumonia and widespread infections common in patients with T-cell defects or during high-dose corticosteroid therapy or TNF-α inhibitors |
| Drug of choice for most patients | All patients with blastomycosis should receive antifungal therapy; itraconazole for mild to moderate pulmonary or disseminated disease Patients with severe pulmonary or disseminated disease, CNS involvement and all immunocompromised hosts should be treated with lipid formulation amphotericin B |
| Alternative therapy | Voriconazole, posaconazole |
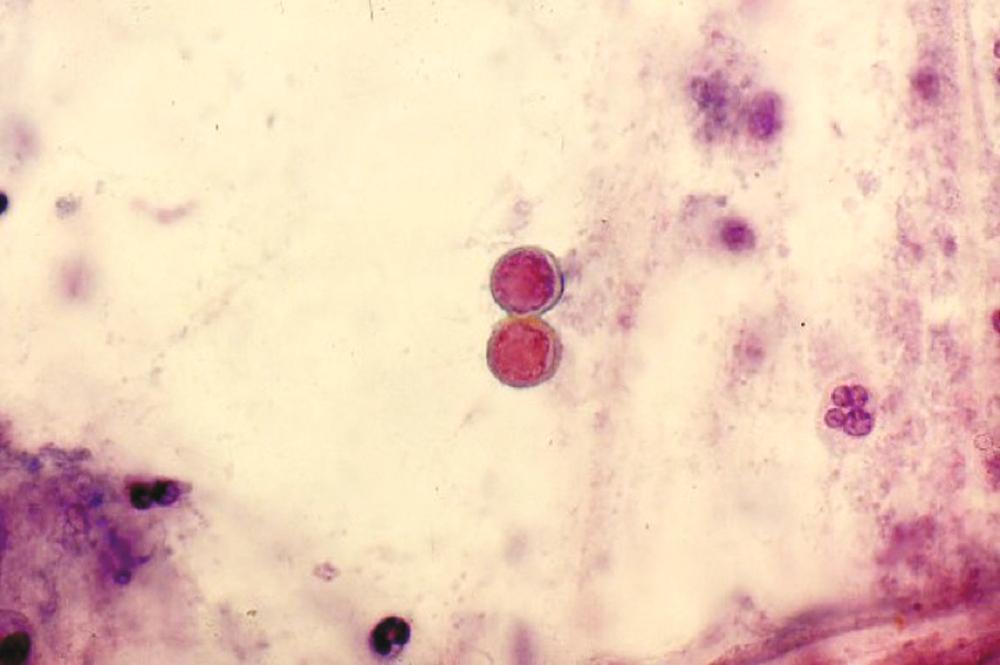
Blastomyces spp. are thermally dimorphic fungi, and numerous new species have been recently identified. In the mold phase in the environment, the organism produces conidia, which when aerosolized and inhaled cause infection. At 37° C on culture media and in tissues, the organism is a distinctive appearing yeast that is 5 to 20 µm in diameter with a thick refractile cell wall, and single broad-based buds ( Fig. 308-1 ).
Blastomyces spp. exist in diverse geographic areas worldwide, but most cases of blastomycosis are reported from the south central and north central regions of the United States and the Canadian provinces surrounding the Great Lakes. Blastomycosis also occurs in Africa and the Middle East. The natural niche of B. dermatitidis is thought to be soil and decaying vegetation, especially in areas associated with rivers and lakes. Although most cases occur sporadically, several well-described outbreaks have occurred, often in association with activities along waterways. The typical patient with blastomycosis is a middle-aged man who has an outdoor occupation or hobby. Blastomycosis develops in both hunters and their dogs in endemic areas.
After the inhalation of conidia, B. dermatitidis transforms into the yeast phase and causes pulmonary infection. Although most patients manifest only pulmonary symptoms, others have cutaneous lesions in the absence of other organ involvement or have disseminated infection. It is likely that most patients have asymptomatic hematogenous dissemination after the initial pulmonary infection, so cutaneous lesions should be viewed as a manifestation of hematogenous spread of the organism. Except in rare instances, blastomycosis is not acquired by inoculation. Cellular immunity involving T lymphocytes and macrophages is an important component of the host response to infection with B. dermatitidis , but neutrophils also play a role. Most patients with blastomycosis are healthy. Patients who are immunosuppressed are more likely to have severe disease. Infection in an immunosuppressed host can occur after new exposure to Blastomyces spp. or less often, from reactivation of a latent focus of infection acquired years earlier.
Most patients with acute pulmonary blastomycosis are asymptomatic or are thought to have community-acquired pneumonia. Patients with acute pneumonia have fever, malaise, a nonproductive cough, and a pulmonary infiltrate that shows lobar or multilobar patchy or nodular infiltrates on chest radiographs. Development of classic, verrucous draining skin lesions is a strong clue for blastomycosis.
Chronic pulmonary blastomycosis must be differentiated from tuberculosis ( Chapter 299 ), other fungal infections, and lung cancer ( Chapter 177 ). Fever, night sweats, weight loss, fatigue, cough, sputum production, hemoptysis, and dyspnea are commonly noted. On chest radiograph, the lesions are cavitary, nodular, fibrotic, or masslike in appearance. Hilar and mediastinal lymphadenopathy and pleural effusions are not commonly observed. Overwhelming pulmonary disease with acute respiratory distress syndrome (ARDS) occurs infrequently.
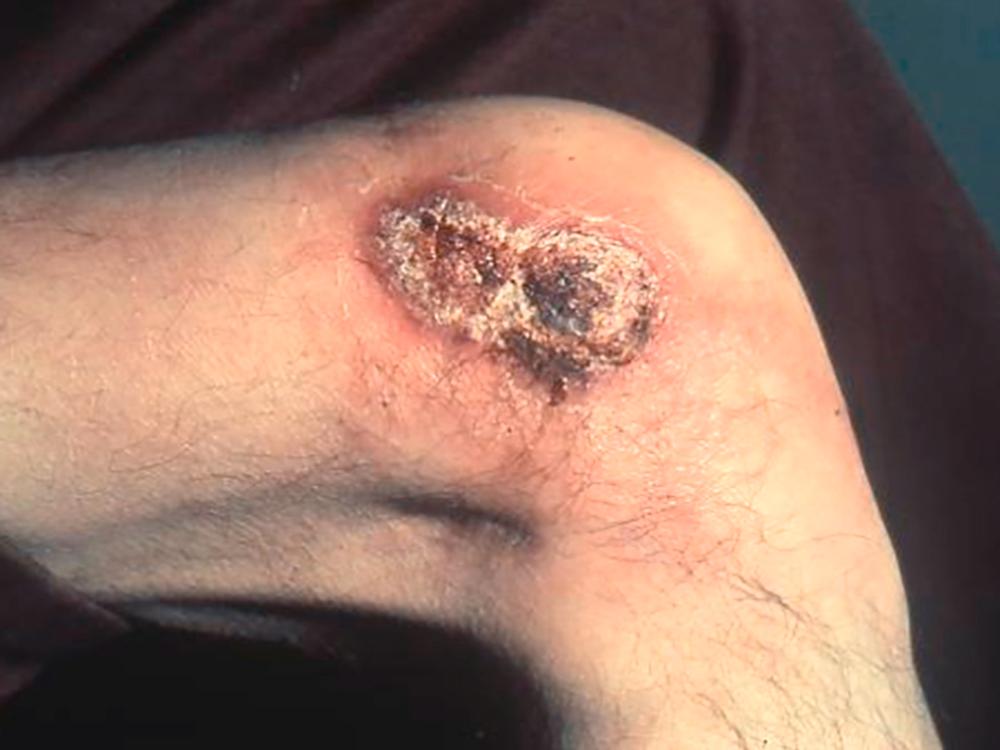
Cutaneous lesions are the most common manifestation of disseminated blastomycosis. The lesions, which can be single or multiple, are usually well circumscribed and painless ( Fig. 308-2 ). Papules, nodules, plaques, and ulcers can occur; often they are verrucous and develop multiple punctate draining areas in the center. The lesions clinically mimic those associated with non-tuberculous mycobacteria, other fungal infections, or pyoderma gangrenosum ( Table 408-5 ). An uncommon manifestation, seen in immunocompromised patients, is the appearance of pustular lesions that readily reveal the organism when aspirated.
Another manifestation of disseminated blastomycosis is osteoarticular involvement. Osteomyelitis can be associated with contiguous skin lesions or can appear at sites distant from cutaneous lesions. Genitourinary involvement may be asymptomatic or be associated with signs of prostatism and the presence of a nodule on digital examination. Infrequent findings include laryngeal and oropharyngeal nodules; ocular lesions; central nervous system (CNS) involvement with either meningitis or intracerebral mass lesions; and dissemination to the liver, spleen, and lymph nodes.
The definitive diagnostic test for blastomycosis is growth of the organism from an aspirate, tissue biopsy specimen, sputum, or body fluid. The mold phase takes several weeks to grow at room temperature. Once growth has occurred, the organism can be rapidly identified as Blastomyces spp. with a highly specific and sensitive DNA probe.
In this setting, the distinctive large, thick-walled yeast with a single broad-based bud (see Fig. 308-1 ) may be identified by: histopathologic examination of cutaneous or pulmonary lesions; cytologic examination of sputum, bronchoalveolar lavage fluid, or other tissue fluids; or calcofluor fluorescent staining of sputum or purulent material from pustular lesions. Identification of characteristic organisms on histopathology allows a tentative diagnosis of blastomycosis and initiation of antifungal therapy before culture results are known.
An enzyme immunoassay for B. dermatitidis cell wall antigens is available for use on serum, urine, or bronchial alveolar lavage fluid. The sensitivity of this assay varies with different reports. Because B. dermatitidis and H. capsulatum share many cell wall antigens, this assay is often positive in patients with histoplasmosis, as well as in those with blastomycosis.
It is helpful to obtain a bone scan in all patients with disseminated blastomycosis because of its propensity to infect bone.
With the exception of patients who have acute pulmonary blastomycosis that has totally resolved before the diagnosis is established, all patients with blastomycosis should be treated with an antifungal agent. Patients who have mild-to-moderate pulmonary or disseminated blastomycosis should be treated with itraconazole (oral solution or capsules 200 mg three times a day for 3 days then once or twice daily for 6 to 12 months) to achieve mycologic cure and prevent relapse. If itraconazole is not tolerated, voriconazole or posaconazole can be used under expert guidance. The echinocandins are not active against B. dermatitidis and should not be used.
Patients who have severe pulmonary or disseminated blastomycosis, all patients with CNS infection, and most immunosuppressed patients should be treated initially with a lipid formulation of amphotericin B. The dosage is 3 to 5 mg/kg IV daily, except for CNS infection, for which 5 mg/kg IV daily should be used. After clinical improvement has occurred, usually within 1-2 weeks, therapy can be changed to itraconazole (oral solution or capsules, 200 mg three times a day for 3 days then twice daily) for a total of at least 12 months of therapy. For all patients who are treated with itraconazole, serum itraconazole levels should be determined when steady state has been reached after 2 weeks of therapy to ensure adequate absorption. Serum concentrations should be greater than 1 µg/mL.
Corticosteroids have been helpful as adjunctive therapy for patients with blastomycosis-associated acute respiratory distress syndrome (ARDS) ( Chapter 90 ), but this practice remains controversial. In patients who remain seriously hypoxic despite mechanical ventilation, extracorporeal membrane oxygenation may be lifesaving.
The prognosis for patients with pulmonary or disseminated blastomycosis is excellent; more than 90% are cured. Most reported deaths occur in patients with overwhelming pneumonia and ARDS. In patients who require mechanical ventilation, the mortality rate is about 40%.
Histoplasmosis is the most common endemic mycosis in the United States. Most infections are self-limited, but the organism has the ability to cause acute and chronic pulmonary infections as well as disseminated infection ( Table 308-2 ).
| CHARACTERISTIC | DESCRIPTION |
|---|---|
| Causative fungi | Histoplasma capsulatum var capsulatum and other Histoplasma spp. |
| Primary geographic distribution | North and Central America. Endemic in the Mississippi and Ohio River Valleys, with extension into the St. Lawrence Basin |
| Primary route of acquisition | Respiratory (inhalation of microconidia) |
| Principal site of disease | Lungs and mediastinal lymph nodes most common; spread to skin, bone marrow, meninges, and other viscera uncommon but serious |
| Opportunistic infection in compromised hosts | Diffuse pneumonia and widespread infections common in patients with T-cell defects or during high-dose corticosteroid therapy or TNF-α inhibitors |
| Drug of choice for most patients | No antifungal is required for uncomplicated pneumonia; itraconazole for mild to moderate pulmonary histoplasmosis. Lipid formulation amphotericin B for moderate to severe/disseminated disease |
| Alternative therapy | Fluconazole, posaconazole |
Histoplasma capsulatum var capsulatum is a thermally dimorphic fungus. In the environment and at temperatures lower than 35° C, it exists as a mold that produces conidia. It produces both tuberculate macroconidia, which are helpful for identification purposes in the laboratory, and microconidia, which are the infectious form. In tissues and at 35 to 37° C, H. capsulatum converts into 2- to 4-µm oval yeasts that reproduce by budding and parasitize macrophages. African histoplasmosis is caused by a different subspecies, H. capsulatum var duboisii , and has different disease manifestations, particularly involvement of skin, subcutaneous tissues, lymph nodes, and bone but only rarely the lungs or other internal organs.
Histoplasmosis, though found worldwide, is primarily a disease of North and Central America. H. capsulatum is endemic in the Mississippi and Ohio River Valleys, with extension into the St. Lawrence Basin; microfoci exist in discrete isolated areas in several eastern states and may represent novel species. Soil, caves, and abandoned buildings that contain high concentrations of bird or bat guano support growth of the organism. Every year, thousands of individuals who live in areas endemic for H. capsulatum are infected, but infections can occur in persons living in essentially every U.S. state. Most cases are sporadic, and the exact source of exposure is unknown. Point source outbreaks occur in association with disruption of soil; cleaning attics, bridges, or barns; renovating or tearing down old structures laden with guano; and spelunking.
After inhalation of microconidia into the alveoli, a localized pulmonary infection ensues. Neutrophils and macrophages phagocytize the organism, which is now in the yeast phase. The organism is able to survive and travels within macrophages to the hilar and mediastinal lymph nodes and throughout the reticuloendothelial system by hematogenous dissemination. Such dissemination, which probably occurs in most persons who are infected, is typically asymptomatic in normal hosts. After several weeks, T-cells specifically sensitized by Histoplasma antigens activate macrophages, which are then able to kill the intracellular fungi.
The extent of disease is determined by both the number of conidia inhaled and the immune response of the host. A small inoculum can cause severe infection in immunosuppressed patients. Persons at greatest risk include patients who have acquired immunodeficiency syndrome (AIDS), lymphoma, or are organ transplant recipients, as well as patients who are taking corticosteroids or tumor necrosis factor inhibitors. Reactivation of latent infection occurs in patients who have deficient cell-mediated immunity, as evidenced by the occurrence of histoplasmosis in immunosuppressed persons who grew up in the endemic area but have not returned to that area for years.
Most individuals infected with H. capsulatum are asymptomatic. Patients who develop symptomatic pulmonary infection usually have a self-limited illness that begins several weeks after exposure and is characterized by fever, chills, fatigue, nonproductive cough, anterior chest discomfort, and myalgias. The chest radiograph typically shows a patchy lobar or multilobar reticulonodular infiltrate.
Acute pulmonary histoplasmosis can be life-threatening. In patients who have experienced heavy exposure to Histoplasma spp., as might occur during the demolition of old buildings or while spelunking in a heavily infested cave, and in those who are immunosuppressed, high spiking fevers, chills, prostration, dyspnea, and cough are prominent, and ARDS can occur.
Chronic cavitary pulmonary histoplasmosis is a progressive, often fatal form of histoplasmosis that develops almost exclusively in older patients who have chronic obstructive pulmonary disease ( Chapter 76 ). Symptoms include fever, fatigue, anorexia, weight loss, cough productive of purulent sputum, and hemoptysis. On chest radiography the usual findings are unilateral or bilateral upper lobe infiltrates with multiple cavities and extensive fibrosis in the lower lobes.
The mediastinal and hilar lymph nodes frequently calcify as the infection resolves; years later they can erode into bronchi and cause hemoptysis and expectoration of broncholiths. Granulomatous mediastinitis is an uncommon syndrome characterized by continuing inflammation and necrosis in the mediastinal lymph nodes. The enlarged nodes are readily apparent on chest radiographs, and computed tomography (CT) shows central necrosis and, in some cases, impingement on adjacent structures, including the esophagus, airways, and blood vessels. Although the symptoms usually resolve without treatment, obstructive syndromes can be severe, and the nodes can persist for years.
Fibrosing mediastinitis is a rare complication of histoplasmosis in which the host responds to the infection with an inappropriate excessive fibrotic response. Obstruction of the airways, superior vena cava, or pulmonary arteries and veins can occur with resultant progressive right heart failure and respiratory insufficiency. Bilateral obstruction of the pulmonary vasculature is less common than unilateral involvement and carries a worse prognosis. Mediastinal widening is seen on chest radiographs, and CT and angiography define the extent of invasion and obstruction of mediastinal structures. Pericarditis ( Chapter 62 ) is a manifestation of a local inflammatory reaction to adjacent histoplasmosis.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here