Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Key concepts in the management of a critically ill patient with valvular heart disease are the use of echocardiography to provide an accurate diagnosis of disease severity and the appropriate use of invasive hemodynamic monitoring to optimize loading conditions. Physical examination is not reliable for diagnosing the presence or severity of valvular heart disease, particularly in patients with acute hemodynamic compensation. Handheld echocardiography may provide clues to the presence of valve disease but does not replace the need for a complete diagnostic study when this diagnosis is suspected. With acute valve regurgitation or prosthetic valve thrombosis, urgent surgical intervention may be necessary. In critically ill patients who are poor surgical candidates, rapid relief of valve obstruction can be lifesaving, including transcatheter balloon valvotomy for rheumatic mitral stenosis or transcatheter aortic valve implantation for calcific aortic stenosis ( Fig. 76.1 ).
Mitral regurgitation may be caused by disease or the distortion of any component of the mitral valve apparatus—the mitral annulus, leaflets, chordae, and papillary muscles—and by alterations in left ventricular (LV) geometry or systolic function ( Fig. 76.2 ). Primary causes of acute mitral regurgitation include endocarditis and myxomatous valve disease (mitral valve prolapse) with spontaneous chordal rupture Bacterial endocarditis results in acute mitral regurgitation because of the destruction of valve tissue, often with leaflet perforation.
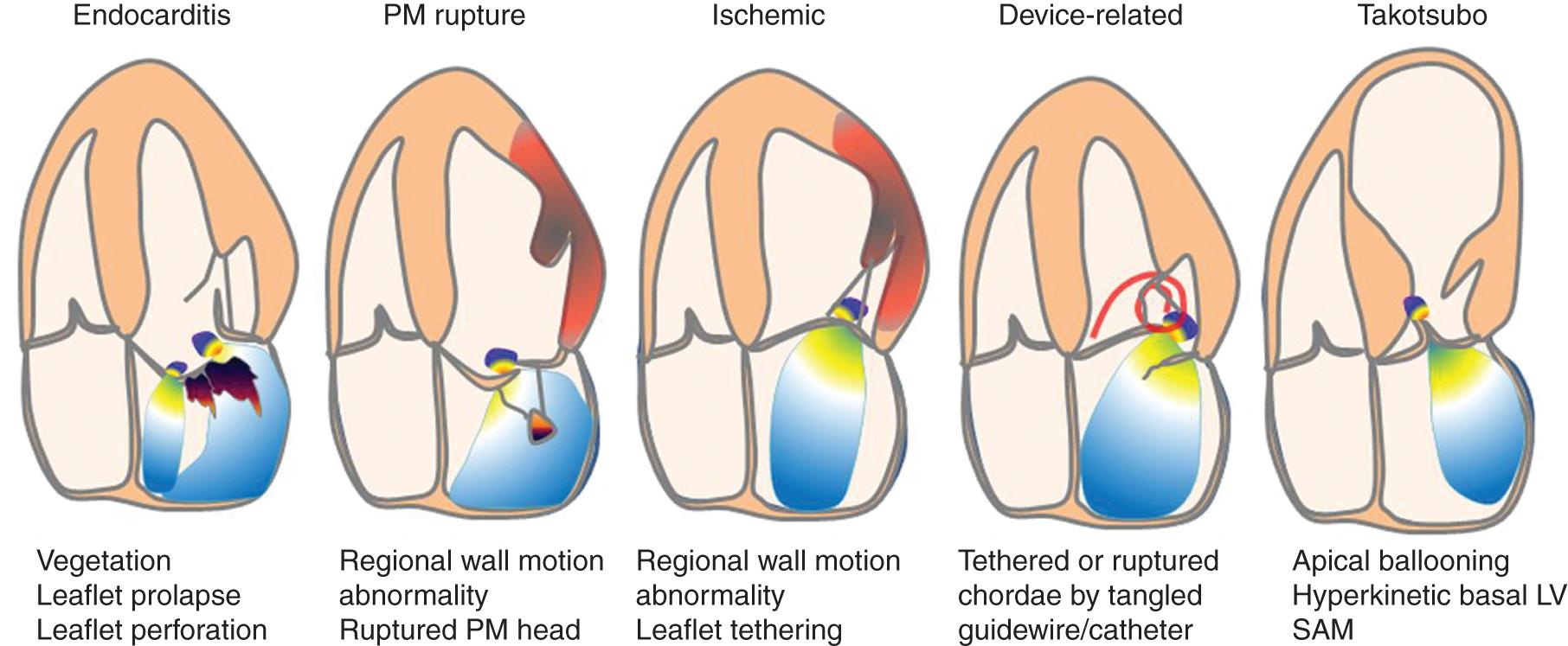
Acute secondary mitral regurgitation may be the result of an acute cardiomyopathy (e.g., takotsubo cardiomyopathy) or coronary disease with acute myocardial infarction or papillary muscle rupture Moderate to severe mitral regurgitation caused by papillary muscle involvement or regional myocardial dysfunction complicates 12% of acute myocardial infarctions and is associated with an increased risk of heart failure or death. ,
Iatrogenic acute mitral regurgitation is a rare complication of balloon mitral valvotomy. Acute mitral regurgitation also may complicate the use of transaortic axial flow ventricular assist devices if the catheter interferes with normal mitral valve closure or disrupts the valve apparatus when the device is removed.
The management of acute mitral regurgitation differs depending on the cause, making the identification of the correct etiology essential during evaluation.
Acute mitral regurgitation presents with acute pulmonary edema and is a surgical emergency ( Figs. 76.3 and 76.4 ). Mitral chordal rupture results in the acute presentation of heart failure, often in patients unaware of the diagnosis of mitral valve prolapse. Patients with mitral valve perforation caused by endocarditis present with pulmonary edema superimposed on the signs and symptoms of endocarditis. Papillary muscle rupture or dysfunction after myocardial infarction (MI) usually presents several days after acute MI; in some cases, the initial presentation is of acute pulmonary edema, with the MI being clinically silent.
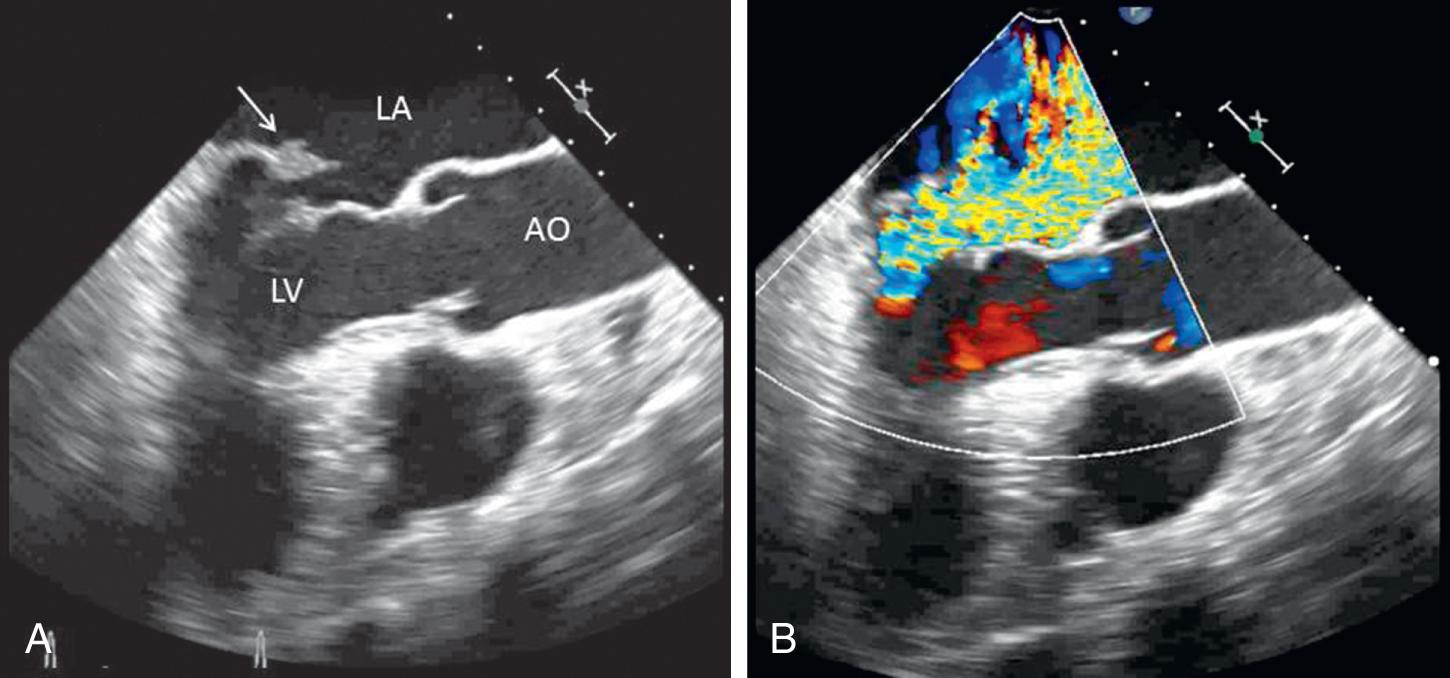
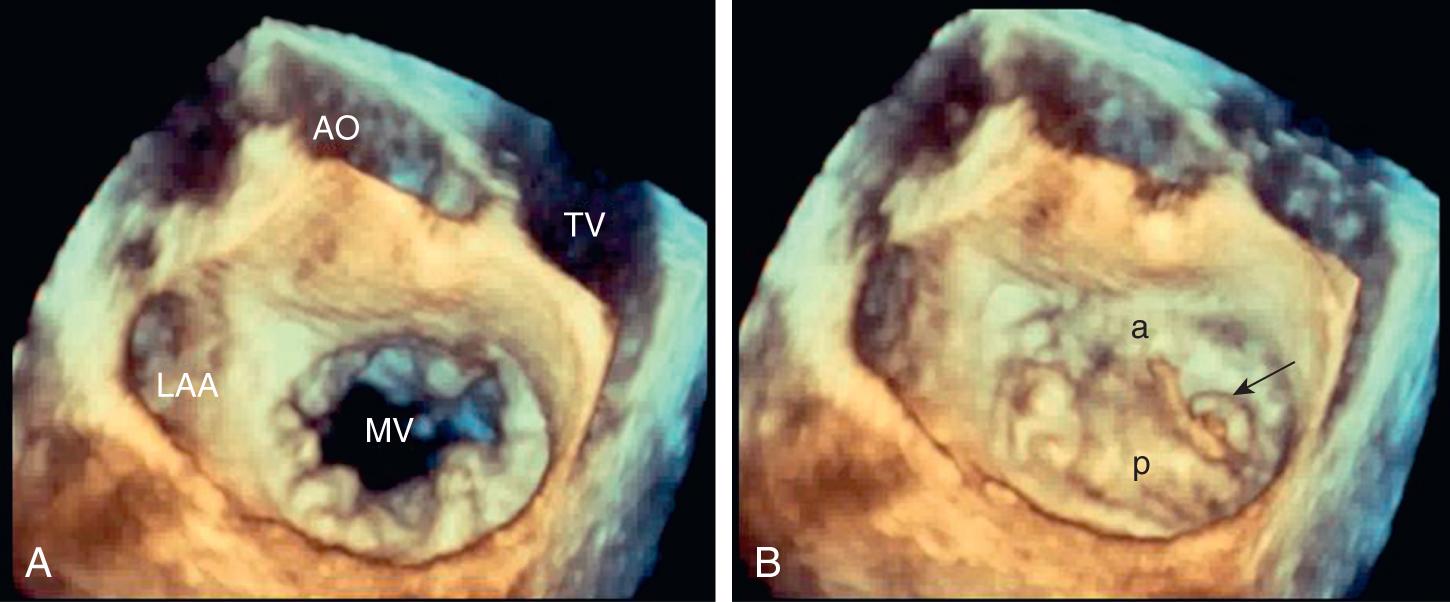
Chronic mitral regurgitation is usually well tolerated even when there is a superimposed hemodynamic load such as systemic infection, pregnancy, or trauma. However, mitral regurgitant severity may acutely worsen by at least two mechanisms. An increase in afterload, for example, with a hypertensive crisis, may increase regurgitant severity caused by an increased driving pressure from the left ventricle to the left atrium. An alteration in the LV geometry, for example, with ventricular dilation caused by decompensated heart failure may change the orientation of the papillary muscles such that leaflet closure is impaired, resulting in a larger regurgitant orifice area. In this situation, a vicious cycle may ensue where LV dilation worsens mitral regurgitant severity, which increases LV dilation.
A high level of clinical suspicion is needed to make a diagnosis of acute mitral regurgitation ( Table 76.1 ). Acute pulmonary edema often obscures the signs and symptoms of the underlying disease process. The classical finding is a holosystolic murmur at the apex, radiating to the axilla. Although there is some correlation between murmur loudness and regurgitant severity with chronic regurgitation, the murmur may be soft with acute severe mitral regurgitation. In patients with severe mitral regurgitation after MI, a murmur cannot be appreciated in up to 50% of patients.
| Physical examination |
|
| Echocardiography (transthoracic) |
|
| Transesophageal echocardiography |
|
| Right heart catheterization |
|
| Chest computed tomography | Sensitive and specific for diagnosis of aortic dissection |
| Angiography | Used when coronary angiography is needed |
Thus in patients presenting with acute pulmonary edema or cardiogenic shock, prompt echocardiography is essential. Transthoracic images are often diagnostic, allowing the identification of the etiology of valve dysfunction, quantitation of regurgitant severity, estimation of pulmonary pressures, and measurement of ventricular size and systolic function. If transthoracic images are nondiagnostic, transesophageal echocardiography (TEE) can be performed at the bedside in the intensive care unit (ICU). TEE provides excellent images of valve anatomy and the Doppler evaluation of valve function.
Other diagnostic tests are based on the clinical presentation. Multiple blood cultures should be obtained in febrile patients with systemic or pulmonary edema to exclude the possibility of endocarditis. In patients with an abnormal electrocardiogram (ECG), chest pain, or a history of coronary artery disease, coronary angiography may be needed.
In patients with acute pulmonary edema or cardiogenic shock after MI, the differential diagnosis includes acute mitral regurgitation, a ventricular septal defect, or a contained rupture of the ventricular free wall. All these possibilities can be diagnosed by echocardiography in an experienced center.
When imaging is nondiagnostic or discrepant with clinical findings, invasive hemodynamic monitoring with a Swan-Ganz catheter for the measurement of pulmonary pressure and cardiac output may be considered in patients with suspected acute mitral regurgitation. At the time of placement, oxygen saturations in the right atrium, right ventricle, and pulmonary artery should be measured. A ventricular septal defect results in a “step-up” in oxygen saturation between the right atrium and ventricle secondary to oxygenated blood from the left ventricle entering the right ventricle. The pulmonary artery balloon-occluded (wedge) pressure tracing should be examined for the presence of a giant “v-wave,” which supports the diagnosis of acute mitral regurgitation but is not always present.
In patients with acute heart failure associated with chronic mitral regurgitation, management is directed at treating the process leading to decompensation and optimizing loading conditions ( Box 76.1 ). For example, in a patient with a systemic infection, treating the infection, controlling fever and tachycardia, and invasive monitoring to optimize preload and afterload are used. Medical therapy typically includes afterload reduction with nitroprusside or other vasodilators and preload reduction with diuretics. The goal is to support the patient through the period of decompensation. Typically, hemodynamics returns to the baseline-compensated state after the acute illness.
Accurate diagnosis with echocardiography; differentiates acute valve dysfunction from acute decompensation with chronic valve disease.
Treat the underlying disease process associated with decompensation (endocarditis, acute myocardial infarction, anemia, etc.).
Optimize loading conditions using diuretics, vasodilators, and other agents with invasive hemodynamic monitoring.
Consult the cardiac surgery team as soon as the diagnosis is made.
Intraaortic balloon pump for acute mitral regurgitation.
Consider surgical or percutaneous intervention.
In contrast, acute severe mitral regurgitation is a medical and surgical emergency. Mortality is extremely high without the restoration of valve competence; even with prompt valve surgery, the 30-day mortality is 23%. Medical stabilization should occur concurrently with consultation by a cardiac surgeon. Acutely, the placement of an intraaortic balloon pump (IABP) provides optimal afterload reduction while improving diastolic coronary blood flow. Advanced circulatory support with extracorporeal membrane oxygenation (ECMO) or a ventricular assist device also has been used successfully for patient stabilization in some cases. , However, valve replacement or repair is the definitive therapy for acute severe mitral regurgitation, with the timing and type of intervention dependent on mitral regurgitation etiology and patient factors.
Spontaneous chordal rupture can usually be treated early with mitral valve repair. Some high-risk patients may be candidates for mitral transcatheter edge-to-edge repair (TEER) with placement of a clip that attaches to both the anterior and posterior leaflet, reducing mitral regurgitation severity while creating a double-orifice valve in diastole.
In patients with endocarditis, acute severe mitral regurgitation is an indication for early surgical intervention (during the initial hospitalization), without waiting for completion of antibiotic therapy. Other indications for early surgical intervention in patients with endocarditis include any valve dysfunction causing heart failure, paravalvular abscess formation, infection caused by highly resistant organisms, or persistent infection despite appropriate antibiotic therapy. In a large prospective multicenter study, early surgery was associated with a lower mortality than medical therapy (12% vs. 21%). Valve repair is preferred but may not be possible, depending on the extent of tissue destruction.
In patients with acute ischemic mitral regurgitation, treatment depends on the exact etiology of valve dysfunction. In patients with acute mitral regurgitation caused by a regional wall-motion abnormality, myocardial function and mitral regurgitation may improve after percutaneous revascularization. In these patients, the use of an IABP and medical therapy may be advantageous during the acute episode, with weaning of therapy as myocardial function improves.
Mitral regurgitation caused by partial or complete papillary muscle rupture requires surgical intervention. Although the risk of surgery is high, with an operative mortality rate of about 50%, the outcome is worse with medical therapy, with a mortality of 75% at 24 hours and 95% within 2 weeks after complete papillary muscle rupture. With the use of echocardiography, partial papillary muscle rupture can be recognized; prognosis in these patients depends on the extent of myocardial damage and severity of mitral regurgitation. With partial papillary muscle rupture, some surgeons prefer to stabilize the patient and delay surgery for 6–8 weeks after MI to avoid operating on the necrotic myocardial tissue. However, many patients cannot be stabilized, so acute intervention must be considered. Risk factors for adverse outcomes with surgery include older age, female gender, and poor LV systolic function.
Given the high risk of surgical intervention in many patients with acute severe mitral regurgitation, other options also should be considered ( Table 76.2 ). Mitral TEER reduces mitral regurgitation by clipping together the two leaflets of the mitral valve, both reducing mitral regurgitation severity and creating a double-orifice valve in diastole with some degree of stenosis. TEER is currently recommended for the treatment of symptomatic severe primary mitral regurgitation when surgical risk is high or prohibitive, active infection has been excluded, and valve anatomy is amenable to this approach. , In patients with acute ischemic mitral regurgitation caused by papillary muscle rupture, prompt surgical intervention continues to be recommended, although TEER might be considered in decompensated patients with a high surgical risk.
| Commonly accepted |
|
| Investigational |
|
The most common causes of acute aortic regurgitation are endocarditis, rupture of a congenital aortic leaflet fenestration, blunt trauma, and acute aortic dissection. , Endocarditis results in aortic regurgitation by the destruction of the valve leaflet tissue, with a high percentage of cases also having paravalvular abscess formation. , Aortic dissection results in acute aortic regurgitation either because of the enlargement of the aortic annulus, resulting in inadequate central closure of the leaflets, or extension of dissection into the valve region, resulting in a flail aortic valve leaflet.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here