Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
In 1657 the physician William Harvey wrote,
“Nature is nowhere accustomed more openly to display her secret mysteries than in cases where she shows tracings of her workings apart from the beaten paths; nor is there any better way to advance the proper practice of medicine than to give our minds to the discovery of the usual law of nature, by careful investigation of cases of rarer forms of disease.”
Three centuries later, the medical geneticist Victor McKusick published the first catalog entitled, Mendelian Inheritance in Man (MIM), a complete index of all the genes and genetic disorders known to physicians in 1966. By 1987, this critically important database was made available online as Online Mendelian Inheritance in Man (OMIM) and is still updated daily, where it remains the world’s largest repository for genetic information on craniofacial disorders. As our understanding of the genetic basis for craniofacial anomalies including clefting improves, so too has our ability to identify malformations earlier during fetal development. The last few decades have deepened our understanding of the molecular and cellular basis for craniofacial conditions; coupling this knowledge with continued surgical innovation will undoubtedly lay the groundwork for better management of the defects created by Nature.
Ralph Waldo Emerson wrote,
“A man finds room in the few square inches of the face for the traits of all his ancestors; for the expression of all his history, and his wants”,
and in doing so he succinctly summarized the significance of the craniofacial complex. This region of our anatomy is how others distinguish us, and it is how we see ourselves and therefore has been the study of intense investigation for hundreds of years.
The craniofacial complex is primarily derived from cranial neural crest cells and mesodermal cells that interact through elaborately choreographed movements to form composite tissues including the skeleton, the musculature, the connective tissues, and the epithelial specializations unique to the head region. Both passive cell displacement and active cell migration are involved in gastrulation and neurulation, which establish the head region and set the stage for craniofacial development. As one might suspect, disruptions that affect the timing, rate, or extent of cell migration often result in craniofacial defects; these will be discussed in detail later in the chapter.
The craniofacial complex also houses the organs responsible for sight, taste, smell, and sound and therefore must integrate these sensory inputs into the architecture of the head.
Like other organs and tissues, the craniofacial complex develops through a synchronized series of reciprocal tissue–tissue interactions. In the head, however, these interactions occur between neural and non-neural ectoderm; between ectoderm and endoderm; and between ectoderm, endoderm, and mesoderm. The molecular signals mediating these interactions have, in large part, been identified and accordingly there is a growing understanding of how perturbations in a particular signaling pathway manifest as a given craniofacial malformation. Clearly, decades of research have provided us with an understanding of the etiologies of many craniofacial deformations.
During gastrulation the head region of an embryo is established. Prior to this stage, a zygote must generate sufficient numbers of cells to eventually form an entire embryo and does so by dividing repeatedly to generate a solid, mulberry-like cell mass known as a morula. It is at this stage that the morula traverses the fallopian tube and enters the uterus. With further cell division the morula becomes a blastocyst, which itself has two components. The first component is the trophoblast, which contributes to the formation of placental structures that support and nourish the developing embryo; the second component is the embryoblast that differentiates into the embryo itself.
Gastrulation begins in the third week of human life and it is an event that is restricted to the embryoblast, and it begins when an invagination that spans the length of the pancake-like human embryo is visible. This invagination is called the primitive streak, and cells from the ectoderm stream into this invagination in a head (cranial) to tail (caudal) gradient. As they migrate past the most caudal extent of the primitive streak the cells pass by an anatomical landmark called Hensen’s node and it is in this moment that cells are exposed to chemical morphogens that will influence their ultimate behavior.
Whereas gastrulation creates the three germ layers – ectoderm, mesoderm, and endoderm – it is neurulation that creates the fourth germ layer, the neural crest. During neurulation the flat neural plate is transformed into a neural tube ( Fig. 18.1 ). This transformation has an important impact of facial development, because the medial domain of the neural plate becomes the ventral surface of the neural tube, and lateral domains of the neural plate constitute the dorsal surface of the neural tube ( Fig. 18.1 ). Some of the most important signaling pathways that control craniofacial development specify medial and lateral domains of the neural plate. For example, the medial domain of the anterior neural plate is patterned by signals in the Hedgehog (HH) family of secreted proteins. Sonic Hedgehog (SHH) is expressed in this midline domain around the time of neurulation and there its primary function is to repress activity of the transcription factor PAX6 (see Fig. 18.1 ). PAX6 is a master transcriptional regulator of eye development. SHH normally represses PAX6 function in this medial domain where the expression domains of the two molecules overlap; as a consequence, a single PAX6-expressing eye domain separated into two bilaterally symmetrical PAX6-positive eye fields. When SHH signaling is lost or blocked, such as happens in congenital cases of holoprosencephaly (HPE), PAX6 expression persists in the medial domain. Consequently, the single PAX6-positive eye field is not subdivided, and the affected fetus exhibits cyclopia ( Fig. 18.1 ). Other midline structures are also affected in HPE : the nose may be completely absent or fetuses may have in place of the nose a tubular-shaped structure called a proboscis. In less severe cases of HPE the affected fetus’ eyes can be very closely set. In milder forms of HPE the affected individual can exhibit hypotelorism ; in a microform of HPE, patients may only exhibit a single central incisor. All of these anomalies share a common feature: they represent a collapse in the mediolateral growth of the craniofacial complex, which can be attributable to disruptions in midline HH signaling.
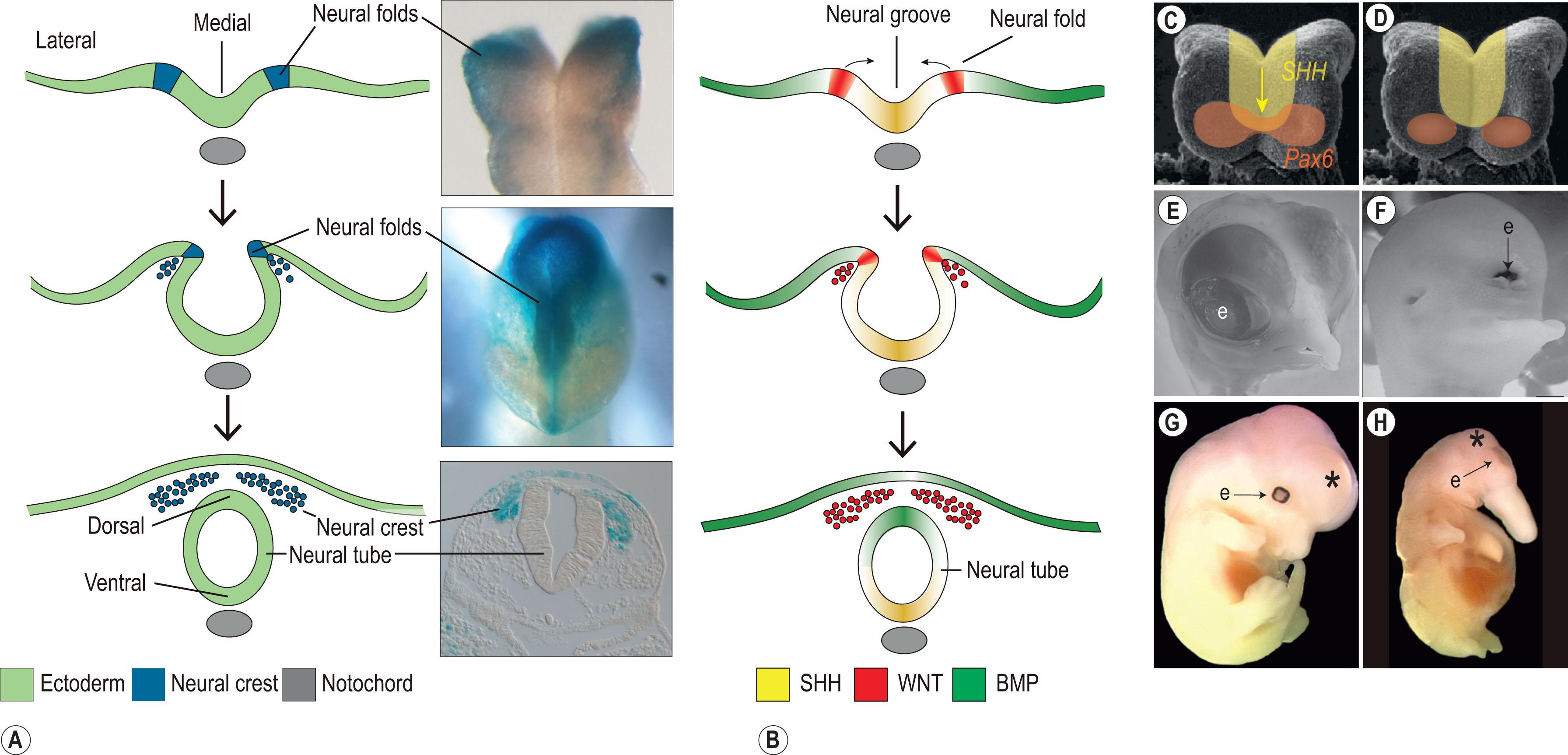
SHH is involved in specifying the medial domain of the neural plate; the lateral domains of the neural plate are specified by signals in the bone morphogenetic protein (BMP) family. Like HH proteins, BMPs are secreted growth factors and a gradient of BMP signaling is instrumental in defining which region of the neural plate will give rise to neuroectoderm (and thus form the brain) and which region will give rise to non-neural ectoderm (and thus form epidermis). Just as loss of SHH can lead to HPE, so too can elevated BMP signaling: BMP signals are normally restrained to the lateral borders of the neural plate but in cases where their domains are expanded they can interrupt medial SHH function; embryos affected in this manner exhibit narrowing of the midfacial region and microform versions of HPE ( Fig. 18.1 ). BMPs are also critically important dorsal signals that specify the roof plate of the developing forebrain.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here