Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The liver develops at 3 to 4 weeks’ gestation as an outgrowing diverticulum of proliferating endodermal cells from the ventral wall of the foregut in response to signals from the adjacent developing heart ( Fig. 71.1 ). In the fourth week, 2 buds can be recognized in the hepatic diverticulum: the cranial bud becomes the liver and the hilar biliary tract, whereas the caudal bud develops into a superior bud that forms the gallbladder and cystic duct and an inferior bud that forms the ventral pancreas.
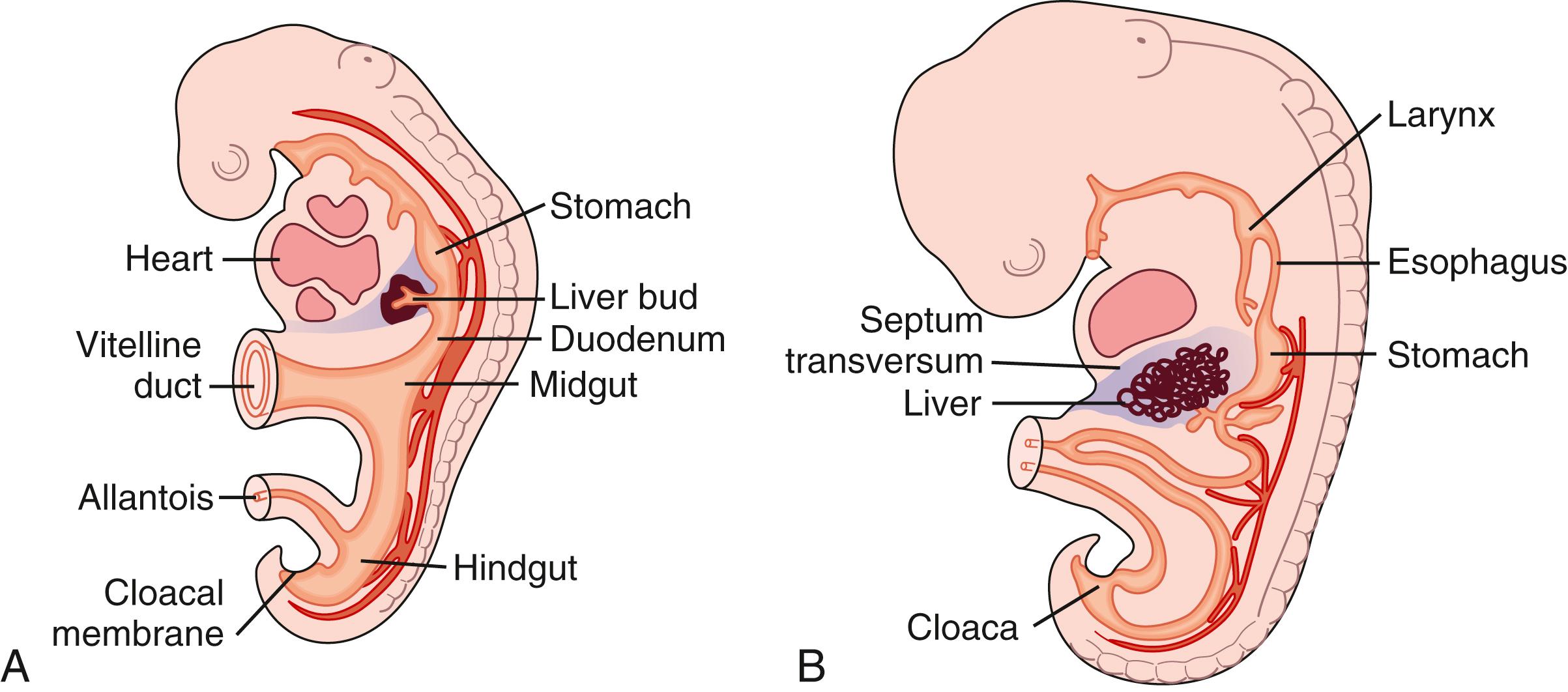
Initially, the liver bud is separated from the mesenchyme of the septum transversum by basement membrane. Shortly, however, this basement membrane is lost, E-cadherin expression is down-regulated in hepatic cells, and cells delaminate from the bud and invade the septum transversum as cords of hepatoblasts—bipotential cells that differentiate into hepatocytes and cholangiocytes. As they invade the septum transversum mesenchyme, hepatoblasts intermingle with endothelial cells, an interaction that appears critical for hepatic morphogenesis.
Hepatic differentiation is highly dependent on signals from the cardiogenic mesoderm and septum transversum mesenchyme, which produce fibroblast growth factor (FGF) and bone morphogenetic protein, respectively. The control of hepatocytic differentiation is complex and involves several transcription factors at various stages of development. For example, GATA4 and forkhead box A (FoxA) are involved in developmental “competence” because they have the ability to interact with compacted chromatin and act as “pioneer” factors that can mark domains of chromatin as competent to be expressed in response to later developmental cues such as FGF Prospero homeobox protein 1 (Prox1) may be involved in down-regulation of E-cadherin, because mutant hepatoblasts maintain high levels of E-cadherin and fail to degrade the matrix surrounding the liver bud. Terminal differentiation of hepatocytes requires the overlapping interaction of a group of transcription factors including hepatocyte nuclear factor (HNF)1β, FoxA2, HNF1α, HNF4α1, HNF6, and liver receptor homolog (LRH)-1. These cross-regulating factors form a dynamic transcriptional network by binding to each other’s promoters and to the promoters of other hepatic transcription factors, creating synergistic interdependence as hepatocyte maturation proceeds. The contribution of Wnt signaling and β-catenin is complex and stage dependent. During early development, canonical Wnt/β-catenin signaling represses hematopoietically-expressed homeobox (Hhex), another early transcription factor in hepatic development; therefore, early in the process, Wnt must be suppressed in the anterior endoderm to facilitate commitment of the endoderm to a hepatic fate. After specification, Wnt signaling promotes hepatogenesis.
By most accounts, the extrahepatic biliary system develops originally as a solid structure that becomes canalized at the end of the fifth week. However, it may develop ab initio as a hollow structure, refuting the concept that biliary atresia results from a failure of the bile duct to canalize. Extrahepatic biliary tract development may require the expression of sex determining region Y- box 17 (SOX17), which is regulated by the homolog of hairy/enhancer-of-split (Hes1). Another transcription factor involved in extrahepatic biliary development is Hhex; in Hhex null embryos, the bile duct is replaced by tissue resembling duodenum. Either the extrahepatic and intrahepatic biliary systems merge at the hepatic hilum or they maintain luminal continuity from the start.
Intrahepatic biliary development begins at 6 weeks when a subset of hepatoblasts close to the portal mesenchyme strongly express biliary-specific antigens (see Chapter 62 ). These biliary precursor cells form a continuous single-layered ring around the portal mesenchyme, called the ductal plate . This plate becomes partly bilayered in the next step with the cells closest to the portal mesenchyme maintaining a biliary phenotype and those closest to the parenchyma resembling hepatoblasts, a process known as transient asymmetry . A period of remodeling follows in which focal dilatations appear between the 2 cell layers and eventually form lumens. The parts of the ductal plate not involved in the formation of ducts regress by apoptosis, and, around the time of birth, the remaining ducts are incorporated into the portal mesenchyme. Incorporation and elongation of ducts begins in the hilum and extends to the periphery of the liver. At birth, the most peripheral small portal tracts require an additional 4 weeks before the ductal plates develop into bile ducts. Similarly, bile canaliculi develop their fully mature appearance during the perinatal and early postnatal period, even though the major bile transporters are expressed at the midgestational age.
The switch in phenotype of hepatoblasts to cholangiocytes requires the coordinated activity of various signaling systems and transcription factors. The earliest sign of biliary differentiation is expression of SOX9, a transcription factor that regulates the timing of biliary duct development. The Wnt/β-catenin signaling system may also play a temporal role in the commitment of hepatoblasts to biliary epithelial cells. HNF-6 and HNF1β also regulate biliary differentiation; mice deficient in these factors show cystic dysgenesis of the biliary tract and abnormalities in the hepatic arterial branches. The developing ducts produce vascular endothelial growth factor (VEGF), which cooperates with angiopoietin-1 produced by hepatoblasts to promote arterial vasculogenesis and recruit mural pericytes to the developing arteries. The maintenance of duct structure during the elongation phase requires that mitoses be aligned uniformly along the axis of the duct, a process called planar cell polarity , which is controlled by noncanonical Wnt signaling and is defective in fibropolycystic liver disease.
Two signaling systems have emerged as critical to biliary differentiation and restriction of biliary differentiation to a periportal location. Transforming growth factor-β (TGF-β) generated by portal mesenchyme stimulates hepatoblasts to switch to a biliary phenotype, and TGF-β signaling is greater near the portal vein and less in the parenchyma. The Notch pathway is also involved in bile duct development; Jagged1 expressed in portal vein mesenchyme interacts with Notch2 on hepatoblasts to induce biliary differentiation at the expense of hepatocyte differentiation. Notch signaling is also instrumental in biliary tubulogenesis. In its absence, formation of the ducts beyond the monolayer ductal plate is impaired. Mutations in the gene that codes for Jagged-1 are associated with Alagille syndrome (see Chapter 62 ).
Mesothelial cells and submesothelial cells derived from the septum transversum migrate inward from the liver surface and give rise to stellate cells, portal fibroblasts, and perivascular mesenchymal cells. Kupffer cells, the tissue-resident macrophages of the liver, arise from yolk sac-derived erythromyeloid precursors rather than from hematopoietic stem cells (HSCs) in the bone marrow. In mice embryos, erythromyeloid precursors develop in the yolk sac, migrate to and colonize the fetal liver, and give rise to fetal erythrocytes, macrophages, granulocytes, and monocytes. Seeding of the liver by monocyte precursors appears to be regulated by sinusoidal endothelial cells. Subsequently, HSC-derived cells replace erythrocytes, granulocytes, and monocytes, but Kupffer cells are only minimally replaced in adult mice. Similarly, the fetal liver is the major site of hematopoiesis in humans before the bone marrow matures.
The existence of hepatic stem cells in the mature liver has been debated, with various cell populations proposed to serve this function. The broader consensus is that the mature liver contains a population of hepatic stem cells that are not equivalent to embryonic stem cells (hepatoblasts) but are similar in that they are self-renewing, proliferative, and bipotential (i.e., capable of generating hepatocytes and cholangiocytes).
These stem cells express epithelial cell adhesion molecule, neural cell adhesion molecule, and cytokeratin 19 and weakly express albumin, but not AFP. They likely also express the Wnt target gene leucine-rich-repeat-containing G-protein-coupled receptor 5. These stem cells are found in the canals of Hering and generate hepatocytes and cholangiocytes in response to liver injury. They decline in number with advancing age. When grown in culture, they produce cords of hepatoblast-like cells that more strongly express albumin, express epithelial cell adhesion molecule, AFP, and intracellular adhesion molecular 1, have reduced cytokeratin 19 expression, and lose neural cell adhesion molecule. By contrast, committed progenitor cells are diploid, unipotent, immature cells that give rise to only one adult cell type. They are either intermediate hepatocytes that express albumin and hepatic enzymes or small cholangiocytes (“oval cells”) that line canals of Hering, intrahepatic bile ducts, and bile ductules. Diploid adult cells can undergo 6 or 7 rounds of division before reaching subcultivation capacity.
During early development, there are 3 major venous systems in the embryo, 2 extraembryonic and 1 intraembryonic. The extraembryonic venous systems are the omphalomesenteric (vitelline) and umbilical (placental) veins, and the intraembryonic system includes the cardinal veins that drain the venous blood of the embryo to the heart. These systems converge into the sinus venosus, a quadrangular cavity that is incorporated into the heart; the vitelline and umbilical veins drain into the sinus venosus via hepatocardiac channels.
The developing liver eventually incorporates the vitelline and umbilical veins, which become enclosed by dividing hepatoblasts and develop asymmetrically. At this time, sinusoids enter from the sinus venosus to form a sinusoidal network. The right umbilical vein regresses, whereas the left umbilical vein forms 2 left-right shunts, one with the right vitelline vein (the portal sinus) and one with the right hepatocardiac channel (the venous duct). These shunts direct placenta-derived arterial blood from the umbilical vein to the inferior vena cava, bypassing the liver. After formation of these shunts, portal vein branches develop from the intrahepatic portions of the vitelline and umbilical veins. The portal sinus and parts of the left umbilical vein give rise to the left portal vein, whereas the right vitelline vein gives rise to the right portal vein. After birth, the obliterated prehepatic segment of the left umbilical vein becomes the round ligament of the liver (ligamentum teres hepatis) in the free edge of the falciform ligament, and the ductus venosus collapses and becomes the ligamentum venosum.
The arterial supply of the liver begins as an offshoot of the celiac trunk at around the eighth week of gestation. By the 10th week, the first arterial radicles are visible in the central portion of the liver, and by the 15th week, they reach the periphery of the liver. As discussed earlier, development of the arterial supply is closely coordinated with bile duct development. The processes of vasculogenesis and vascular remodeling are dependent on stage-specific expression of angiogenic growth factors VEGF and angiopoietin by ductal plate cells and hepatoblasts, respectively, and by their receptors in developing endothelial and perivascular smooth muscle cells.
Sinusoidal endothelial cells are derived in part from a common endothelial/blood cell progenitor called “hemangioblasts,” initially located in the vitelline and umbilical veins, and in part from the endocardium of the sinus venosus. Endothelial cell maturation occurs between the 5th and 12th week of gestation. During that time, sinusoidal endothelial cells between the hepatocyte plates acquire fenestrae, lose expression of typical endothelial markers CD34 and CD31, and become invested by a perisinusoidal matrix rich in tenascin and poor in laminins. These alterations may be necessary to adapt the liver to its hematopoietic function during fetal life.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here