Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Arrhythmias with triggered mechanisms are often initiated from repetitive depolarizations that occur after membrane repolarization.
Atrioventricular reciprocating tachycardia requires an accessory pathway to initiate and maintain the supraventricular arrhythmia.
Catheter-based ablation is a reasonable therapeutic intervention for atrial fibrillation with either radiofrequency or cryotherapy.
When esophageal temperature monitoring is limited in ablation procedures, core temperature monitoring at an alternative site such as the nasopharynx is reasonable.
Although hybrid approaches for atrial fibrillation ablation are effective for persistent atrial fibrillation, further trials are required to confirm these benefits.
Catheter-based ablation for atrial flutter is a reasonable choice either as initial therapy or as rescue therapy if pharmacologic therapy is ineffective.
The Wolff-Parkinson-White syndrome presents with an arrhythmia in the setting of a typical electrocardiographic pattern and is often resolved with catheter-based ablation.
Leadless pacing systems offer multiple clinical advantages, including freedom from complications associated with the generator pocket and leads.
A subcutaneous implantable cardiac defibrillator is an attractive clinical option for suitable patients with inadequate vascular access and high infectious risk.
The risk of lead extraction is considered low if it was implanted less than a year ago.
Indications for lead removal include infection, thrombosis, pain, perforation, persistent arrhythmias, and the need for magnetic resonance imaging or radiation therapy.
Cardiac arrhythmias are a common and frequent source of morbidity and mortality with treatment options constantly evolving. This chapter will discuss the pathophysiology, diagnostic criteria, and treatment modalities for commonly encountered cardiac arrhythmias in the perioperative setting.
Supraventricular tachycardia (SVT) originates in the conducting system from the bundle of His or above, and describes tachyarrhythmias with atrial and/or ventricular rates above 100 beats per minute (bpm) at rest. A typical subset of SVT is paroxysmal supraventricular tachycardia (PSVT), characterized by a sudden onset of rapid heart rate and then termination. The prevalence of SVT and PSVT are 2.29 per 1000 persons in the general population and 36 per 100,000 persons per year, respectively, with higher rates in women and the elderly. The classification of SVT includes consideration of the QRS complex on the electrocardiogram (ECG). A narrow complex rhythm is defined as a QRS duration below 120 milliseconds. However, SVTs with concomitant aberrant ventricular conduction, such as in intermittent bundle branch block, will display a widened QRS complex. Variable conduction at the level of the atrioventricular (AV) node can result in irregular SVT.
The mechanisms of SVT may be focal or reentrant ( Box 24.1 ). Cardiac cells with automaticity lack a true resting membrane potential and undergo slow diastolic depolarization ( Fig. 24.1 ). This diastolic depolarization results in a more positive transmembrane potential that reaches the threshold to trigger cellular excitation. Cardiac cells with automaticity can be found in the sinoatrial node, subsidiary atrial foci, AV node, and His-Purkinje system. Alterations in diastolic potential, threshold potential, and/or the depolarization speed will influence the rate of the threshold potential, resulting in an increase or a decrease in automaticity ( Fig. 24.2 ). Altered automaticity occurs when cardiac cells with automaticity change the rate of pacemaker firing. SVT may be triggered in the setting of repetitive depolarizations after membrane repolarization that reach the threshold potential to trigger depolarization. This triggered activity is often considered a type of enhanced automaticity.
Sinoatrial node
Subsidiary atrial foci
Atrioventricular node
His-Purkinje system
Unidirectional block is necessary
Slowed conduction in the alternate pathway exceeds the refractory period of cells at the site of unidirectional block
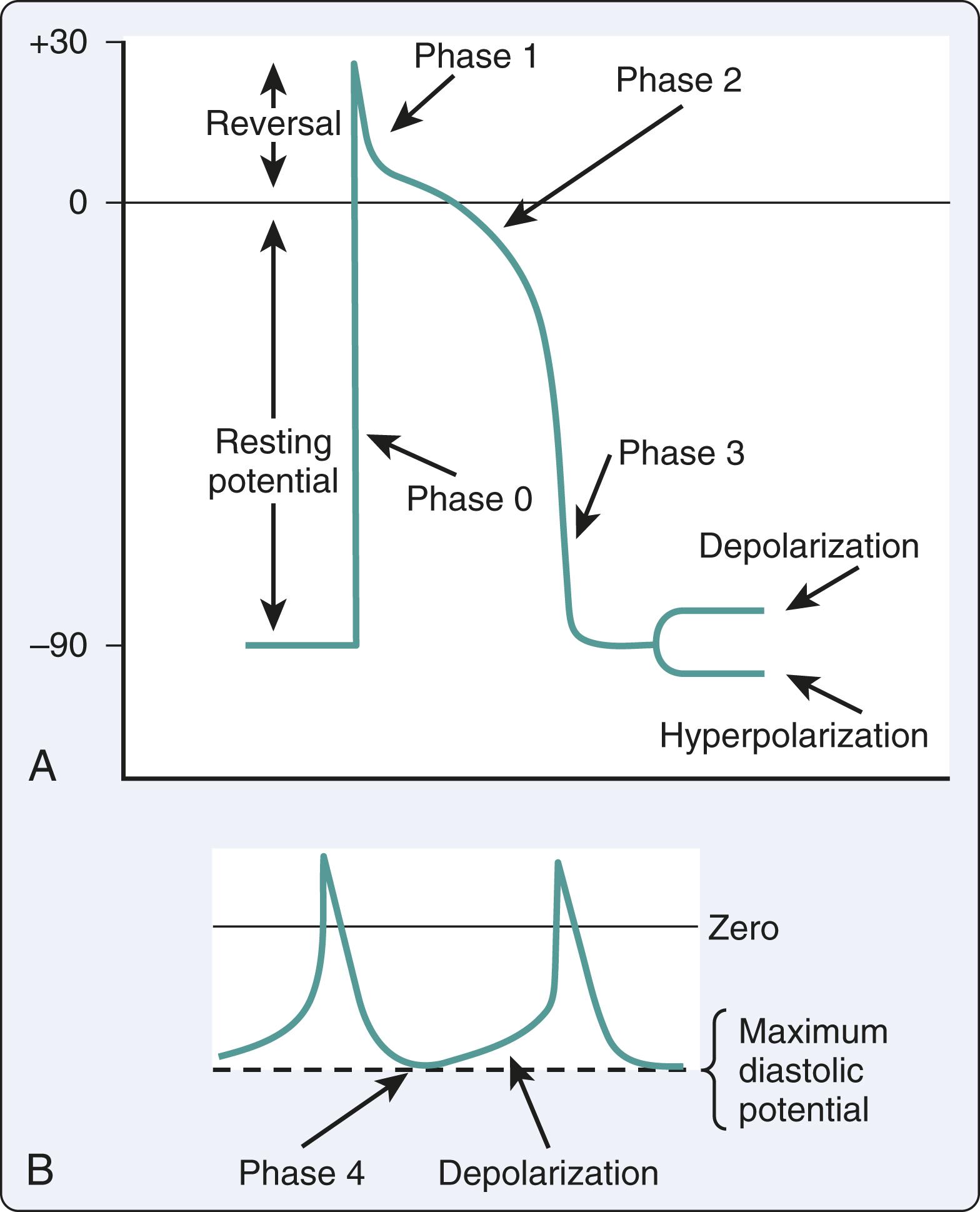
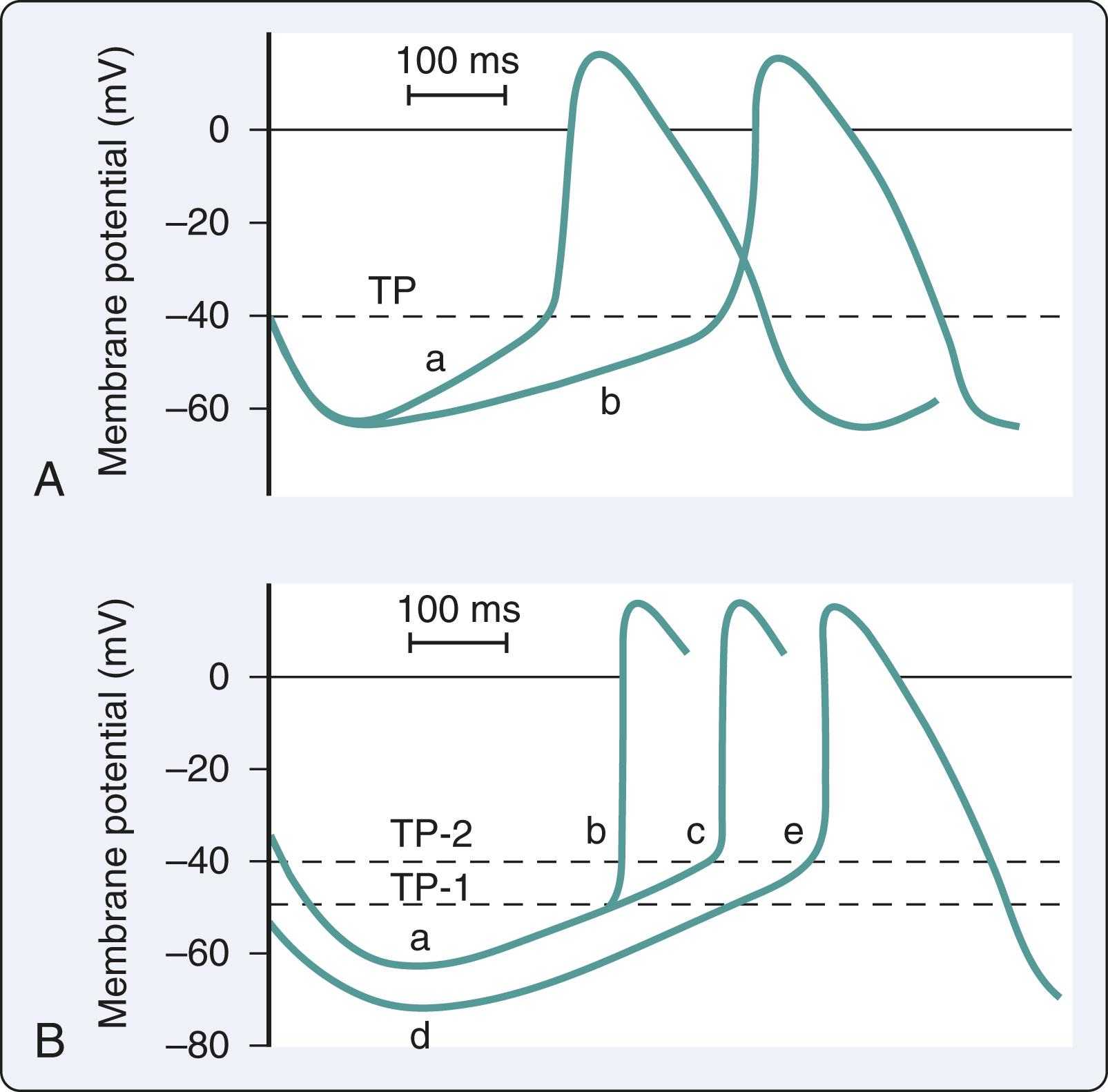
Reentry occurs when a cardiac impulse persists and reexcites the myocardium that is no longer refractory. The reentry mechanism requires a unidirectional block of impulse conduction such as differences in membrane refractoriness. After repolarization, previously refractory membranes become available for depolarization if the initial impulse has found an alternate propagation route and returns to the prior site of conduction block. Reentry with depolarization can occur due to slowed conduction in the alternate pathway that exceeds the refractory period at the site of the block. Both focal and reentrant mechanisms are relevant in the main types of SVT, namely atrioventricular reciprocating tachycardia (AVRT), atrioventricular nodal reentrant tachycardia (AVNRT), and atrial tachycardia.
AVRT requires an accessory pathway to bypass the standard conduction pathway through the AV node and beyond. , The accessory pathway both initiates and maintains the SVT, resulting in two different pathways that exist and connect the atrium to the ventricle. Accessory pathways are abnormal strands of myocardium connecting the atria and ventricles across the AV groove, providing alternate routes for conduction that bypass the AV node and bundle of His ( Box 24.2 ). These accessory pathways may be based on anatomical location, electrocardiographic manifestations, and conduction properties of the accessory pathways.
Concealed: accessory pathway displays retrograde conduction.
Manifest: accessory pathway displays antegrade conduction. Pathways often exhibit retrograde conduction.
Orthodromic: antegrade conduction from atria to ventricles occurs through the normal atrioventricular (AV) nodal conduction system and retrograde conduction through the accessory pathway.
Antidromic: antegrade conduction from atria to ventricles occurs through the accessory pathway and retrograde conduction through the AV nodal pathway.
Abnormal pathways are treated with percutaneous radiofrequency ablation.
Abnormal pathways are treated surgically from the endocardium to epicardium by transection and/or cryoablation.
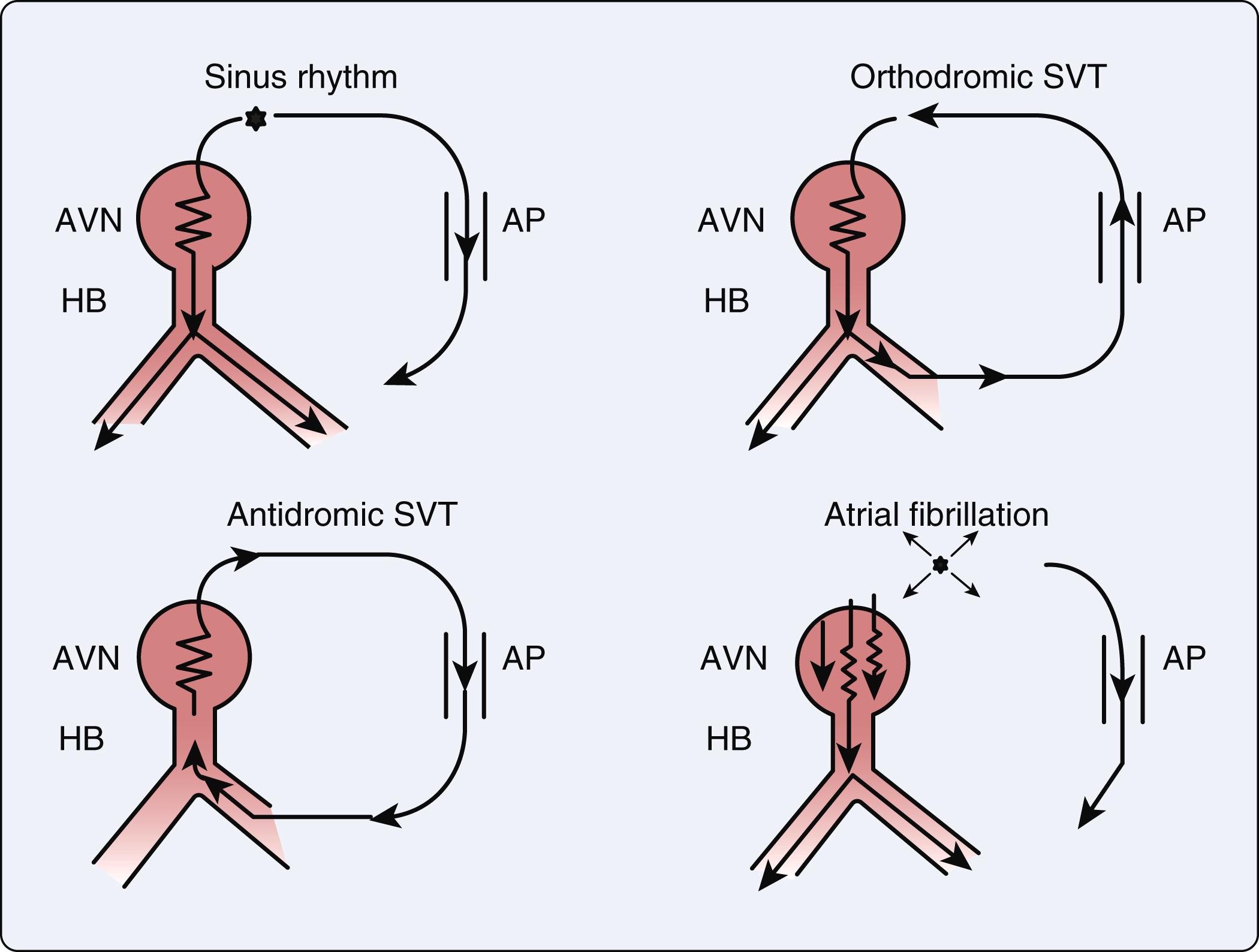
Orthodromic conduction comprises 95% of AVRT in which the cardiac conduction initially proceeds in a normal anterograde fashion to the ventricles ( Fig. 24.3 ). The impulse then completes the circuit traveling retrograde via the accessory pathway back to the atria. Regardless of the beat origination, the impulse will travel in an antegrade direction through the AV node and retrograde through the accessory pathway. , Antidromic conduction in AVRT comprises the remaining 5% of AVRT in which impulses travel retrograde to the AV node system and then antegrade through the accessory limb ( Fig. 24.3 ). The predominant orthodromic pattern appears as a narrow-complex tachycardia, whereas the antidromic pattern has a wide complex appearance that resembles ventricular tachycardia (VT). ,
AVNRT is an SVT with a reentry circuit in the atrioventricular node with fast and slow pathways. It is the most common PSVT occurring primarily in young women with otherwise structurally normal hearts. Typical AVNRT accounts for up to 90% of cases in which a premature atrial complex arriving at the atrioventricular node accesses a slow pathway with a shorter refractory period for further anterograde propagation that is indicated by a relatively longer PR interval. If the fast pathway is recovered and ready for depolarization, the slow pathway impulse can travel retrograde toward the atria via the fast pathway. Nevertheless, due to the longer refractory time of the fast pathway, the impulse will travel antegrade once again on the slow pathway. In the less common atypical AVNRT, anterograde conduction can occur via the fast pathway with retrograde conduction through the slow pathway.
Atrial tachycardia is characterized by an atrial rate greater than 100 bpm, but it does not originate within the sinus node. Since this arrhythmia originates from a focus in either the right or left atrium, it is appropriately referred to as focal atrial tachycardia. Focal atrial tachycardias typically arise from an area smaller than 2 cm. These atrial foci occur in predictable anatomic clusters ( Box 24.3 ). The left-sided atrial foci are typically clustered around the pulmonary veins. The right-sided atrial foci may be related to the tricuspid valve annulus, crista terminalis, coronary sinus ostium, perinodal tissues, or the right atrial appendage. , Although the ECG may provide clues about location based on the P-wave axis, the site of atrial tachycardia is more precisely identified by catheter-based electrophysiologic (EP) investigation.
Foci occur in predictable anatomic clusters.
Left-sided foci occur around the pulmonary veins.
Right-sided foci can be in several locations:
Tricuspid annulus
Crista terminalis
Coronary sinus ostium
Perinodal tissues
Right atrial appendage
Patients with SVT most commonly experience palpitations that may be associated with diaphoresis and dizziness. Furthermore, shortness of breath and chest pain have been reported in the presence of cardiac comorbidities such as coronary artery disease or valvular disease. Overall, patients are usually hemodynamically stable because the ventricular heart rate does not compromise cardiac output.
An abrupt onset and termination of palpitations are consistent with a paroxysmal SVT. A history of syncope typically adds some urgency for further evaluation. Whether palpitations are regular or irregular can differentiate SVT from atrial fibrillation (AF). Patients with a known accessory tract should be promptly evaluated to assess for underlying AF, which can lead to sudden death in this setting ( Fig. 24.3 ). The comprehensive clinical assessment typically includes an ECG for rhythm analysis and echocardiography to screen for associated structural abnormalities.
Further diagnosis of the mechanism and location of a given SVT may require invasive EP testing with catheters typically positioned in the high right atrium, bundle of His, coronary sinus, and the right ventricle (RV; Fig. 24.4 ). , The electrical mapping is detailed and can refine the diagnosis with respect to mechanism and location, including suitability for catheter-based ablation. The catheters are most often introduced through femoral vessels with prior infiltration of local anesthesia. Systemic heparinization is required, particularly when catheters are planned for the left atrium or left ventricle. The complications from this invasive EP testing include those related to vascular access, arrhythmias, hypotension, hemorrhage, deep venous thrombosis, embolic phenomena, infection, and cardiac perforation ( Box 24.4 ). , Proper application of adhesive cardioversion electrodes before the procedure facilitates rapid cardioversion-defibrillation in the event of persistent or hemodynamically unstable tachyarrhythmias resulting from stimulation protocols. ,

Hypotension
Hemorrhage
Deep venous thrombosis
Embolic phenomena
Infection
Cardiac perforation
Contemporary EP mapping includes sophisticated, multichannel, computerized systems that can acquire multiple epicardial, intramural, and endocardial electrograms from a single depolarization. The multichannel, computerized mapping allows rapid identification of arrhythmia pathways, mechanisms, and components ( Fig. 24.5 ). , The resulting insights into arrhythmia mechanisms have led to the maturation of catheter-based mapping and ablation that have therapeutic applications in supraventricular and ventricular arrhythmias. , Despite these advances, the mapping and ablation process can be laborious and time-consuming for the precise localization of the EP substrate responsible for the arrhythmia and ablation of the pathway. The catheter tip is localized with three-dimensional (3D) fluoroscopy and/or advanced mapping software and precisely controlled within the myocardial area of interest with these navigational systems.
Catheter-based EP analysis will often focus on identifying the site of earliest electrical activity for focal, automatic, or triggered SVT and localizing the critical component responsible for ongoing reentrant arrhythmias. , The therapeutic goal is to interrupt the pathogenesis of SVT by interposing scar tissue in the conduction pathway of the arrhythmia. This is accomplished by applying a form of energy from a catheter to a particular myocardial area resulting in localized injury and electrically inert myocardial tissue. The energy sources for ablation include laser, microwaves, radiofrequency current, and extreme cold (cryoablation). A common choice is radiofrequency energy that scars the myocardium by resistive heating. Therapeutic success is determined by the volume and depth of tissue injured, which is determined by factors such as delivered power, and catheter tip size. The measurement of tissue impedance during application of radiofrequency ablation ensures that transmural injury occurs. The interruption of conduction across the lesion after an applied stimulus indicates successful pathway interruption.
A typical ablation strategy for the fast-pathway ablation in AVNRT is performed by positioning the catheter adjacent to the AV node. The catheter is withdrawn until the atrial electrogram is larger than the ventricular electrogram and the His recording is small or absent. The electrographic tracing is closely monitored as radiofrequency energy is applied for either PR prolongation or heart block. The focused energy is delivered until there is PR prolongation or the retrograde fast-pathway conduction is eliminated. The therapeutic efficacy is then confirmed with interruption of conduction at the lesion site and noninducibility of AVNRT.
The anesthesia team must be familiar with the preoperative electrophysiology study results and the characteristics of the associated SVT (rate, associated hemodynamic disturbances, syncope), including current therapies. A standardized focused preprocedure evaluation should be completed, including cardiopulmonary comorbidities such as coronary artery disease, valvular disease, morbid obesity, sleep apnea, pulmonary hypertension, and lung disease ( Box 24.5 ). The focused assessment should also consider the patient’s ability and comfort level to be supine for an extended period of time. Oral anticoagulation and/or antiplatelet agents may not be held before ablation. Preoperative discussion with the EP team should address procedural considerations including arrhythmia characteristics, ablation plan, and patient factors to inform the anesthetic plan.
Preoperative focused assessment to account for patient and procedural factors
Periprocedural discussion with the procedural team to customize the anesthetic
Routine noninvasive monitors—consider indications for invasive monitoring
Moderate sedation—consider indications for general anesthesia
Adapt the anesthetic plan to facilitate arrhythmia detection and mapping
Manage complications including bleeding and cardiac perforation
In addition to standard noninvasive monitoring, invasive blood pressure monitoring should be considered for extended procedures and monitoring of activated clotting times. Continuous blood pressure monitoring for known unstable SVT will allow for more precise titration of vasopressor and inotropic therapy. The EP team may administer provocative agents such as isoproterenol or adenosine to identify triggers for the arrhythmia of interest. These agents may induce acute hemodynamic changes that can be accurately tracked with continuous monitoring of systemic blood pressure. ,
The typical anesthetic plan for SVT ablation is monitored anesthesia care with sedation, titrated to the sensitivity of the specific arrhythmia and degree of desired patient cooperation. Indications for general anesthesia include inability to tolerate the supine position, an extended or complicated ablation procedure, and chronic arrhythmias. Anesthetic agents may provide the required sedation but may also affect cardiac conduction. , The selected anesthetics should facilitate patient comfort during long EP studies. The SVT needs to be induced for diagnostic purposes and catheter ablation, but sedatives often directly affect conduction and alter the ability to map and treat the SVT. Sevoflurane, midazolam, and fentanyl have minimal clinical effects on conduction and inducibility of SVT from the sinoatrial or atrioventricular nodes. Propofol also is well suited for EP procedures, except in pediatric ectopic atrial tachycardia in which it may significantly alter nodal activity. , In contrast, dexmedetomidine may have significant conductive effects including lengthened nodal conduction, increased atrial refractory period, and diminished atrial excitability. In sedative doses, midazolam, propofol, fentanyl, and remifentanil do not significantly affect the inducibility of reentrant tachycardias.
Anesthetic agents may affect the autonomic nervous system that plays a significant role in certain arrhythmias. , During increased stress, the autonomic nervous system shortens the effective refractory period and increases conduction velocity in SVT. As a result, sympathetically driven SVT may not be inducible even with a small dose of midazolam. Therefore, changes in adrenergic tone may dampen the ability to induce and map the arrhythmia of interest (see Box 24.5 ).
Ongoing communication with the EP team during the procedure can guide the dynamic adaptation of the anesthetic plan to adjust for considerations such as hemodynamic stability, arrhythmia characteristics, autonomic sensitivity, and inducibility of SVT. If the arrhythmia of interest cannot be induced, the anesthetic depth should be lightened to assist the detection and mapping of the arrhythmia. Further procedural maneuvers to assist in this setting include proarrhythmic agents such as isoproterenol, epinephrine, adenosine, and programmed stimulation. After the arrhythmia has been accurately localized, the depth of anesthesia can then be increased for patient comfort and procedural stability. ,
Obstructive sleep apnea may be a significant challenge in the electrophysiology laboratory. , Patients under minimal sedation are still at risk for hypoventilation that can be recognized early with trending of carbon dioxide via capnography. For extended procedures or intense stimulation, more profound sedation will be required. In addition to the increased risk hypoventilation poses to the patient, hypoxia and hypercapnia can interfere with detection and inducibility of SVT due to effects on the atrioventricular node producing slowed conduction and prolongation of the refractory period.
Although moderate sedation is often a successful anesthetic choice for SVT ablation, it must be titrated to balance patient comfort and procedural success. At times, sedation must be acutely deepened to navigate short but intensely stimulating parts of the procedure, including cardioversions. Conversion to general anesthesia may be required to facilitate patient comfort and procedural goals. , The anesthetic should be customized to meet the patient’s needs and procedural requirements, including consideration of relevant comorbidities, patient cooperation, procedural length, and electrophysiologist preferences. Further adaptations are also required in the setting of procedural complications (see Box 24.4 ) and the COVID-19 pandemic (see Chapter 4 ).
Ventricular Tachycardia and ventricular fibrillation (VF) often lead to sudden cardiac death. Although a common cause of VT is ischemic heart disease, further etiologies include congenital heart disease, electrolyte imbalances such as hypokalemia and hypomagnesemia, cocaine exposure, long QT syndrome, and cardiomyopathies. Although VT is a wide complex tachycardia, it is classified according to duration and morphology. Nonsustained VT is defined as three or more consecutive ventricular beats at a rate of at least 100 bpm for less than 30 seconds. , However, if VT continues beyond 30 seconds or hemodynamic instability ensues, the arrhythmia is categorized as sustained VT. Sustained VT is monomorphic if it has a stable QRS morphology, whereas the QRS complex morphology changes during polymorphic VT in a beat-to-beat fashion ( Fig. 24.6 ). Polymorphic VT in the setting of a prolonged QT-interval may be torsades de pointes. VT storm is another critical clinical condition characterized by a patient who experiences three or more episodes of sustained VT in 24 hours. Ventricular flutter is VT with a rate of 300 bpm that has a sinusoidal monomorphic appearance and no isoelectric interval between successive QRS complexes ( Fig. 24.6 ). VF has a rate above 300 bpm with irregular electrical activity and variability in electrocardiographic waveform.
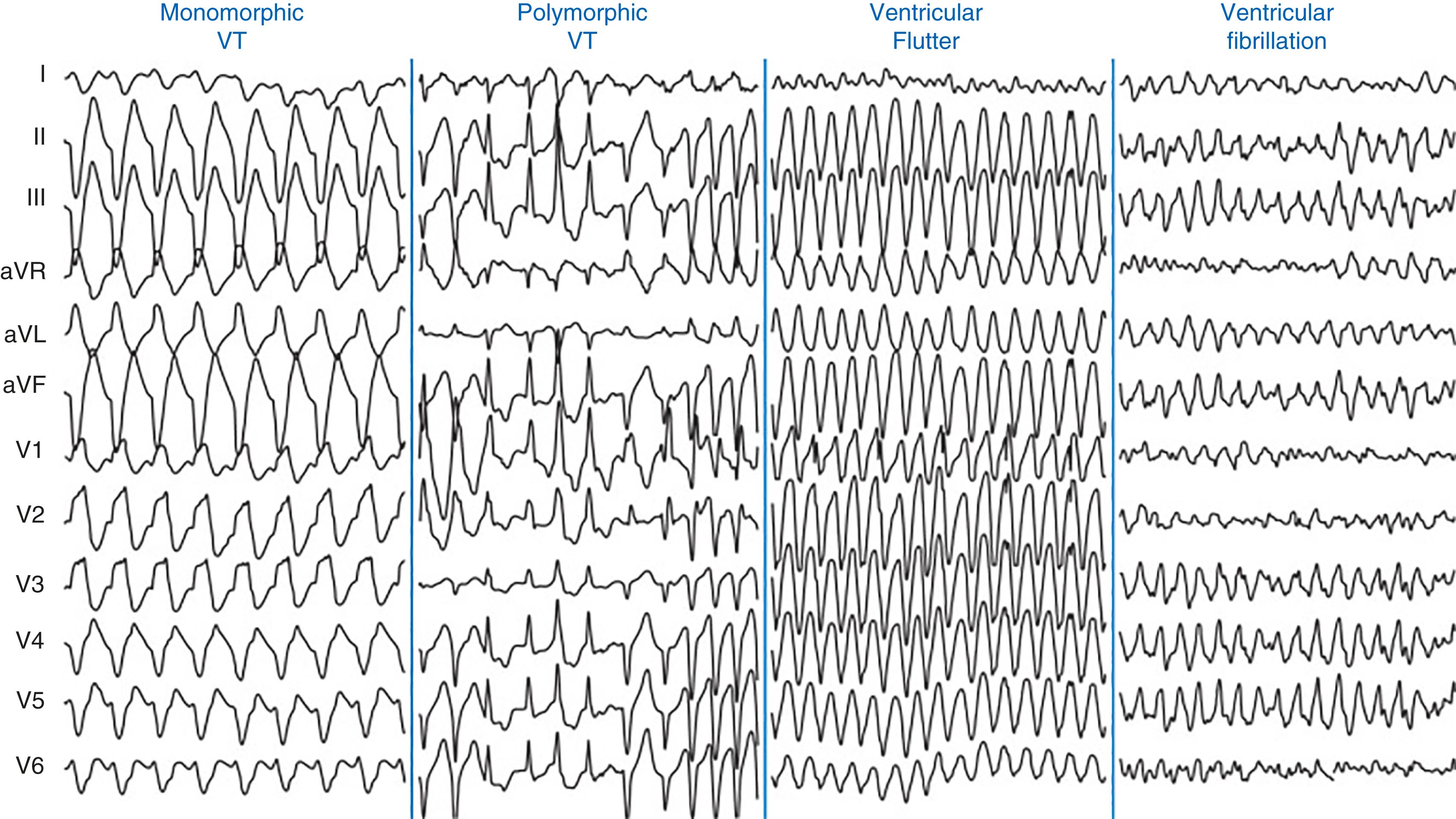
Monomorphic VT is commonly seen during the healing of prior myocardial infarction. Monomorphic VT with underlying structural heart disease is most commonly due to myocardial reentry between ventricular scar and normal myocardium. , Fibrosis helps facilitate reentry by slowing conduction. In addition, there are fewer functional gap junctions at the scar border, creating a vulnerable area for arrhythmia. This area between normal and scar tissue forms reentrant circuits that sustain VT. , Without any structural heart disease, VT is often a primary electrical disorder caused by a focal, triggered mechanism that may originates from the RV outflow tract or apical septum.
The clinical presentation of VT will depend on its duration and its effects on cardiac output. Consequently, its presentation can be mild with palpitations to severe with sudden cardiac death. However, VT can also exacerbate underlying coronary or valvular disease, potentially complicating its clinical presentation. Common symptoms of VT include dyspnea, chest pain, palpitations, and/or general malaise. , If the VT impairs cardiac output, syncope could deteriorate into cardiac arrest.
The management will significantly depend on hemodynamic stability as reflected by vital signs and level of consciousness. Initially, stable patients may become unstable if the heart rate exceeds 200 and/or there is significant underlying cardiac disease. Stable arrhythmias should be treated with an intravenous antiarrhythmic agent such as lidocaine, amiodarone, or procainamide for pharmacologic cardioversion (see Chapter 8 ). Although there is limited consensus on which agent to administer first, lidocaine is a reasonable choice since it does not cause hypotension. Although amiodarone has a slower onset of action, it is typically more effective for conversion of refractory VT. Procainamide will slow VT even if it fails to abort it. If pharmacologic cardioversion is unsuccessful, then electrical cardioversion should be considered after adequate sedation. Associated underlying conditions such as electrolyte imbalances, proarrhythmic medications, and myocardial ischemia should be considered and addressed. The management options for VT have been comprehensively covered in recent guidelines. The option of an implantable cardioverter-defibrillator should be considered (see Chapter 3 ). The contemporary options for ablation of VT include surgical and catheter-based procedures. Given its contemporary importance in the management of VT, catheter-based approaches will be discussed later. Transcatheter ablation can treat VT both with focal and reentrant mechanisms. ,
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here