Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Dr. Gross serves as a consultant to Medtronic and NeuroPace, Inc., and receives compensation for these services. Medtronic and NeuroPace develop products related to the research described in this chapter. The terms of this arrangement have been reviewed and approved by Emory University in accordance with its conflict of interest policies.
This chapter includes an accompanying lecture presentation that has been prepared by the authors: ![]() .
.
Epilepsy affects 1% of the world’s population, and 30% to 40% of cases are refractory to medical therapy. Electrical stimulation offers a nonablative treatment approach for these patients.
Epileptic networks appear to propagate through common neural circuits, allowing targeted inhibition of common epileptogenic foci.
Deep brain stimulation of the anterior nucleus of the thalamus has the strongest evidence for deep brain stimulation in epilepsy, though targeting of the cerebellum, hippocampus, centromedian nucleus of the thalamus, and subthalamic nucleus all remain potential targets.
Vagus nerve stimulation affords a minimally invasive method for seizure reduction and has been supported by two randomized controlled trials.
Responsive neurostimulation allows for real-time detection and inhibition of seizure propagation and has particular utility in patients with multifocal seizure onset zones or in patients with onsets in eloquent cortex.
Electrical stimulation for epilepsy rarely results in seizure freedom but has demonstrated strong evidence for seizure reduction. Further strategies to improve the efficacy of these techniques are needed.
Epilepsy affects 1% of the world’s population, and 30% to 40% of cases are resistant to medications. Although a significant proportion (10%–50%) of medically refractory cases involve patients who are candidates for resective surgery, with postoperative seizure freedom rates of 40% to 90%, millions of patients remain who are not candidates for resective surgery or who have recurrent seizures after surgery. , Very few of these patients respond to additional medication trials, and less than 10% achieve seizure freedom with vagus nerve stimulation (VNS). Thus there is a pressing need for alternative therapies for medically refractory epilepsy.
Electrical stimulation of the nervous system has rapidly developed as an adjunct to medical therapy for epilepsy. VNS has been approved since 1997 for reducing the frequency of seizures in patients older than 12 years of age with partial onset seizures refractory to pharmacologic therapy. Deep brain stimulation (DBS) has been proven to be extraordinarily effective and safe for the treatment of movement disorders such as Parkinson disease, dystonia, and essential tremor. , These successes have inspired the application of DBS to an ever-broadening range of neurological and psychiatric disorders including depression, obsessive-compulsive disorder, and Gilles de la Tourette syndrome and increasingly to epilepsy as well. In this chapter, we examine the use of electrical stimulation in epilepsy including potential targets, mechanisms of neuromodulation and seizure control, clinical evidence and recent clinical trials, and future directions and novel therapies.
Focal or partial onset seizures vary considerably in their epileptogenic zones and in their semiology (i.e., clinical manifestations), but they appear to propagate along common neural circuits such as the cortical-striatal-thalamic network and the limbic circuit of Papez , ( Fig. 100.1 ). These pathways provide common nodes at which neuromodulatory tools may influence the propagation and dissemination of neural information including the pathologic oscillations mediating the behavioral effects of seizures.
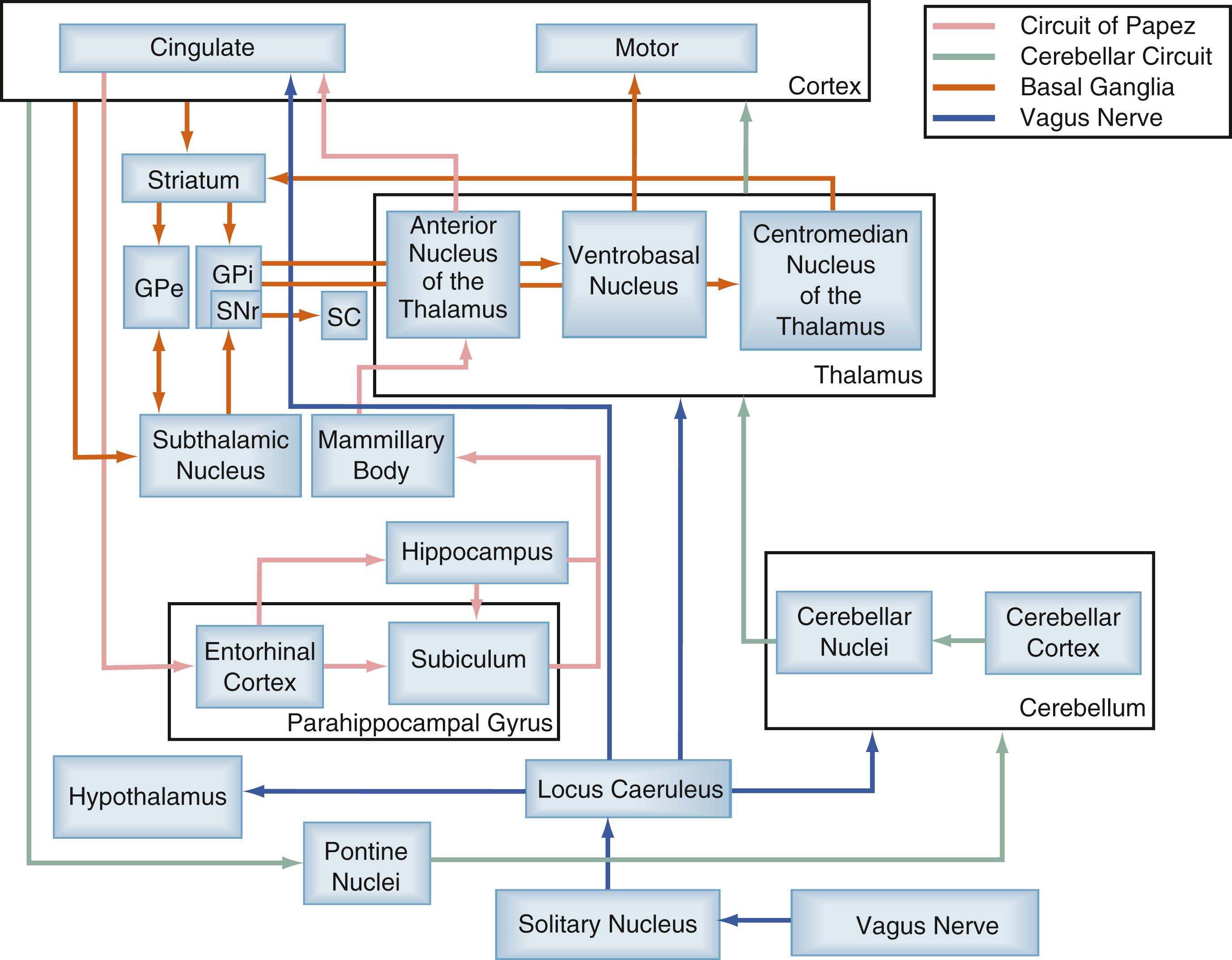
One of the most well studied of these epileptic networks is the circuit of Papez, which is critical for emotion and memory. This network is well known to be involved in the generation and propagation of limbic (e.g., mesial temporal lobe) seizures. , The circuit originates from the hippocampus and subiculum, projecting via the fornix to the mammillary body, then traveling via the mammillothalamic tract to the anterior nucleus of the thalamus (ANT) (see Fig. 100.1 ). The ANT then projects to the cingulate gyrus and in turn to the parahippocampal gyrus, followed by the entorhinal cortex, which finally projects via the perforant pathway back to the hippocampus. , Lesioning and high-frequency electrical stimulation have been studied at several locations within this circuit and demonstrated some effectiveness—including in the hippocampus, mammillary bodies, subiculum, and ANT.
Cortical-thalamocortical excitatory loops have been shown to be involved in absence epilepsy and motor cortex seizures. In a nonhuman primate model of chronic focal motor seizures, thalamotomy restricted to the anterior part of the ventral posterior lateral nucleus was able to produce long-lasting benefit and in most cases led to nearly complete seizure suppression. Thalamic relays are also thought to mediate the benefits from lesioning and electrical stimulation of the cerebellum for epilepsy, but this circuit and its influences are less clearly defined. The thalamocortical network is the subject of further investigations in epilepsy and has provided several potential targets for the neuromodulation of seizures. Further work with these targets will require more precise understanding of the mechanisms mediating the effects of electrical neuromodulation.
The stimulation parameters used in many clinical trials have been informed less by complete understanding of the mechanisms of action of electrical stimulation than by empirical and historical considerations ( Table 100.1 ). More recently, more complex parameters have begun to be entertained in experiments with human patients. Increased effectiveness may also result from a deeper understanding of the mechanisms of electrical stimulation on the nervous system, followed by implementation of the most efficacious parameters into clinical practice.
| Study | Target | Design | N | Seizure Type | Stimulation Parameters | Follow-Up | Results | Adverse Events |
|---|---|---|---|---|---|---|---|---|
| Cooper et al., 1973 ; Cooper et al., 1976 | Cerebellum | Open-label | 15 | Variable (6 CPSz, 6 GTC, 3 myo) | Variable (most 10 Hz, 10 V; 1-min epochs alternating hemispheres) | 11–38 mo | 4 of 15 SF at ≥30 mo (2 CPSz, 1 GTC, 1 myo) 6 of 15 improved (2 CPSz, 2 GTC, 2 myo) 5 of 15 no change |
1 broken lead |
| Van Buren et al., 1978 | Cerebellum | Double-blind crossover | 5 | Variable | 10 Hz, 10–14 V, 8 min “on” R/“off” L, 8 min “on” L/“off” R | 24–29 mo | 0 of 5 SF 0 of 5 improved |
3 CSF leaks 1 increased CSF pressure |
| Levy and Auchterlonie, 1979 | Cerebellum | Open-label | 6 | Variable | 10 Hz, 2–4 V, 8 min “on” R/“off” L, 8 min “on” L/“off” R | 7–20 mo | 0 of 6 SF 2 of 6 improved |
1 infection resulting in explantation All had headaches |
| Wright et al., 1984 | Cerebellum | Double-blind crossover | 12 | Variable | 10 Hz, 5–7 mA, 1 min “on” R/“off” L, 1 min “on” L/“off” R | 6 mo | 0 of 11 SF 0 of 11 improved |
6 patients >1 operation 2 postoperative wound infections, 1 resulting in explantation 4 reoperations 1 lead repositioning 1 device failure |
| Velasco et al., 2005 | Cerebellum | Double-blind crossover | 5 | Variable | 10 Hz, 3.8 mA, PW 450 μs, 2.0 μC/cm , 4 min “on” B/l, 4 min “off” B/l | 24 mo | 3 mo: mean seizure reduction 33% 6 mo: mean seizure reduction 41% |
3 reoperations for migration 1 wound infection resulting in removal 1 ataxia and dysmetria 4 electrode migrations |
| Velasco et al., 2000 | HC | Open-label | 16 | TLE | 130 Hz, 0.2–0.4 mA, PW 450 μs | 2 wk | 7 of 10 SF after 6 days 3 of 3 chronic stimulation improved Interictal spikes decreased |
N/A |
| Tellez-Zenteno et al., 2006 | HC | Double-blind crossover | 4 | MTLE | 190 Hz, 1.8–4.5 V, PW 90 μs | 6 mo | 0 of 4 SF 3 mo: median seizure reduction 15% |
None reported |
| Velasco et al., 2007 | HC | Open-label | 9 | MTLE | 130 Hz, 0.3 mA, PW 450 μs 1 min “on” B/l, 4 min “off” B/l |
18 mo | 4 of 9 SF 5 of 9 improved |
3 skin erosion and local infection, 1 requiring hospitalization 2 explantations |
| Boon et al., 2007 | HC | Open-label | 12 | TLE | 130 Hz, 2–3 V, PW 450 μs | 15–52 mo | 1 patient exited trial before stimulation 1 of 11 SF 9 of 11 improved (6 of 11 >50%) 3 of 11 SF after additional leads |
1 asymptomatic hemorrhage |
| McLachlan et al., 2010 | HC | Double-blind crossover | 2 | MTLE | 185 Hz, “subthreshold,” PW 90 μs | 9 mo | 0 of 2 SF 3 mo: mean seizure reduction 33% |
None reported |
| Cukiert et al., 2011 | HC | Open-label | 6 | Variable (5 TLE) | 130 Hz, 4 V, PW 300 μs | Acute stimulation only | Clinical outcomes pending 4 of 6 with interictal spikes suppressed |
None reported |
| Boëx et al., 2011 ; Bondallaz et al., 2013 | HC | Open-label | 8 | MTLE (2 HS) | 130 Hz, 0.5–2 V, PW 450 μs | 10–74 mo | 2 of 8 SF 4 of 8 improved (50%–90%) |
1 electrode displacement resulting in reimplantation 1 electrode fracture 2 reversible memory deficits with stimulation |
| Tyrand et al., 2012 | HC | Open-label | 12 | TLE (6 HS) | 130 Hz, 1 V peak-to-peak, PW 210 or 450 μs | Acute stimulation only | No seizure outcomes reported HS patients demonstrated 51.8% decrease in epileptiform discharges with biphasic stimulation |
N/A |
| Lim et al., 2016 | HC | Open-label | 5 | MTLE | 1V, PW 90–150 μs 2 patients with HS: 5 Hz 3 with normal MRI: 125 Hz |
Mean 38.4 mo | Mean 45% reduction 2 patients with HS fared better: 54% and 72% reduction |
None reported |
| Jin et al., 2016 | HC | Open-label | 3 | MTLE | 130–170Hz, PW 450 μs, 1–2.5 V | 26–43 mo | Patient 1: 95% reduction Patient 2: 92% reduction Patient 3: 91% reduction |
None reported |
| Cukiert et al., 2017 | HC | RCT | 16; 8 stimulation, 8 sham | TLE | 130 Hz, PW 300 μs, 2 V | 6 mo | 4 SF, 7/8 “responders” (>50% reduction) Patients with evidence of HS on MRI had better response |
2 local skin erosions |
| Benabid et al., 2002 ; Chabardès et al., 2002 | STN | Open-label | 5 | Variable | 130 Hz, 0.8–5.2 V, PW 90 μs | 30 mo | 0 of 5 SF 3 of 5 improved (67%–80%) |
1 infection 1 postimplantation subdural hematoma |
| Handforth et al., 2006 | STN | Open-label | 2 | CPSz | 185 Hz, <3.5 V, PW 90 μs | 27 mo | 2 of 2 improved (33%–50%) | 1 repeated surgery 1 hardware failure |
| Vesper et al., 2007 ; Wille et al., 2011 | STN | Open-label | 5 | myo | 130 Hz, 3.0 V, PW 90 μs | 12–42 mo | 1 of 5 SF 4 of 5 improved (>30%) |
|
| Capecci et al., 2012 | STN | Open-label | 2 | Variable | 130 Hz, 2–3 V, PW 60 μs | 12–48 mo | 1 of 2 improved (65%) | 1 patient demonstrated mild balance impairment, dysarthria, severe abulia, apathy, and mood changes under chronic stimulation |
| Sramka and Chkhenkeli, 1990 ; Chkhenkeli and Chkhenkeli, 1997 ; Chkhenkeli et al., 2004 | Caudate | Open-label | 57 | Variable | Variable | Variable | Unclear | N/A |
| Velasco et al., 1987 ; Velasco et al., 2000 ; Velasco et al., 2006 | CMT | Open-label | 18 | Variable | 60 Hz, 0.5–0.6 mA, 1 min “on” R/“off” L, 4 min “off” B/l, 1 min “on” L/“off” R, 4 min “off” | 18 mo | Lennox-Gastaut: 2 of 13 SF, 8 of 13 improved (50%–80%) Partial seizures: 2 of 5 improved (>80%) |
2 patients explanted owing to repeated skin erosions |
| Fisher et al., 1992 | CMT | Double-blind crossover | 6 | Variable | 65 Hz, 0.5–10 V, PW 90 μs, 1 min “on”/4 min “off” × 2 h/day | 9 mo | 30% mean seizure reduction With stimulation 24 h/day, 3 of 6 improved (>50%) |
1 connection repair 1 minor hemorrhage with no symptoms or complications |
| Andrade et al., 2006 | CMT | Open-label | 2 | Variable | 100–185 Hz, 1–10 V, PW 90–120 μs | 20–80 mo | 1 of 2 improved (>50%) | 1 intermittent nystagmus with stimulation 1 patient with possible auditory hallucinations and anorexia during stimulation |
| Valentín et al., 2013 | CMT | Single-blind | 11 | Variable (6 PGE, 5 FLE) | 130 Hz, <5 V, PW 90 μs | 6–72 mo | PGE: 5 of 6 improved (>50%) FLE: 1 of 5 improved (>50%) |
1 infection resulting in explantation 1 transient agraphia |
| Son et al., 2016 | CMT | Open-label | 14 | Variable | Mean 2.2 V, PW 124.4 μs, 129.3 Hz | Mean 18.2 ± 5.6 mo | Mean 68 ± 22.4% reduction (range 25%–100%) 1 patient SF Mean coordinates in the superior portion of anterior ventrolateral CMT Coordinates not significantly associated with seizure outcomes |
1 patient misplacement of B/l leads due to intraoperative brain shift resulting replacement |
| Valentín et al., 2017 | CMT | Open-label | 2 | Variable | Not reported | Patient 1: 4 yr Patient 2: 18 mo |
Patient 1: >90% reduction, stimulation parameters had to be changed at 6 mo to maintain response Patient 2: No response |
|
| Hodaie et al., 2002 ; Andrade et al., 2006 | ANT | Single-blind | 6 | Variable | 100–185 Hz, 1–10 V, PW 90–120 μs | 50–70 mo | Difficult to interpret; 6 of 6 improved (>50%) by implantation; no further improvement with stimulation | 1 skin erosion requiring wound revision 1 lethargy with continuous stimulation |
| Kerrigan et al., 2004 | ANT | Open-label | 5 | Variable | 100 Hz, 1–10 V, PW 90–330 μs | 6–36 mo | Difficult to interpret; nonsignificant improvement in 4 of 5 | 1 reimplantation for incorrect positioning |
| Lim et al., 2007 | ANT | Open-label | 4 | Variable | 90–110 Hz, 4–5 V, PW 60–90 μs | 33–48 mo | 4 of 4 improved (37%–75%) | 1 resolved mild left-hand weakness associated with hemorrhage 1 scalp erosion resulting in explantation |
| Osorio et al., 2007 | ANT | Single-blind | 4 | Variable | 145 Hz, 4.1 V, PW 90 μs, 1 min “on” B/l, 5 min “off” B/l | 36 mo | 4 of 4 improved (53%–92%) | None reported |
| Fisher et al., 2010 (SANTE) | ANT | Double-blind parallel-group | 110 | Partial onset | 145 Hz, 5 V, PW 90 μs | 4 mo (blinded phase) 13–37 mo (open) |
4 mo: Median seizure reduction 40.4% with active stimulation, 14.5% with sham stimulation 13 mo: 2 of 110 SF; 43% with >50% response 25 mo: 6 of 81 SF; 54% with >50% response |
808 reported in 109 participants, 55 in 40 categorized as serious, 238 of 808 events considered device-related 18.2% paresthesias 14.8% depression during blinded phase 13.0% memory impairment during blinded phase 12.7% implant site infection 10.9% implant site pain 8.2% replaced leads for poor placement 4.5% nonsymptomatic hemorrhages 5 deaths 5 status epilepticus |
| Piacentino et al., 2015 | ANT | Open-label | 6 | Complex partial | 4 V, 140 Hz, PW 90 μs | Minimum 3 yr | >50% reduction in patients with limbic system epileptic origin, no response in patients with origins elsewhere | None reported |
| Van Gompel et al., 2015 | ANT | Open-label | 2 | B/l TLE | 7 Hz, 4 V, PW 90 μs | 12 wk | Both patients >50% reduction | None reported |
| Krishna et al., 2016 | ANT | Open-label | 16 | Complex partial with secondary generalization (68.8%) Generalized (25%) |
Varying voltage, 130 Hz, PW 90 μs | 4.3 ± 3.8 yr | 11/16 (68.8%) reported >50% reduction Stimulus in anteroventral ANT close to mammillothalamic tract was associated with long-term benefits |
2 infections (1 superficial, 1 deep which was removed) 1 patient transient postoperative psychosis due to electrolyte imbalance 1 patient postoperative agitation with stimulation, stimulation discontinued |
| Lehtimäki et al., 2016 | ANT | Open-label | 15 | Variable | 140 Hz, 5V, PW 90 μs, 1 min “on”/5 min “off” | Variable | 10 of 15 with >50% reduction Stimulus in anterosuperior aspect of ANT was associated with better response |
None reported |
| Koeppen et al., 2019 | ANT | Open-label | 10 | 10 focal, 7 with focal to generalized tonic clonic | 145 Hz, 5V, PW μs, 1 min “on”/5 min “off” | Mean 21.5 mo (range 12–42 mo) | Median reduction 70% Stimulus response better when 5 mm lateral to wall of third ventricle, lead tip 10 mm dorsal to midcommissural plane, covering diameter of mammillothalamic tract |
None reported |
| Sitnikov et al., 2018 | ANT | Open-label | 12 | Variable | 4 V, 130 Hz, PW 90 μs | Range 7 mo to 5.2 yr | Mean reduction 80.3% 5 patients SF |
1 patient infection of extension cables resulting in explanted pulse generator 1 patient R-side subcortical hematoma with L-side hemiparesis, resolved in 4 weeks |
| Herrman et al., 2019 | ANT | RCT | 18; 8 patients active, 10 sham, then 6 mo open-label | Variable | 5 V, 150 Hz, PW 90 μs | 1 yr | At 6 mo active group 23% reduction, no significant difference between groups At 6 mo total stimulation for both groups, mean reduction 22% from baseline (statistically significant) 4 patients with >50% reduction |
1 patient internal capsule affected resulting in explantation and replantation 1 patient dysarthria and L cranial VII palsy, resolved in 1 wk 1 patient recurrence of GTC seizures with stimulation |
| Kowski et al., 2015 | Nucleus accumbens | Randomized crossover | 4 | Partial | 125 Hz, 5 V, PW μs, 1 min “on”/5 min “off” | 3 mo blinded stimulation, 3 mo more with ANT stimulus | 3 of 4 patients >50% reduction during blinded phase | 1 patient new-onset depression, Staphylococcus aureus infection of leads resulting in explantation and replantation |
| Schaper et al., 2020 | ANT | Retrospective case-control | 20 | None reported | 145 Hz, 5 V, PW 90 μs | 1 yr | Median 46% seizure reduction, 50% responders, 20% seizure; r = 0.24 for seizure response based on electrode proximity to MMT-ANT junction | None reported |
| Guo et al., 2020 | ANT | Retrospective case-control | 19 | None reported | 130 Hz, PW 90 μs 1.5–3.1 V | Mean 23.8 mo | Mean 64.3% seizure reduction Lead proximity to ANT was associated with better outc omes |
None reported |
Despite the extensive use of electrical stimulation in neuromodulation, its mechanism of action remains poorly understood. The initial observation that high-frequency (>50 Hz) DBS mimicked the effects of ablative procedures suggested that DBS was inhibitory in nature and induced a reversible, functional lesion. Increasingly, however, the action of electrical stimulation on neural circuits has been recognized to be more complex and multifaceted. Stimulation amplitude, frequency, and pulse width play major roles in determining the effects of stimulation on the nervous system; manipulating other parameters such as waveform and polarity can have a significant impact as well. , Early work by Ranck indicated that electrical fields have differential effects on different neuronal structures. Activation thresholds are lowest in myelinated axons, with increasing thresholds found in unmyelinated axons, dendrites, and cell bodies. More recent work from Histed and colleagues using low-current 250-Hz electrical microstimulation with concomitant two-photon calcium imaging to identify the location of electrically activated neurons has supported these hypotheses. Using multicompartment cable models of neurons coupled to a finite element model of extracellular electrical fields, McIntyre and colleagues suggested that the majority of cells within approximately 2 mm of the electrode will entrain efferent (axonal) output at the stimulus frequency, whereas the cells stimulated at subthreshold levels will be suppressed. Electrical stimulation may consequently be overriding, or overwriting, the neural circuit—and in the case of pathologic circuitry, blocking and replacing abnormal neural activity. Indeed, we found evidence of entrainment of downstream (both orthodromic and antidromic) neuronal firing by DBS in a patient with Parkinson disease. The effects of modified efferent output on downstream circuits thus depend on their neural connections.
Other mechanisms—for example, neurochemical interactions and gene and protein expression—may also prove critical. The anticonvulsant effects of low-frequency stimulation have been correlated with changes in adenosine receptor expression, and VNS has been associated with alterations in a variety of neurotransmitters and hormones in cerebrospinal fluid. Furthermore, the progressive improvement in outcome associated with electrical stimulation for movement disorders as well as epilepsy , suggests that synaptic, neurochemical, and/or protein expression changes occur in response to electrical stimulation of the pathologic neural network.
A wide variety of anatomic targets, stimulation parameters, and outcome measures have been investigated for epilepsy, primarily in small case series (see Table 100.1 ). We will highlight and summarize the results of these investigations as categorized by anatomic target. In general, the results are described in terms of complete freedom from seizures (seizure free), a clinically significant reduction in seizure frequency (reduction, response, improvement), or no response (unresponsive, no benefit).
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here