Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Until 1987, infections by members of the family Anaplasmataceae, including the genera Ehrlichia, Anaplasma, and Neorickettsia, were known mainly as veterinary diseases ( Table 192.1 ). Canine ehrlichiosis was first described in 1935 by Donatien and Lestoquard in Algeria. This disease is produced by Ehrlichia canis, which is transmitted to dogs by Rhipicephalus sanguineus ticks. The disease is characterized by fever associated with the presence of clusters of small Giemsa-stained organisms in circulating monocytes. Ehrlichia spp. generally have a tick vector and tropism for macrophages, granulocytes, or sometimes endothelial cells, where they grow within cytoplasmic membrane–bound vacuoles. Consequently, Ehrlichia was recognized as distinct from other genera of obligately intracellular bacteria of medical importance ( Rickettsia, Coxiella, and Chlamydia ). In 1937 the genus name Ehrlichia was suggested in honor of the German bacteriologist Paul Ehrlich. Subsequent phylogenetic studies have shown that two other economically important veterinary pathogens, Anaplasma marginale (described in 1910) and Ehrlichia (formerly Cowdria ) ruminantium (described in 1925), are also ehrlichiae. The first human disease demonstrated to have an ehrlichial cause was sennetsu neorickettsiosis, an infectious mononucleosis–like illness recognized to have occurred only in western Japan, Malaysia, and Laos. Although human infections caused by all members of the reorganized family Anaplasmataceae have been generically referred to as “ehrlichiosis,” and the causative agents are referred to as “ehrlichiae,” it is increasingly apparent that the clinical manifestations and causative agents are distinct. Because of the confusion arising from omitting species-specific diagnosis, more clarity in reporting the results of diagnosis is needed in order to eliminate a substantial burden of “undetermined” cases.
| CAUSATIVE AGENT | MAMMALIAN HOST | MAJOR TARGET CELL | VECTOR, TRANSMISSION |
|---|---|---|---|
| Ehrlichia chaffeensis | Humans, deer, dogs, coyotes, marsh deer | Monocytes and macrophages | Ticks (Amblyomma americanum, Dermacentor variabilis, Ixodes pacificus) |
| Ehrlichia ewingii | Dogs, humans, deer | Granulocytes | Ticks (A. americanum, D. variabilis) |
| Ehrlichia muris subsp. muris and subsp. eauclairensis | Humans, Apodemus mice, voles, white-footed mice | Macrophages, endothelial cells? | Ticks (Ixodes persulcatus, Ixodes scapularis, Haemaphysalis flava) |
| Ehrlichia canis | Dogs, humans | Macrophages | Ticks (Rhipicephalus sanguineus) |
| Ehrlichia ruminantium | Cattle, sheep, wild ruminants | Endothelial cells | Ticks (Amblyomma variegatum) |
| Anaplasma phagocytophilum | Humans, white-footed mice, wood rats, bank voles, wood mice, yellow-necked mice, horses, dogs, cats, sheep, cattle, white-tailed deer, roe deer, red deer, fallow deer | Granulocytes | Ticks (I. scapularis, I. pacificus, Ixodes ricinus, I. persulcatus), Haemaphysalis longicornis |
| Anaplasma platys | Dogs | Platelets, macrophages | Rhipicephalus ticks |
| Anaplasma marginale | Cattle, wild ruminants | Erythrocytes | Ticks (e.g. Rhipicephalus ) |
| Anaplasma centrale | Cattle, wild ruminants | Erythrocytes | Ticks (e.g. Rhipicephalus ) |
| Anaplasma capra | Humans, goats | Unknown | Ticks ( I. persulcatus ) |
| Candidatus Neoehrlichia mikurensis | Wild rodents, dogs, humans | Endothelial cells, neutrophils? | Ticks (Ixodes ovatus, I. ricinus, I. persulcatus, Haemaphysalis concinna) |
| Neorickettsia sennetsu | Humans | Macrophages | Possibly ingestion of raw fish infested by digenean trematodes |
| Neorickettsia risticii | Horses | Macrophages, enterocytes, mast cells | Ingestion of aquatic insects (e.g., mayflies) infested by digenean trematodes |
| Neorickettsia helminthoeca | Dogs | Macrophages | Ingestion of trematode-infested salmon |
The first diagnosed case of human ehrlichiosis in the United States occurred in a 51-year-old man who became ill in April 1986, 12 to 14 days after tick bites in rural Arkansas. Although the disease was initially thought to be caused by the canine pathogen E. canis , the main causative agent of human monocytotropic ehrlichiosis (HME) is Ehrlichia chaffeensis, which was finally described in 1990. In 1994, Anaplasma phagocytophilum was identified as the causative agent of a distinctly different infection, now called human granulocytotropic anaplasmosis (HGA). Subsequently, human infections have been documented as caused by E. canis, Ehrlichia ewingii, and Ehrlichia muris subspecies eauclairensis ; by a bacterium related to E. ruminantium that is called Panola Mountain Ehrlichia ; by a bacterium recently identified and assigned to a new genus, Candidatus Neoehrlichia mikurensis ; by the newly recognized Anaplasma capra ; and, in a single case of infection, by Anaplasma ovis in a patient from Cyprus.
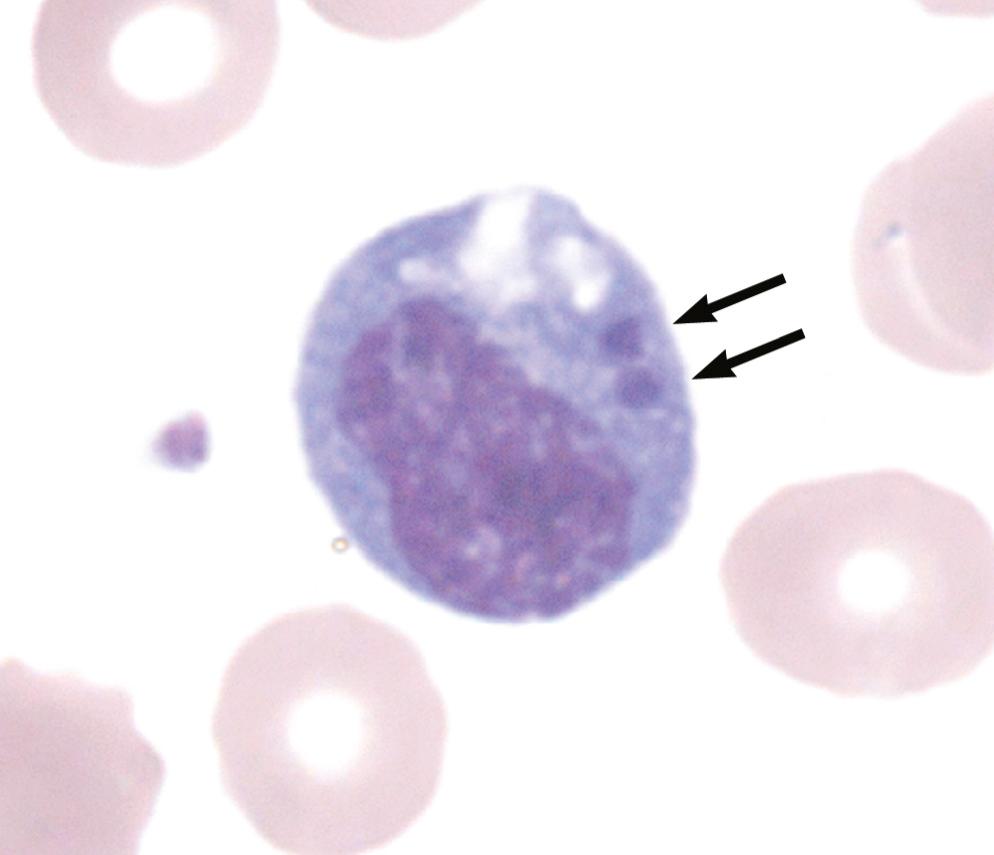
Members of the family Anaplasmataceae are defined predominantly by their genetic similarities and differences but also by phenotypic characteristics and host affinities (see Table 192.1 ). These are small (0.5 µm) gram-negative bacteria. Their clustered inclusion-like appearance of a microcolony in the host cell vacuole is called a morula, from the Latin word for mulberry ( Fig. 192.1 ).
The taxonomic relationships of Ehrlichia, Anaplasma, Neorickettsia, Neoehrlichia, Wolbachia, Orientia, Rickettsia, Coxiella, and Chlamydia have been clarified through molecular and metabolic studies. The evolutionary relationships determined by comparison of whole genomes and the genes rrs (16S ribosomal RNA gene) and groESL indicate that Ehrlichia, Anaplasma, Neorickettsia, Neoehrlichia, Wolbachia, Orientia, and Rickettsia evolved from a common ancestor ; in contrast, Coxiella and Chlamydia are phylogenetically unrelated to ehrlichiae. Ehrlichiae and chlamydiae superficially resemble one another in that both reside within cytoplasmic vacuoles. Unlike chlamydiae, however, ehrlichiae are able to synthesize adenosine triphosphate through metabolism of glutamine, a metabolic characteristic shared with members of the genus Rickettsia, and lack the pathogen-associated molecular patterns lipopolysaccharide and peptidoglycan . Because of the increasing use of molecular methods for genetic identification, the diversity and number of potential clades within Anaplasmataceae exceeds 323 entries within the NCBI Taxonomy database (see https://www.ncbi.nlm.nih.gov/Taxonomy/Browser/wwwtax.cgi ), spanning established genera described here, but also including the genera Candidatus Xenohaliotis, Candidatus Xenolissoclinum, and Candidatus Cryptoplasma. Whether any of these molecular “isolates” are bacteria with the capacity to cause human disease is unknown. The family Anaplasmataceae contains at least five genera that are actually very different from one another. E. chaffeensis shares many antigens and genetic sequences with the predominantly canine pathogens E. canis and E. ewingii ; E. muris and closely related species found in Japanese wild mice, voles, and ticks; and more recently in humans, a bacterium with a close genetic relationship to the ruminant pathogen E. ruminantium. A second genus includes a granulocytotropic bacterium, A. phagocytophilum ; an organism of uncertain tropism, A. capra ; and a suspected erythrocytic bacterium, A. ovis, which can infect humans. All of these are related genetically to the veterinary pathogens Anaplasma platys, Anaplasma bovis, and A. marginale. The third contains Neorickettsia sennetsu , which is closely related to Neorickettsia risticii, Neorickettsia helminthoeca, and an unnamed organism found in Japanese fish flukes. The fourth genus, Neoehrlichia, contains two species: Candidatus N. mikurensis and Candidatus Neoehrlichia lotoris. The former was first identified in Ixodes ricinus ticks in Europe and then in Ixodes ovatus ticks and wild rats from Japan, and it is the only species identified as a human pathogen in the genus. The final genus, Wolbachia , contains bacterial endosymbionts of invertebrates, including insects and helminths, some of which may contribute to disease in human filariasis.
Ehrlichia and Anaplasma have two ultrastructural forms, a larger reticulate cell and a smaller, dense core cell, and the cell wall differs from that of Rickettsia spp., with thinner outer and inner leaflets reflecting the absence of lipopolysaccharide and lipooligosaccharide. Genes coding for the enzymes required for the biosynthesis of peptidoglycan and lipopolysaccharide are not present in the E. chaffeensis or A. phagocytophilum genome. The genomes of these obligately intracellular bacteria are small, ranging from 1.2 to 1.6 Mb to as low as 900 kb for Neorickettsia. Bacteria in the Anaplasmataceae family possess multiple genes that are members of the pfam01617.8, Surface_Ag_2 gene family, encoding major surface porin proteins responsible for antigenic variation and host cell adhesion in genera as diverse as Neisseria, Brucella, and Pseudomonas. E. chaffeensis, E. canis, E. ewingii, and A. phagocytophilum have gene families that encode more than 19 paralogous, surface-exposed pfam01617.8 proteins of 22 to 30 kDa (p28/Omp-1 family) for Ehrlichia and approximately 105 paralogous genes encoding 41 to 49 kDa (major surface protein-2 [Msp2]) for A. phagocytophilum. In accordance with the pfam01617.8 predictions, roles have been shown for A. phagocytophilum Msp2 in antigenic variation, and Msp2/p44 and p28/Omp-1 could function as porins in both A. phagocytophilum and E. chaffeensis. Antigenic diversity within a single strain is based on the presence of hypervariable regions in the Ehrlichia p28/omp1 family, where each gene is transcribed independently yet is dominated by the expression of a single protein in mammalian infection, and a different protein is expressed in ticks. Reinfection with different E. chaffeensis and A. phagocytophilum strains has been reported, underscoring the role of antigenic diversity in immune protection. Similarly, Msp2/p44 of A. phagocytophilum is characterized by conserved domains that flank a hypervariable region, but expression requires gene conversion into a single genomic site. This condition may be further complicated by segmental conversion that generates even greater antigenic complexity. Infection in vivo yields a large number of transcriptional and antigenic variants that presumably contribute to persistence in reservoir hosts.
E. chaffeensis undergoes a 72-hour developmental cycle in which the infectious dense core stage attaches, enters, and converts into the reticulate stage, which replicates numerous times by binary fission in an acidified early endosome and converts into the dense core stage, which is released from the host macrophage. The Ehrlichia genome encodes more than 20 outer membrane proteins (OMPs) that are porins, only one of which is expressed in humans and another in ticks, and several proteins that contain a series of tandem repeat units (TRPs). TRP120 and TRP47 are expressed exclusively on dense core ehrlichiae. These play a role in adhesion to the target cell, in part regulated by bacterial response regulator CtrA. Attachment of TRPs is most likely to host Wnt receptors and of EtpE to DNAX, triggering internalization via caveolae. Ehrlichiae residing in early endosomes acquire nutrients by induction of autophagy by Etp-1 and block lysosomal fusion via protein expression controlled by a two-component regulatory system. TRPs translocate to the nucleus of the infected cell, where they bind DNA at sites that are differentially transcribed with infection to reprogram host cell gene expression, downregulating interleukin (IL)-12 and IL-18 and upregulating apoptosis and cyclin inhibitors that favor ehrlichial growth. TRP120 also interacts with multiple host proteins involved in cell signaling, protein trafficking, and actin cytoskeleton organization, providing multiple opportunities to reprogram the host cell's functions. Ehrlichiae also modulate host Wnt, Notch, and Jak/Stat signaling and SUMOylation pathways, resulting in downregulated pattern recognition receptor expression and innate proinflammatory immune responses, further creating an environment for ehrlichial survival.
Both E. chaffeensis and A. phagocytophilum express functional type IV secretion systems, and at least one A. phagocytophilum substrate, AnkA, is translocated into the infected host cell, where it regulates bacterial entry by interacting with Abl-interactor 1 (ABI1), which influences epidermal growth factor receptor signal transduction and cytoskeletal changes. AnkA eventually translocates to complex with host nuclear heterochromatin, where it binds widely throughout the genome to intergenic regions sequestered into the nuclear lamina and to gene promoters and by recruiting histone deacetylase alters histone acetylation, chromatin structure, and ultimately transcriptional activity. Both bacteria also express two-component histidine kinase regulatory systems that influence trafficking of the parasitophorous vacuole after bacterial entry.
Human ehrlichioses are tick-borne zoonoses. Most patients give a history of tick exposure during the month before the onset of illness. The seasonality of HME, with peak incidence in May to August, reflects the tick-transmitted epidemiology. Exposures are predominantly rural and suburban and involve recreational, peridomestic, occupational, and military activities. More than 60% of patients are male. Documented cases of HME have been reported in 47 states, particularly in the south-central and southeastern United States. This region conforms to the distribution of the Lone Star tick, Amblyomma americanum, the range of which is expanding northward and westward; along with white-tailed deer, this maintains the ehrlichiae in nature through acquisition of E. chaffeensis during feeding as a larva or nymph on persistently infected deer or by cofeeding with infected ticks and subsequently transmitting ehrlichiae to nonimmune deer. The pathogen is transmitted transstadially from larvae to nymphs and from nymphs to adults, but not transovarially. Organisms closely related to E. chaffeensis and evidence of human ehrlichial infections have also been reported in South America, Africa, and eastern Asia.
Between 1987 and 2017, 15,527 cases of HME were reported in Morbidity and Mortality Weekly Reports ( MMWR; Centers for Disease Control and Prevention [CDC]). From 2008 to 2012, the incidence of HME was 3.2 cases per million population in the United States, a fourfold increase from 2000, with hospitalization in 57%, a life-threatening complication in 11%, and a case-fatality rate of 1% (4% in children <5 years and 3% in those ≥70 years of age). An active, prospective, 3-year study in Cape Girardeau, Missouri, revealed 29 cases in a family practice of 7000 patients, an average annual incidence of 138 cases per 100,000 population. Similarly, selected communities have prevalence rates as high as 330 cases per 100,000 population under permissive ecologic circumstances.
It is presumed that most infections are not diagnosed, because cross-sectional seroprevalence in endemic regions ranges from 1.3% to 12.5% for HME. Thus, subclinical seroconversion could reflect exposure to other Ehrlichia or Anaplasma species or other antigenic stimuli. E. canis, which induces cross-reactive serologic responses, also infects patients in South and Central America. E. muris subsp. eauclairensis and E. ewingii are known to cause milder infections that result in serologic cross-reactions with E. chaffeensis. A single case of infection by an E. ruminantium –like bacterium, called the Panola Mountain Ehrlichia , has been identified in a 31-year-old man in Georgia. Human infection by E. muris subsp. eauclairensis has, to date, occurred only in Wisconsin and Minnesota. Transfusion-transmitted HME is possible, and transfusion-related E. ewingii infection has been reported, but both are probably rare. Candidatus N. mikurensis is found in Ixodes ticks throughout Europe, Asia, and Africa. Surveillance with polymerase chain reaction (PCR) of asymptomatic forest workers in Central Europe found an infection rate of 1.6%. Life-threatening transplant-associated disease occurred in both recipients of kidneys from the same deceased donor.
Human granulocytic anaplasmosis (HGA) also has a seasonal occurrence, peaking in June but continuing through November, in accordance with the activity of nymphal and adult stages of Ixodes scapularis ticks. Although risk for HGA is associated with outdoor activity, a substantial proportion of cases occurs in suburban areas of northeastern and upper Midwestern cities. HGA occurs in specific geographic locations; 92% of US cases are reported from New England and the Upper Midwest, with the highest rates in Wisconsin, Minnesota, and Rhode Island. Infection rates increased from 2008 to 2012 and increased with age, and the geographic range expanded. The majority of diagnoses were made by means of PCR. The distribution is almost identical to that of Lyme disease because of the shared Ixodes spp. tick vectors. HGA is documented throughout Europe, particularly in Slovenia, Sweden, and Norway, and in China, Korea, Japan, and other parts of Asia. Serologic studies suggest a global distribution in the Northern Hemisphere for HGA, A. phagocytophilum, and its tick vectors.
Between 1995 and 2017, a total of 30,759 cases of HGA were reported by the CDC in MMWR. The incidence of HGA nationally was 8.0 cases per million person-years in 2012, but above 50 cases per million person-years in Minnesota, Wisconsin, and Rhode Island ; however, active case collection has yielded an incidence of 14 to 16 cases per 100,000 population in the upper Midwest between 1990 and 1995, with rates as high as 24 to 58 cases per 100,000 population in some northwest Wisconsin counties in 1994–1995 and in Connecticut in 1997–1999. Cross-sectional seroprevalence studies have shown that up to 15% of the population in northwestern Wisconsin, 1% of Connecticut residents and US. military personnel, 17% of Slovenians, and 12% of the population of Sweden's Koster Islands have antibodies reactive with A. phagocytophilum in the absence of antecedent clinical evidence for HGA. Alternatively, in some centers in Sweden and Finland, the rate of asymptomatic seroconversion from bites of infected ticks is probably rare. The demonstration of mildly affected patients who recover spontaneously, even in the absence of specific therapy, suggests that HGA could frequently be subclinical. Transfusion-transmitted HGA has become an increasing threat in spite of leukoreduced blood products in the United States and in Europe.
From 4% to 36% of patients with serologic evidence of A. phagocytophilum infection also have serologic evidence of Borrelia burgdorferi or Babesia microti infection, possibly representing serial infections with each agent; both agents are also transmitted by Ixodes spp. tick bites. Concurrent HGA and Lyme disease, documented by isolation of both agents, has been reported, although a prospective study has shown only a 2% incidence of coinfection in patients with erythema migrans and Lyme disease. Coinfection with tick-borne encephalitis virus has been demonstrated in Europe and is likely in the United States with the increasing incidence of Powassan and deer tick virus infections. Whether concurrent infection with these agents results in increased severity, prolonged duration of illness, or more frequent and severe sequelae has yet to be definitively determined.
A. phagocytophilum is transmitted to humans by the bites of nymphal and adult I. scapularis ticks in the eastern United States, Ixodes pacificus in California, I. ricinus in Europe, and presumably Ixodes persulcatus in parts of Asia. However, A. phagocytophilum DNA was also found in Haemaphysalis longicornis ticks examined in conjunction with 62 cases of HGA in Shandong Province in China. Although transstadial transmission of the infectious agent occurs, A. phagocytophilum is not maintained transovarially, and thus natural maintenance requires horizontal (tick-mammal-tick) transmission. A major proven reservoir host is the white-footed mouse, Peromyscus leucopus ; however, other small mammals, such as sciurids (squirrels) in the western United States, and ruminants have been found naturally infected or have serologic evidence of infection, including voles, wood rats, white-tailed deer, red deer, and roe deer. Current serologic evidence suggests that larval ticks acquire A. phagocytophilum after feeding on small mammals infected earlier in the season by nymphal ticks. White-footed mice develop immunity to A. phagocytophilum after a period of bacteremia that may last from several days to weeks; prior immunity reduces small mammal reservoir competence and transmission. Small mammals are not adversely affected by the infection, and some may become persistently infected. The contribution of persistently infected ruminants and cervids as reservoir hosts for A. phagocytophilum requires further investigation. In northern China, where A. capra infects goats, 28 of 477 (6%) persons with a history of tick bite had PCR evidence of infection, and all had a history of a nonspecific febrile illness, including 10 with rash or eschar.
After entering the skin by tick bite inoculation and being spread presumably through lymphatic and blood vessels, ehrlichiae invade target cells of the hematopoietic and lymphoreticular systems. Morulae of E. chaffeensis are observed mainly in macrophages and monocytes, less frequently in lymphocytes, and rarely in polymorphonuclear leukocytes. Ehrlichial morulae have been identified in peripheral blood, bone marrow, hepatic sinusoids, lymph nodes, splenic cords, splenic sinusoids, splenic periarteriolar lymphoid sheaths, cerebrospinal fluid (CSF) macrophages, and macrophages in perivascular lymphohistiocytic infiltrates in organs such as the kidney, appendix, and heart.
The best studied tissue in HME is bone marrow, largely because of the frequency of leukopenia, thrombocytopenia, and anemia. Frequent findings include granulomas, myeloid hyperplasia, and megakaryocytosis. Erythrophagocytosis and plasmacytosis occur in smaller proportions of patients with HME. Focal hepatocellular necrosis; hepatic granulomas, including ring granulomas; cholestasis; splenic and lymph node necrosis; diffuse mononuclear phagocyte hyperplasia in the spleen, liver, lymph nodes, and bone marrow; perivascular lymphohistiocytic infiltrates of various organs, including the kidney, heart, liver, meninges, and brain; and interstitial mononuclear cell pneumonitis are also observed. It is worthy of emphasis that direct endothelial injury and thrombosis have not been described. The observation of erythrophagocytosis, myeloid hyperplasia, and megakaryocytosis in the bone marrow of patients with HME suggests peripheral consumption of blood elements and a compensatory response. In contrast, evidence in murine models exists for myelosuppression, which suggests either direct bacterium-related or indirect chemokine effects on bone marrow.
Although E. chaffeensis causes a direct cytopathic effect when grown in cell culture, it appears that the host responses account for most of the clinical manifestations. The toxic shock manifestations of HME are likely to be the systemic effects of increased levels of proinflammatory cytokines, including tumor necrosis factor-α (TNF-α), IL-1α and IL-1β, and IL-6; defective production of Th1 cytokines, including interferon-γ (IFN-γ) and IL-2; the increased production of antiinflammatory IL-10; and increased levels of chemokines. TNF-α is produced by natural killer T cells and CD8 T lymphocytes in murine models and possibly in humans and, together with perforin expressed by these cells, results in killing of CD4 T lymphocytes, significant reductions in IFN-γ expression, and apoptotic tissue injury. IFN-γ stimulates macrophage killing of E. chaffeensis through the sequestration of iron, and opsonization with immune serum enhances the destruction of ehrlichiae by macrophages. E. chaffeensis circumvents host defenses by inhibiting the fusion of infected phagosomes with lysosomes and inhibiting the signal transduction pathway of IFN-γ–mediated anti-ehrlichial activity. There is evidence that E. chaffeensis also evades immunity through downregulation of other host defense genes of the infected macrophage and manipulation of the host cell as a niche favorable to its survival and growth.
A. phagocytophilum is observed predominantly in neutrophils in peripheral blood and tissues from infected individuals. Dramatic histopathologic findings involve the presence of opportunistic pathogens, especially severe fungal and viral infections, which account for most fatalities. Pathologic findings in humans and animal models include normocellular or hypercellular bone marrow, erythrophagocytosis in mononuclear phagocytic organs, hepatocellular apoptoses and periportal lymphohistiocytic infiltrates, focal splenic necrosis, mild interstitial pneumonitis, and pulmonary hemorrhage. Vasculitis, endothelial injury, granulomas, and meningeal inflammation have not been described.
A. phagocytophilum disseminates to blood, bone marrow, and spleen after a tick bite, likely through local infection of neutrophils attracted to the tick bite wound in the dermis. In the bone marrow, progenitors of myeloid and monocytic lineages can be infected. Endothelial cells can be infected in vitro, but evidence for in vivo infection is lacking. With neutrophil infection, the bacteria attach to the cell surface P-selectin glycoprotein ligand-1 (CD162) and perhaps other ligands, enter a vacuole that is altered by secreted A. phagocytophilum effectors to avoid autophagy, mimic recycling endosomes by accumulating an array of Rab guanosine triphosphatases (GTPases) to the membrane, and thereby preclude lysosome fusion and replicate.
In vitro, A. phagocytophilum survives by deactivation of the neutrophil antimicrobial response through AnkA-mediated silencing of granulocyte RAC2 and CYBB (gp91 phox ) transcription and prolonged downregulation of phagocyte oxidase activity. Additional pathogenetic processes modified in infected granulocytes include delayed apoptosis, ineffective binding to and transmigration of activated endothelium, and inhibition of phagocytic activity. However, infection also paradoxically stimulates an inflammatory response with neutrophil activation, chemokine secretion, and degranulation. Increased proinflammatory activity, in part mediated by TLR2 and NALP4 inflammasome activation of macrophages and by transcriptional upregulation of chemokine genes, allows the recruitment of new neutrophil host cells and localized tissue injury that further exacerbates inflammatory stimulation when neutrophils cannot generate effective antimicrobial responses. These findings are consistent with the dissociation of bacterial burden and histopathologic evidence of tissue injury in mouse and horse models, suggesting a role for host immunity in disease. A. phagocytophilum infection is initially controlled by IFN-γ, which results in macrophage activation and marked increases in inflammatory tissue injury in mouse models, paralleling evidence of macrophage activation as a mechanism of increased severity in humans ; downregulation of IFN-γ expression in animal models results in a higher bacterial burden but less inflammatory tissue injury and lessened clinical disease. Paradoxically, infection of Stat1-deficient animals results in ample IFN-γ production with massive inflammatory consequences and marked increases in bacterial burden. Although involved in inflammatory injury with infection, host innate and adaptive immune mechanisms contribute little to control of A. phagocytophilum infection except for CD4 T lymphocytes.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here