Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The skeleton has a variety of functions, including mechanical support, protection of soft tissues, locomotion, hematopoiesis, energy homeostasis, and acid–base buffering. The skeleton is the largest reservoir for calcium and phosphate in the body.
Chronic gastrointestinal (GI) and liver disorders can affect skeletal mass, size, and structure through perturbation of bone cell function. Bone-forming cells (osteoblasts) and bone-resorbing cells (osteoclasts) regulate bone mass, the most important determinant of bone strength. Osteocytes (derived from osteoblasts) embedded in the calcified bone matrix are the master regulators of bone cell activity. In children with chronic digestive and liver diseases, bone mass is predominantly affected by deceleration of linear growth, lower bone turnover, loss of skeletal muscle (which normally applies anabolic mechanical tension to bone), and endocrine disturbances (e.g., delayed puberty, growth hormone [GH] resistance, and sick euthyroid syndrome). Because growing children have actively remodeling bones, they may be particularly vulnerable to the effects of chronic diseases on the skeleton. However, restoration of health in children offers the prospect of full skeletal reconstitution, especially in those with growth potential.
This chapter summarizes current knowledge on how digestive and liver diseases affect bone in children and offers suggestions for treatment of GI and liver disorders to optimize the chances of achieving peak bone mass in growing children. To achieve this goal, we first review basic bone biology, then the assessment of bone mass and bone metabolic activity; we then review current knowledge on the effects of digestive and liver diseases on bone metabolism and bone mass in children and available therapies to enhance bone mass.
Bone size, shape, and architecture evolve continuously in growing children (“bone modeling”) ( Fig. 91.1 ). While linear growth ceases after epiphyses fuse in late puberty, bone continues to acquire mineral content until the early part of the third decade of life. Trabecular bone architecture becomes more robust in early adulthood, transitioning from rod-type trabeculae to plate-like trabeculae. Parathyroid levels rise transiently, probably as an adaptation to increase the synthesis of active vitamin D and intestinal calcium absorption. An array of systemic endocrine and bone paracrine factors regulate this normal physiologic process. These systems act on osteoblasts, cells derived from mesenchymal precursors, and osteoclasts, which develop from hematologic precursors. Osteoblasts undergo a sequence of developmental events controlled by hormones, microRNAs, and transcriptional factors that ensure the proper expression of their mature phenotype and functional properties. , The main function of mature osteoblasts is to form a protein matrix that is rich in type I collagen and contains bioactive factors such as transforming growth factor β, osteonectin, and osteopontin. Under normal circumstances, calcium phosphate crystals mineralize the collagen matrix to form mature bone tissue. The inflamed intestine and liver release a variety of bioactive molecules that can affect osteoblast activity.
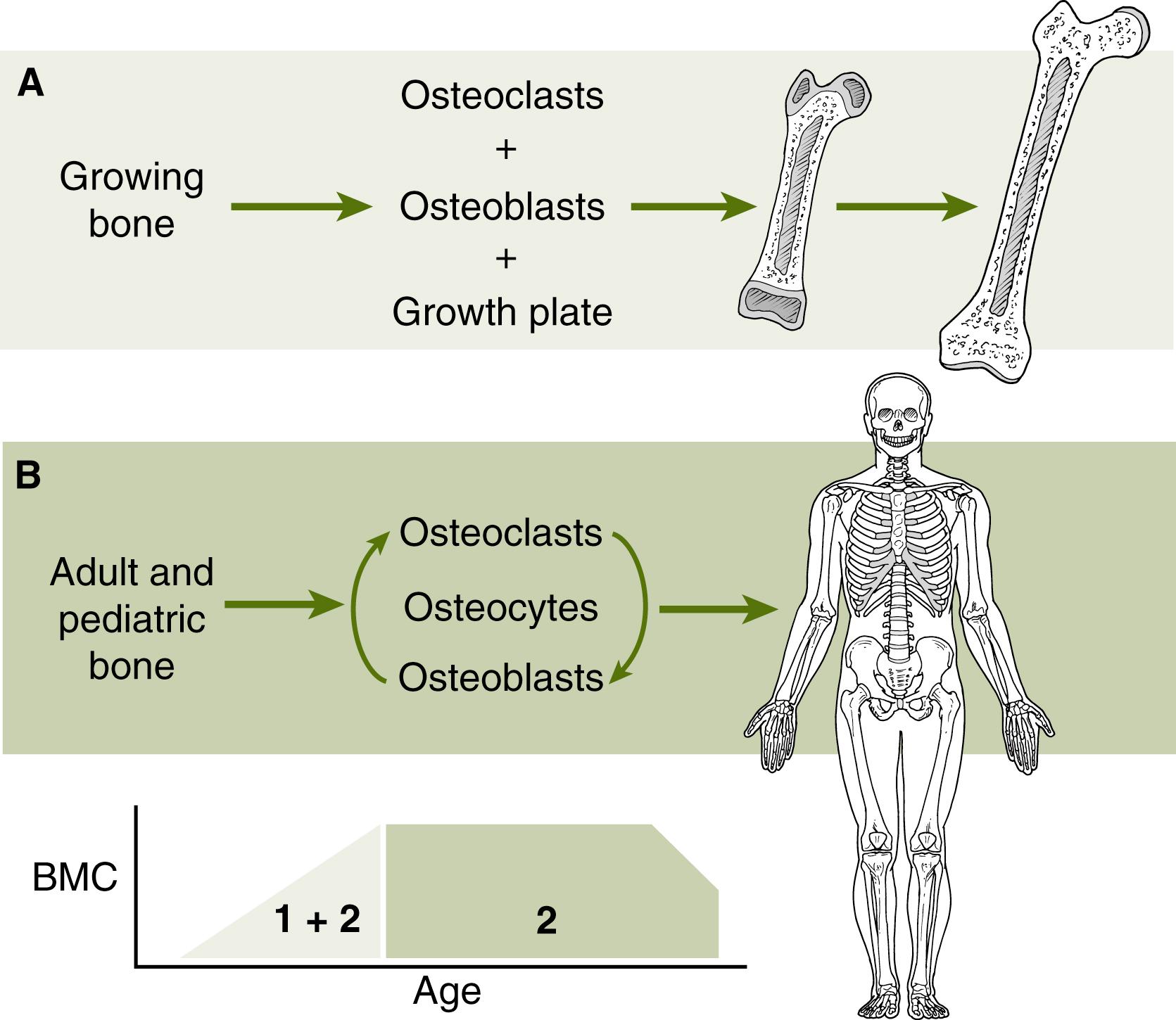
Most osteoblasts die by apoptosis, but some osteoblasts survive and become embedded in the mineralized matrix and become osteocytes. Osteocytes develop radiating processes that form a network that senses mechanical stress. Osteocytes are the most abundant bone cell type and are master directors of both osteoblasts and osteoclasts. , ,
Osteoclasts require receptor activator of nuclear factor κβ ligand (RANKL) to differentiate and become active. Osteoblasts, osteocytes, stromal cells, and activated T cells produce RANKL, which serves as the final common mediator for osteoclast development. , Membrane-bound and soluble RANKL binds to its receptor RANK on osteoclast precursors, stimulating proliferation, differentiation into mature osteoclasts, and bone resorption. Mice lacking RANKL or RANK have abnormally dense bones because of lack of osteoclasts. These mice also fail to develop lymph nodes, functionally linking the immune system and bone cell biology. Interestingly, cytokines associated with inflammation such as tumor necrosis factor (TNF)-α can increase osteoclast formation directly , and in synergy with RANKL. , Interleukin (IL)-17 stimulates osteoclastogenesis directly and via RANKL. On the other hand, interferon (IFN)-γ, IL-4, and IL-12 are potent inhibitors of osteoclast formation by inhibiting RANKL function. Osteocytes secrete RANKL to stimulate osteoclast differentiation and sclerostin, an inhibitor of osteoblast development, thus holding ultimate control over bone remodeling. ,
Unlike bone modeling where bone grows rapidly and changes in shape, bone remodeling eliminates and replaces small amounts of damaged or old bone over a period of several months. The activities of osteoblasts and osteoclasts are coupled during bone remodeling. Therefore, increased osteoclast activity leads to osteoblastogenesis and vice versa. Consequently, diseases and therapies that decrease osteoblast activity eventually result in decreased bone resorption, a state referred to as low bone turnover. In this state, bone mass is lost primarily because of a decrease in osteoblast function. Conversely, bone resorption induces osteoblast activity. However, because bone formation is much slower than bone resorption, osteoblasts cannot keep up with bone resorptive activity. In this setting, bone mass is lost, and bone microarchitecture is compromised. This remodeling state is known as high bone turnover (e.g., which occurs after menopause). In this context, it makes sense to decrease osteoclast activity pharmacologically to stall bone loss. However, in children, digestive and liver diseases in general arrest linear growth, delay puberty, and reduce muscle mass, leading to a decrease in bone modeling and low bone turnover. This has important implications for the approaches used in children to foster skeletal reconstitution in the setting of chronic GI and liver diseases (please see therapies ahead).
Structurally, bone tissue can be compact or trabecular. Compact bone, which forms the shafts of long bones, constitutes approximately 80% of the bone mass. An array of tightly packed mineralized cylinders, each with an axis formed by a vein, an artery, and a nerve that runs parallel to the long axis of the bone (the osteon), forms compact bone. Each cylinder is made of concentric layers of mineralized matrix in which there are embedded osteocytes. On the other hand, trabecular bone is made by interconnecting mineralized struts and plates (trabeculae), making a three-dimensional lattice, and is located in the epiphyses of long bones, vertebral bodies, and flat bones. Each trabecula is made of mineralized matrix containing osteocytes and lined by osteoblasts and osteoclasts. Trabecular bone is intimately associated with the bone marrow. In adults, trabecular bone is the most metabolically active bone tissue, accounting for 80% of its metabolic activity. In children, both cortical bone and trabecular bone are metabolically active. Chronic pediatric diseases may affect both trabecular bone and cortical bone.
Peak bone mass is the major determinant of bone strength over the life of the individual. Heredity is the primary determinant of peak bone mass. , Therefore, a family history of osteoporosis and fractures is a major risk factor for fragility fractures. Other factors that influence bone mass include body weight, muscle mass, physical activity, diet, genetically determined later puberty, and ethnicity. Rapid gain of bone mass occurs throughout childhood, especially during puberty when bones grow rapidly in length, volume, and strength. However, bone mass accretion and gains in bone architectural strength end early in the third decade of life. Peak bone mass is achieved earlier in women than in men because women complete their sexual maturation earlier. , There may be a narrow, fixed window of opportunity during puberty and early adulthood when peak bone mass can be acquired. If this window is missed, the individual may not attain genetically programmed peak bone mass. , However, some data suggest that healthy male individuals with delayed puberty eventually catch up and show normal bone mass in early adulthood. This has important practical implications for children with GI and liver diseases in whom delayed puberty is common.
After late adolescence (about 20 years of age), bone remodeling maintains bone mass and structure, but later in life, bone formation can no longer keep up, and bone mass is lost at a steady rate. Eventually, in some individuals, bone weakens to a point where it may be unable to sustain the stresses of daily living, fail structurally, and fracture with ordinary physical activity or minor trauma.
Every effort should be made to minimize the impact of the underlying GI and liver disease on nutrition, pubertal maturation, muscle mass, and physical activity to give the best chance to achieve peak bone mass.
The most widely available method to measure bone mass is dual x-ray absorptiometry (DXA). In DXA, an x-ray source generates fan beams of two different energies. Bone and soft tissues differentially attenuate these two x-ray energies ( Fig. 91.2 ). After traversing the body, an array of detectors captures the residual x-ray energy. DXA scans the patient from head to toe with a C-arm, where the source of x-ray is underneath the patient and the detector array is above. DXA scanners measure bone mineral content (BMC, in grams) and bone area (in square centimeters). Bone mineral density (BMD) is a calculated value (BMC divided by bone area, in grams per square centimeter). BMD measured by DXA is therefore a projection of the three-dimensional skeleton and not a true volumetric density (in grams per cubic centimeter). In addition, DXA obtains percent fat and fat-free mass (which approximates lean mass). DXA can also obtain an image of the lateral spine to look for compression fractures (vertebral fracture analysis [VFA]), but VFA has limitations in children owing to the incomplete vertebral mineralization, which can be erroneously interpreted as a vertebral compression factor.
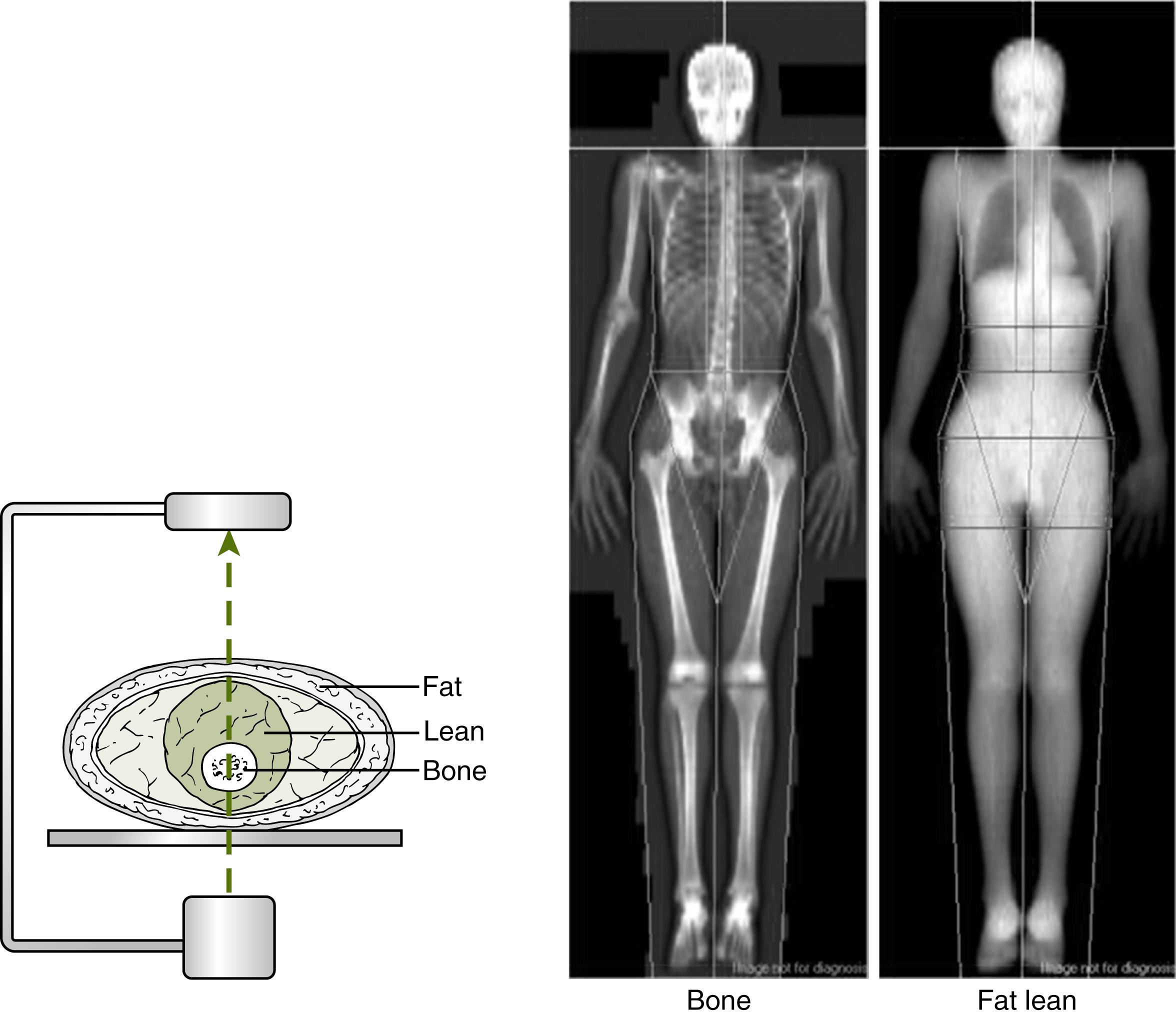
DXA involves minimal radiation and time and is reproducible. DXA use has been validated in children, and there are appropriate reference data for various DXA instruments. , DXA in growing children should only be performed in the total body (minus head) and lumbar spine and not in the hip or femoral neck (as in adults). In children with contractures or other issues that preclude proper positioning for DXA, BMD can be measured in lower extremities and results extrapolated based on established normative data. , DXA scanners are not interchangeable, so longitudinal measurements should be performed in the same instrument whenever possible, using the same software version.
DXA does not measure the dimension of depth of bone, only bone area. Therefore, bone density by DXA will appear to increase in growing bones, even if the true volumetric density of bone remains constant. Special caution should be taken to interpret DXA results properly in children with growth retardation and/or delayed puberty, a common complication of GI and liver diseases in children. DXA tends to underestimate BMD in children who are small for age, whereas it overestimates BMD in children with larger skeletons. To overcome this limitation, volumetric bone density can be calculated using geometrical assumptions to generate a value called the bone apparent mineral density (BAMD) , or can be measured with peripheral quantitative computed tomography (pQCT; see the following section). Alternatively, BMD can be adjusted to height z score to adjust for short stature.
In children, BMD should always be expressed as a z score, which measures the deviation of the observed BMD from the mean for age, sex, and race. T scores, which are used in older adults, should never be used in children because they compare measured BMD with that of young adults and therefore grossly underestimate BMD in children.
As with any ancillary test, DXA should be performed if its results are anticipated to influence the medical management of the patient. Children with chronic GI and liver disorders who have risk factors for low BMD, such as continuous use of corticosteroids for more than 3 months, malnutrition, amenorrhea (in girls), and chronic active inflammation or who have a history of fragility fractures (vertebral compression, extremity fractures with low trauma, or more than two fractures at any site) should have DXA. , In children with low BMD (z score < −2), DXA may be repeated to assess the result of medical interventions as early as in 3 months if there is active linear growth or more commonly every 6 to 12 months. ,
Peripheral QCT of the radius and tibia can determine bone mass, bone architecture, and certain mechanical properties of bone. It involves minimal radiation exposure (comparable to that of DXA). It has several advantages over DXA: pQCT measures volumetric bone density, differentiates compact from trabecular bone, and directly visualizes limb muscle and fat compartments. Parameters of bone strength, stiffness, and bone fragility can be inferred from pQCT measurements. pQCT is currently used primarily in research centers but may be adopted more widely with standardization of measurements at different skeletal sites, improved precision, and generation of normative data.
HRpQCT can image volumetric bone density and bone geometry of trabecular and cortical bone, thanks to reduced voxel size compared with pQCT. It is currently available only in bone research centers.
Bone biopsy can be used to assess bone remodeling and diagnose bone metabolic disease. Transiliac bone biopsy is performed after timed administration of oral tetracycline to label the mineralizing front in the bone so bone formation rates can be calculated. There are normative data to interpret bone biopsies in growing children. Although it is considered the “gold standard” to assess bone metabolic activity, a major disadvantage of bone biopsy for routine pediatric use is its invasive nature.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here