Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Arrhythmogenic right ventricular cardiomyopathy (ARVC) is an inherited disorder causing fibrofatty replacement of the right ventricular myocardium. , This pathological change results in right ventricular (RV) dilatation and dysfunction, myocardial scarring, and risk of sudden cardiac death (SCD) caused by ventricular arrhythmia. Increasingly, there is appreciation of left heart involvement in ARVC, to the extent that some authors suggest excluding the RV specification and renaming the disease “arrhythmogenic cardiomyopathy.” ,
Desmosomal mutations in plakophilin 2, desmoplakin, desmoglein 2, desmocollin 2, and plakoglobin can result in the phenotype of ARVC. Most mutations follow autosomal dominant transmission with variable penetrance, although recessive variants exist. Desmosome mutations result in abnormalities in cell–cell adhesion, cellular signaling (intra- and intercellular), and gene expression related to adipogenesis and fibrogenesis. Fibrofatty scarring of the myocardium then provides the substrate for scar reentry based ventricular arrhythmias. Nondesmosomal mutations that are also important include transmembrane protein 43, lamin A/C, desmin, titin, alpha-T-catenin, N-cadherin, and phospholamban.
The diagnosis of ARVC is multifaceted, but cardiac imaging plays a central role. Although there is an increasing focus on cardiac magnetic resonance imaging (CMRI), echocardiography is widely considered the first-line diagnostic imaging modality in light of its availability and cost efficiency. This chapter outlines the echocardiographic approach to the assessment of ARVC, including a description of traditional techniques and a discussion about emerging methods, including strain quantification. The role of nonechocardiographic imaging modalities in establishing the diagnosis is subsequently reviewed.
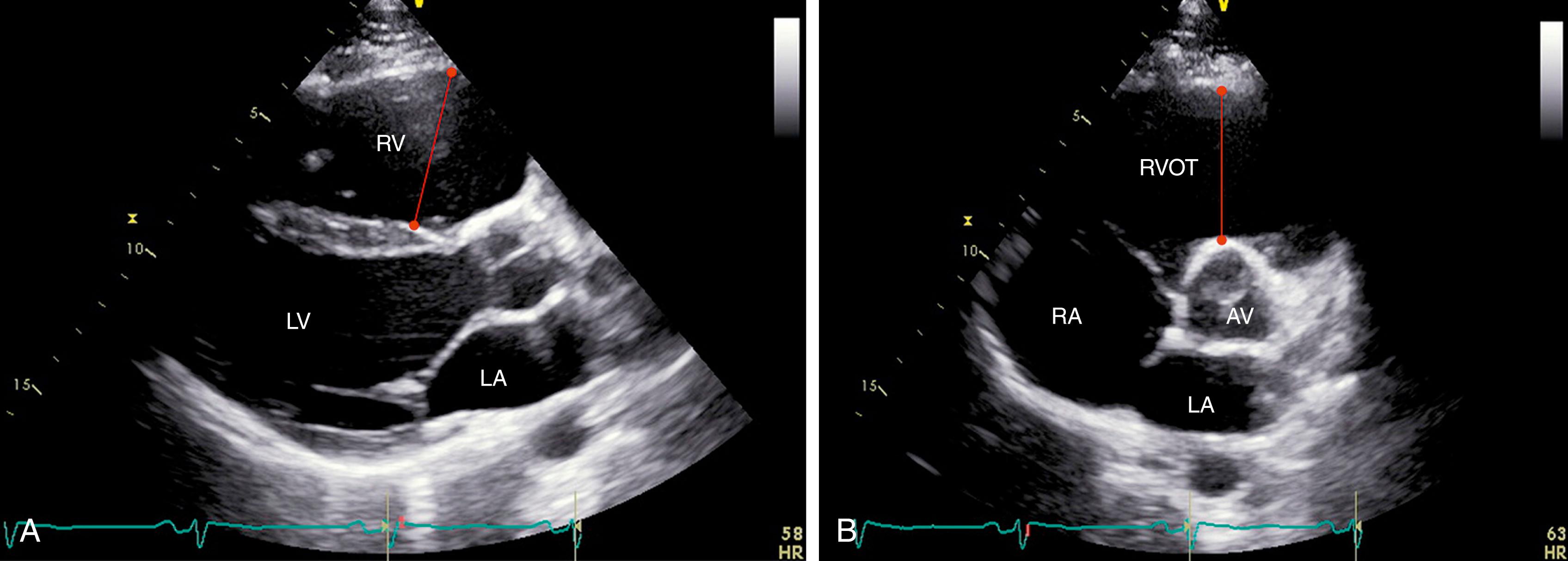
RV dilatation is almost uniformly noted in ARVC ( Fig. 74.1 ). All patients in the original descriptions of the disease had either local or global RV dilatation. RV outflow tract (RVOT) dilatation was found in 100% of probands from the North American Multidisciplinary Study of ARVC. According to the 2010 revised Task Force Criteria ( Table 74.1 ), a major criterion for ARVC is fulfilled when there is RVOT dilatation seen in the parasternal long-axis (PLAX) view 32 mm or greater or parasternal short-axis (PSAX) view 36 mm or greater in conjunction with impaired RV systolic function or localized aneurysms (akinesia or dyskinesia). Sensitivity and specificity for the diagnosis of ARVC are 75% and 95% for the PLAX view and 62% and 95% for the PSAX views, respectively.
| Global or Regional Dysfunction and Structural Alterations |
|---|
| Major |
|
| Minor |
|
Either global or regional RV systolic impairment is commonly seen in ARVC, particularly in more advanced stages of the disease. A total of 79% of probands in the North American Multidisciplinary Study had qualitative abnormalities in regional RV systolic function. The anterior wall and apex were most commonly affected. Because of the asymmetric geometry of the right ventricle and difficulty visualizing the entire RV endocardium, estimation of RV volume (and hence RV ejection fraction) by two-dimensional (2D) echocardiography is challenging. In cases in which endocardial definition is poor, the administration of an ultrasound-enhancing agent can facilitate assessment of global and regional RV systolic function.
RV fractional area change (FAC) from the apical four-chamber view has been shown to be a useful correlate of RV systolic function and is decreased in individuals with ARVC compared with control participants ( Fig. 74.2 ). In the proposed Task Force Criteria modification, the optimal cutpoint for FAC (≤33%) was determined using data from the Multidisciplinary Study coupled with a large group ( n = 450) of normal individuals. In the presence of regional wall motion abnormality, an FAC of 33% or less had a sensitivity of 55% and specificity of 95% for the diagnosis of ARVC. Importantly, FAC also has prognostic value, predicting an increase in major adverse cardiac events in this population. , Although measuring FAC can be difficult without adequate RV endocardial delineation, given its importance in diagnosis and prognosis, it should be quantified whenever possible.
![Figure 74.2, Measurement of fractional area change (FAC) of the right ventricle in a patient with known arrhythmogenic right ventricular cardiomyopathy using end-diastolic ( A ) and end-systolic ( B ) areas. FAC is calculated as the ratio of the difference between end-diastolic area (EDA) and end-systolic area (ESA) to EDA (i.e., FAC = [EDA-ESA]/EDA). It is important to carefully delineate pacemaker or implantable cardioverter-defibrillator wires (arrows) from the endocardial border of the right ventricle. Figure 74.2, Measurement of fractional area change (FAC) of the right ventricle in a patient with known arrhythmogenic right ventricular cardiomyopathy using end-diastolic ( A ) and end-systolic ( B ) areas. FAC is calculated as the ratio of the difference between end-diastolic area (EDA) and end-systolic area (ESA) to EDA (i.e., FAC = [EDA-ESA]/EDA). It is important to carefully delineate pacemaker or implantable cardioverter-defibrillator wires (arrows) from the endocardial border of the right ventricle.](https://storage.googleapis.com/dl.dentistrykey.com/clinical/EchocardiographyinArrhythmogenicRightVentricularCardiomyopathy/1_3s20B9780323698306000742.jpg)
Conventional measurements of RV systolic function, such as tricuspid annular plane systolic excursion and RV peak systolic annular velocity (RV S′), tend to become abnormal only in advanced stages of disease. They are also limited by angle dependency of this measurement relative to longitudinal contraction. This can be problematic late in the course of disease when dilatation and displacement of the tricuspid annulus alter the plane of annular motion.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here