Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Dupuytren disease (DD) is the most common heritable fibrotic disease. Dupuytren is a chronic disease that can cause progressive fibrosis and finger flexion contractures. The etiology is a “two-hit” process of genetic predisposition and environmental stressors triggering onset and progression, similar to other fibrotic diseases. Dupuytren affects 10 million Americans and varies widely in biologic severity. The majority of those affected have no contractures. The majority with contractures have mild disease biology, needing one or two corrective procedures during their lives. A small minority—3% to 5%—have a treatment-resistant phenotype with progressive hand impairment despite interventions. Undertreatment, overtreatment, and retreatment are common.
DD is not well understood. Conventional Dupuytren wisdom includes misconceptions and oversimplifications that need debunking. For example, Dupuytren is not a “disease of the fascia.” It is not a “Viking” disease. Meaningful Dupuytren prevalence figures require age, gender, and ancestry context. Recontracture incidence is not a simple percent. Dupuytren “recurrence” has no standard definition in common use and is a misleading term. There is little consensus on what defines Dupuytren diathesis or its impact before treatment. Posttreatment Dupuytren splinting has no demonstrable long-term benefit. Open proximal interphalangeal joint release for Dupuytren has no predictable long-term benefit. Alcoholism, smoking, manual labor, diabetes, and local trauma are, at most, minor risk factors. Dupuytren inheritance is not merely autosomal dominant with variable penetrance. Short-term results do not predict long-term outcomes in DD. This chapter addresses these and related issues.
Dupuytren contracture (DC) is only one aspect of DD. Even without measurable contracture, many Dupuytren patients struggle with local tenderness, sensory complaints, , and a subjective loss of dexterity. DC is associated with a range of other benign diseases, carcinoma, sarcoma, and early death. ,
Dupuytren procedures reduce angular contracture severity. Open procedures such as fasciectomy and dermofasciectomy remove shortened tissues. Minimally invasive procedures such as percutaneous needle fasciotomy (PNF) or collagenase clostridium histolyticum (CCH) injection disrupt fibrotic tissue continuity in situ. Current treatment guidelines vary based on individual risk factors, local anatomy, disease history, and the surgeon’s and patient’s preferences. Dupuytren management still follows the original 1800s model: Wait for a bent finger, do something to make it less bent, ignore all other aspects of the disease, and repeat as needed.
Long-term Dupuytren treatment outcomes have not changed since the introduction of dermofasciectomy in the mid-1900s. Currently, fasciectomy is the most common treatment. Fasciectomy has a higher risk of permanent complications and treatment failure over time than operations of similar complexity for other benign hand diagnoses. Failed Dupuytren surgery is the most common indication for elective finger amputation, with an amputation rate of 8% reported for fasciectomy performed after dermofasciectomy.
Many aspects of DD lack clarity: enigmatic biology; unpredictable natural history; lack of standard terminology either for severity, outcome, recontracture, or patient satisfaction; lack of an animal model; lack of a unique biomarker; variation in biologic aggressiveness; and intermittent or slow progression, often over many years. Proper management can be rewarding, and treatment options have grown in the last decade, yet “two issues remain unsolved relevant to Dupuytren’s disease: its cause and its cure.” The real hardship of Dupuytren is felt less by surgeons than by patients, who may give up rather than risk additional treatment complications for temporary partial improvement. Our goal is to minimize the lifetime burden of both DD and the complications of our treatment.
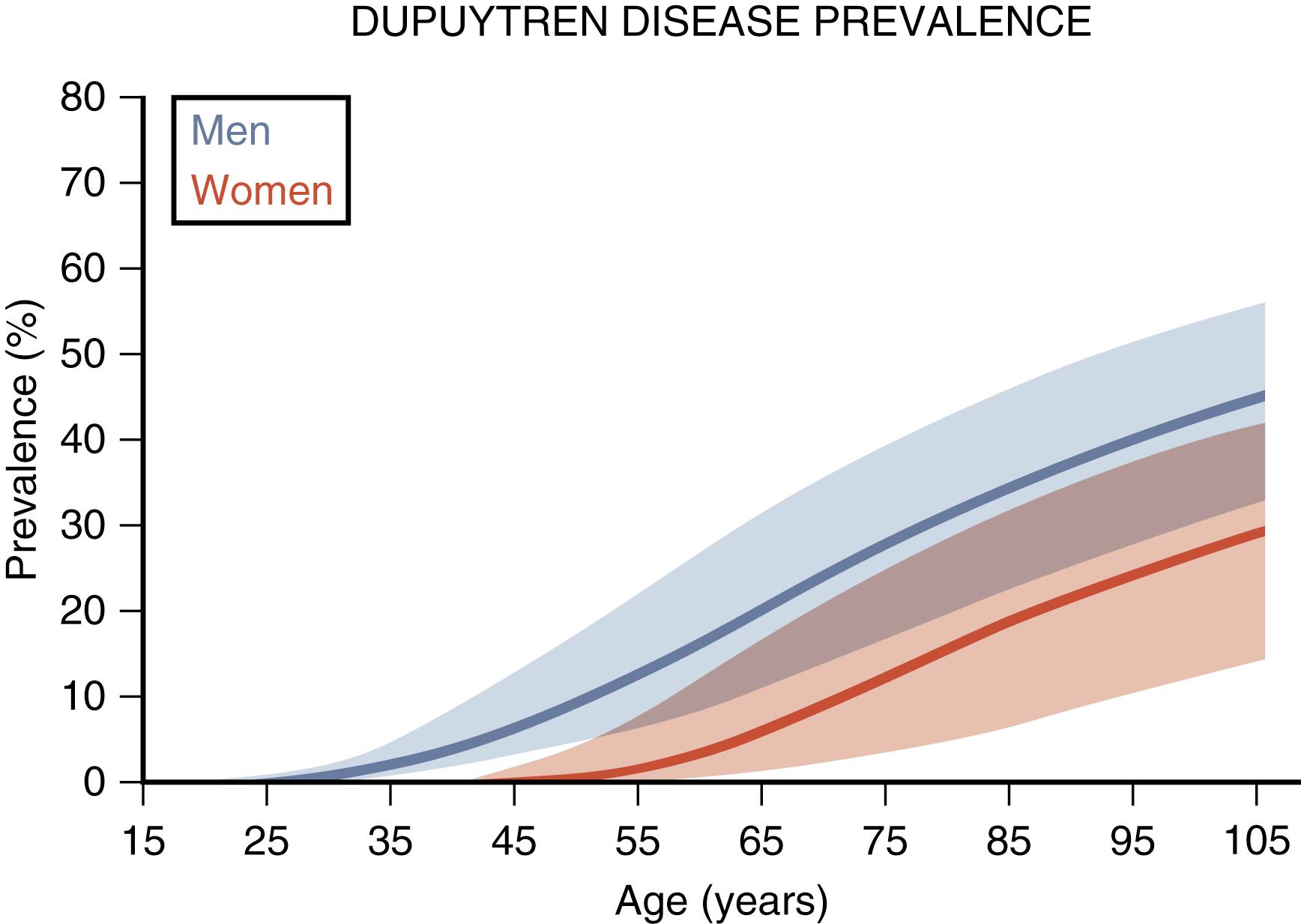
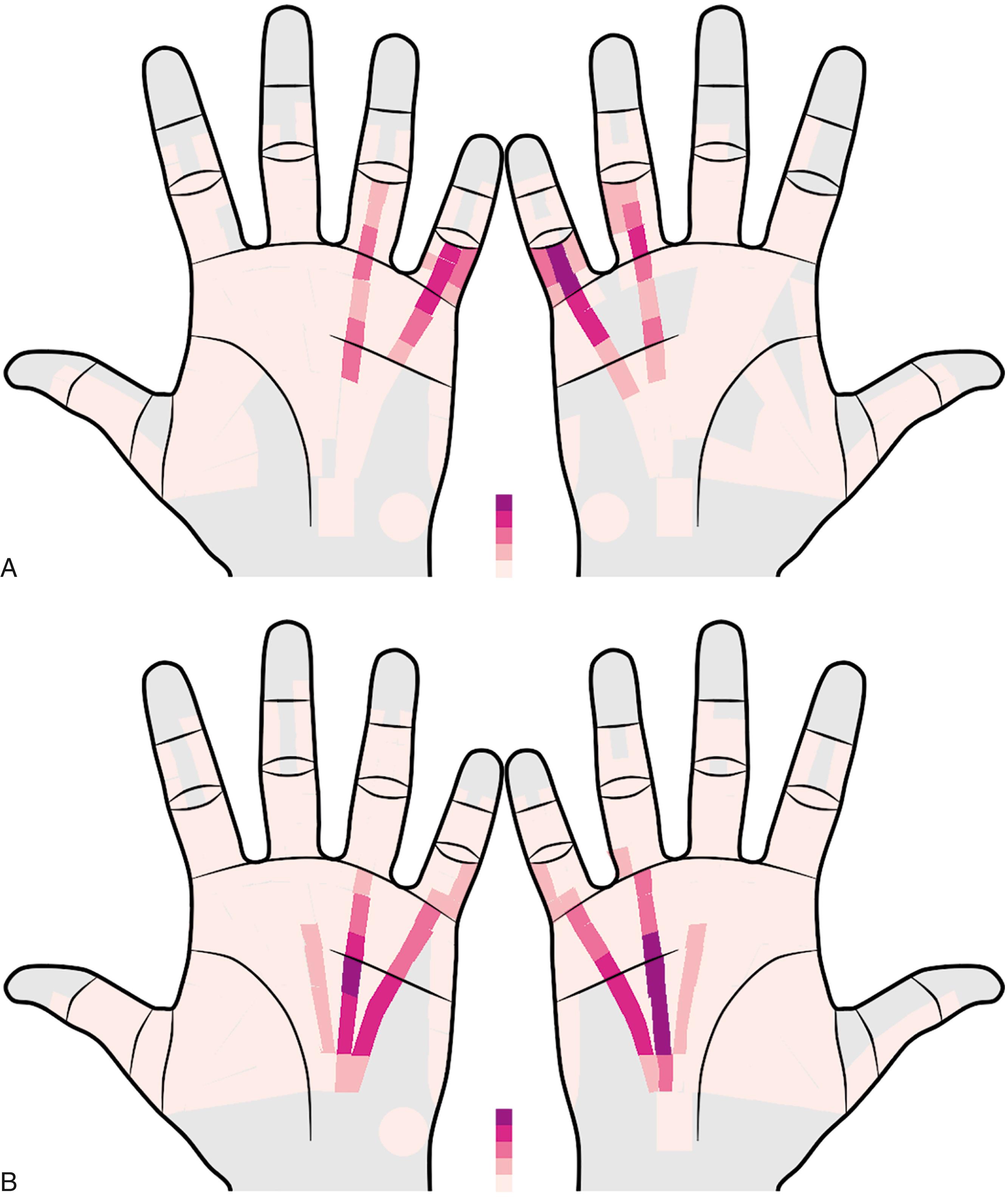
DD is most prevalent in senior Caucasian men with a family history of the condition. Clinical onset is rare in childhood and is uncommon before age 40. After 50, the prevalence increases with each decade, as shown in Fig. 4.1 . The mean prevalence in Caucasians is 12% at age 55, 21% at age 65, and 29% at age 75. The most common age range at diagnosis is the early 50s to the early 60s. The incidence in women parallels that of men but lags by an average of 10 to 15 years, resulting in a male-to-female prevalence ratio that decreases with age. Prevalence in men decreases after 70 and in women after 80 due to increased DD patients’ mortality compared with the general population. The ring or little fingers are most often involved. Overall, half of those affected have bilateral disease, but this varies with age: Initially, only one in five have bilateral disease, but three in four will eventually have bilateral involvement.
Dupuytren is a polygenic disease. Studies have reported autosomal dominant, X-linked, mitochondrial, and recessive genes , ; copy number variations ; and epigenetic changes. It is unhelpful to describe Dupuytren as an isolated or geographic disease. The idea that Dupuytren is a “Viking disease” began as a 1985 speculation and was widely repeated without evidence. Genetic research has discounted this along with the notion that Vikings were genetically homogenous blue-eyed blonds. Dupuytren is a Caucasian disease. In Caucasians, Dupuytren is as common in Bosnia as it is in Norway. Compared with Caucasian Americans, Dupuytren is one-third as common in Hispanic Americans, one-fifth as common in African Americans, and one-tenth as prevalent in Asian Americans, roughly paralleling the percent Caucasian heritage of these American groups. Dupuytren is just one of many fibrotic diseases influenced by ancestry. For example, African ancestry correlates with an increased risk of nephrosclerosis, keloid, and scleroderma. Caucasian descent increases the risk of Dupuytren, Ledderhose, frozen shoulder, pulmonary fibrosis, and primary biliary cholangitis.
Self-reported family history studies underreport Dupuytren prevalence because of late-onset disease and lack of awareness of mild involvement in senior relatives. Nearly half of DD patients know of an affected relative. However, over two-thirds will have an affected living relative if one examines the extended family tree. Positive family history is the strongest predictor of developing the disease and is associated with early onset and early age of first treatment. , On average, patients with both parental lines affected have an earlier age of onset than those with only one affected line, who, in turn, have an earlier age of onset than those with no family history. An affected sibling triples one’s risk of developing DD. These findings strongly suggest a genetic basis. It is likely that Dupuytren, like diabetes, is not one disease but a phenotypic group involving multiple genes and environmental triggers.
In Dupuytren patients, similar local soft tissue changes can arise in areas outside the palm. Nodular fibrotic changes of the extensor surfaces of finger or toe joints (knuckle pads), plantar tissues (Ledderhose), shoulder joint capsule (frozen shoulder), and tunica albuginea of the penis (Peyronie) correlate with the presence of DD. Other locations of Dupuytren-linked nodular pathology include the gastrocnemius fascia, Achilles tendon, forearm, palmaris longus, Guyon canal, flexor carpi ulnaris, , flexor carpi radialis, , and upper arm. Because these findings may or may not present with palm disease, “ectopic Dupuytren disease” is a misnomer. For example, the author has followed a patient for over a decade with bilateral knuckle pads, Ledderhose, and frozen shoulder, but unaffected palms. Referring to this group of related fibrotic manifestations as Dupuytren spectrum disease better reflects demographic , , , and genomic correlations and the need for a systemic research focus.
The term diathesis was first used in 1891 to distinguish idiopathic from “false” (posttraumatic) DC. Houston reintroduced the term in 1961 to describe a recontracture-prone Dupuytren phenotype associated with a family history of Dupuytren, knuckle pads, bilateral disease, Ledderhose disease, and “younger average age” at treatment. However, he repeatedly changed the criteria and, three decades later, cautioned against efforts to quantify diathesis-based predictions.
Efforts to quantify the correlation between recontracture and diathesis factors have yielded conflicting results. Statistical correlations between recontracture and family history of Dupuytren have been reported as significant , and not significant ; knuckle pads significant , and not significant ; Ledderhose significant , , and not significant, , early-onset disease significant, , not significant, and significant negative risk ; any ectopic lesions (knuckle pads, Ledderhose, Peyronie) significant , , , and not significant , ; bilateral involvement significant and not significant , , , ; male gender significant , and not significant ; younger age at first treatment significant, , , , , not significant, , and significant negative risk ; more than two involved rays significant and not significant ; more than three involved rays significant and not significant ; radial hand involvement significant , , and not significant. , Predictive combinations of risk factors have also been reported , , and disputed. Despite these uncertainties, diathesis factors of family history of DD, onset younger than 50, Ledderhose, and knuckle pads are in everyday use to guide recommendations. The bulk of evidence suggests the strongest biologic predictor of recontracture is younger age at the first corrective procedure. Diathesis factors do not correlate with either histologic staging, Dupuytren genetic risk score, or 5-year functional outcomes.
Reported DD comorbidities include hypercholesterolemia, diabetes, smoking tobacco, alcoholism, diabetes, psoriasis, epilepsy, regional trauma, chronic hand vibration exposure, and a lower-than-average body mass index. Whether these associations cause, aggravate, or relate to a common origin requires a better understanding of the molecular biology of disease onset and disease persistence. The literature is mixed, with conflicting reports of either correlation or lack of correlation for most of these factors. ,
Dupuytren risk categories include (1) risk of ever developing DD, (2) risk of progression from a nodule to contracture, and (3) risk of recontracture after a corrective procedure. These are not interchangeable. For example, diabetes and hyperlipidemia , correlate more with the risk of developing DD than with the risk of progressing to DC. The opposite is the case for chronic hand vibration exposure. Alcoholism correlates with a greater risk of diagnosis and contracture but not the age at first treatment.
Spurious correlations are an issue in the study of Dupuytren and other chronic diseases. For example, frequent medical visits for a chronic illness increase the chance of diagnosing an unrelated chronic disease such as DD and falsely associating the two. , In contrast, patients with multiple medical comorbidities are less likely to choose treatment for recontracture, biasing associations between comorbidities and postprocedure progression. Measurements of angular contracture severity without considering disease duration may also yield spurious correlations. For example, chronic heavy alcohol use correlates with greater contracture severity but may also delay treatment presentation, leading to greater contracture severity at presentation. Disease awareness from affected family members lowers the average self-reported age of onset, which increases the self-reported duration of disease.
Rheumatoid arthritis and obesity are negative risk factors for Dupuytren: They correlate with a lower risk of developing DD. Overlapping genetic loci relating to both body mass index (BMI) and DD , points to the potential of investigating biologically different comorbidities to guide further research into the origins of DD.
It is also important to avoid conflating statistical significance (the chance that there is a correlation) and effect size (how influential is the correlation). An association may have high statistical significance but a low numerical effect on the entire cohort. For example, family history correlates strongly with and has a large effect size on the risk of developing Dupuytren and younger age at the first corrective procedure. Smoking also correlates significantly with the risk of developing Dupuytren. However, tobacco’s effect size is so small relative to a positive family history that the increased risk from smoking is only demonstrable in those without DD in the family. Nongenetic conditions that correlate with DD should be considered minor risk factors.
Anecdotal associations between mechanical injury and Dupuytren date back 400 years to Plater’s description of the contracture appearing after an acute injury. Guillaume Dupuytren suggested an inciting effect of repeated palm trauma in those predisposed to the disease. Whether Dupuytren is an occupational injury or provoked by acute injury has been debated by as many lawyers as physicians since the 1800s. Statistical challenges include lack of controls, the common occurrence of unreported hand injuries, lack of measures of nonoccupational manual activities, and lack of a biomarker to assess the real-time effects of injury or strenuous hand use on Dupuytren biology. Summarizing a long and unresolved debate, local injury or prolonged high force manual activities may provoke changes in the palm resembling early DD. In some patients, these changes progress to contracture. The effect size of injury or heavy manual activities on Dupuytren onset or rate of progression remains unknown.
Dupuytren correlates with flexor tendinitis , for reasons unknown. The association may represent shared risk factors, aggravating effects of tendinitis-related inflammation on Dupuytren, or shared biology. Mild proximal interphalangeal (PIP) contractures associated with chronic flexor tendinitis can confuse the assessment of concurrent Dupuytren. More problematic, Dupuytren-related flare reaction can worsen the morbidity and outcome of trigger finger surgery.
With slowly progressive chronic diseases, there are often lengthy periods between onset, diagnosis, and first treatment. As a group, 9 out of 10 patients with palmar DD have no contracture, and many are unaware that they have a condition. On average, 2 years pass between a patient’s first awareness of the disease and their first medical evaluation for DD. The involved palmar surface area increases over time, but this does not correlate well with angular contracture progression. Patients with mild DD may not progress to DC. A rough 10-year prediction for patients presenting with isolated nodules is as follows: 10% will have spontaneous nodule involution, 20% will develop contracture requiring treatment, and 70% will either be unchanged or progress to cord without contracture. , The presence of knuckle pads correlates with earlier contracture progression in patients with isolated nodules and no contracture.
Conversely, patients may develop contractures without first noticing nodules: Cords or contractures are the sentinel finding in nearly one-quarter of patients. Clinical behavior stratifies patients into four prognostic risk groups. The likelihood of future corrective procedures for DC, in groups of increasing risk, are people with (1) no clinical DD, (2) untreated DD without DC, (3) untreated DC, and (4) treated DC.
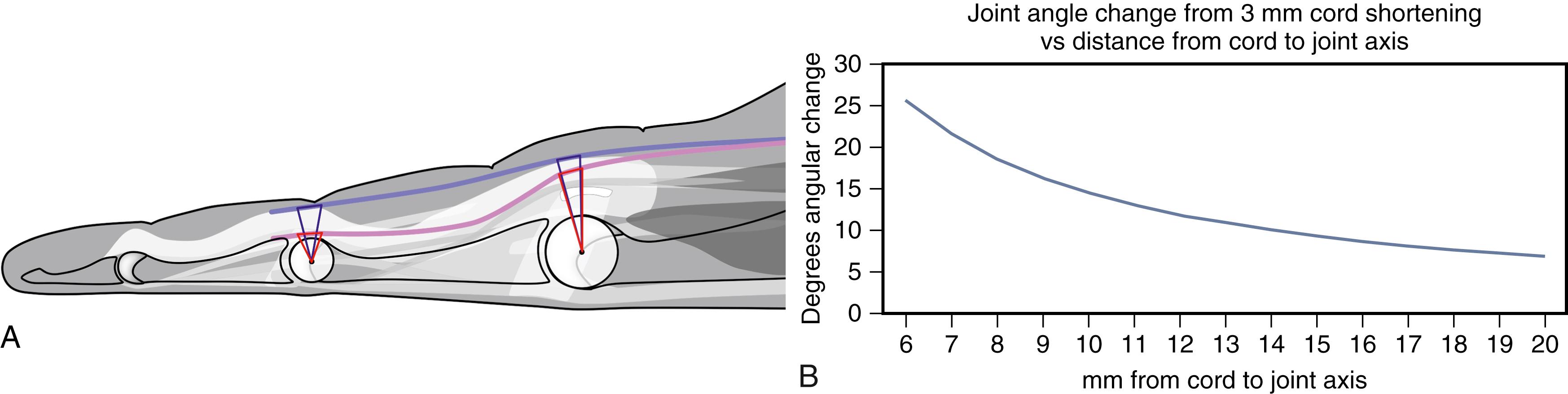
Contracture progression is often episodic and unpredictable. The surface area of involvement, number of affected rays, and total angular contracture tend to increase over time. Dupuytren diathesis is not associated with more rapid contracture progression in untreated patients. Instead, anatomic geometry influences the rate of angular contracture. Dupuytren causes linear soft tissue contracture, but we only measure secondary angular joint changes. Angular change from cord shortening depends on the length of shortening and increases logarithmically with decreasing distance between a cord and a joint’s axis of rotation ( Fig. 4.2 ). This geometry explains location-specific differences. Lateral cords, PIP cords, and smaller joints cords are comparatively closer to the joint axis than midline cords, metacarpophalangeal (MCP) cords, or cords of larger joints. Also, lateral cords are more common at PIP joints than MCP joints. These factors alone predict faster contracture rates of PIP joints than MCP joints and faster contracture rates for small finger contractures than the larger digits. These predictions are consistent with the author’s series of 2046 previously untreated patients showing average rates of angular PIP contractures greater than twice that of MCP joints and overall small finger contracture rates twice that of the other fingers.
The rate of DC progression is inexplicably slow compared with our current understanding of fibrotic tissue contracture progression. Skin wound contraction proceeds at 0.65 ± 0.25 mm per day, or 1 to 3 cm per month. Myofibroblast matrix contraction models predict rates of about 1 cm per month. A Dupuytren cord would produce a 90-degree joint contracture at these rates in 1 to 3 months, depending on location. This contracture rate is rare, suggesting our current disease model is a poor fit of the basic disease biology.
Despite a wide range of observed Dupuytren-related genomic and proteomic abnormalities, there is no unique biomarker profile yet of Dupuytren biologic activity in blood or tissue. Dupuytren biomarker research is challenging because Dupuytren biology is dynamic and spans immune, inflammation, and soft tissue remodeling pathways. A circulating Dupuytren biomarker profile could reveal systemic disease dynamics and quantify real-time therapeutic drug response.
Genetic Dupuytren is a polyclonal polygenic disease. The most extensive genome-wide association studies of Dupuytren to date report variants in over two dozen genetic loci associated with fibrosis, inflammation, and extracellular matrix regulation. , A weighted genetic risk score based on this work correlates with increased recontracture risk after contracture treatment. However, this genetic risk score does not correlate with clinical risk (diathesis) scores. It has a smaller effect size than diathesis risk factors early age of onset, bilateral disease, ectopic disease, and positive family history. Overlap of gene variants in pulmonary fibrosis, arthrofibrosis, and DD suggest Dupuytren predisposition is a subset of many genetic factors predisposing to fibrosis.
Gene expression studies are a promising addition to Dupuytren DNA research but have a Dupuytren-specific challenge. Gene expression of DD affected tissues are exquisitely sensitive to their mechanical environment. Dupuytren tissues respond within minutes to changes in mechanical tension, , resulting in potential gene expression artifacts in both cell culture , and xenograft tissue transplant models. Studies correlating gene expression with clinical outcomes have yielded mixed results. ,
The genetic relationship between DD and gender is complicated. Androgen receptors are overexpressed in the palmar fascia of Dupuytren patients. TIMP-1, the gene that expresses tissue inhibitor of metalloproteinase 1 (implicated in DD), is located on the X-chromosome. Estrogen suppresses transforming growth factor beta one (TGFβ1)-induced myofibroblast differentiation, collagen synthesis, , and matrix metalloproteinase gene expression in response to acute mechanical stress. Estrogen might explain why onset in women is delayed on average compared with men until after menopause.
Dupuytren-related proteomic abnormalities have been identified in plasma, extracellular matrix, cell membranes, cytoplasm, and cell nuclei. These include acute phase proteins, chemoattractants, cytokines, cytoskeletal proteins, , enzymes, , , , , enzyme inhibitors, , , growth factors, , , , , extracellular matrix components , , , , , , , , membrane proteins, , , , , , , , cell metabolism proteins, , , , , oxidative stress proteins, signaling proteins, , , , , , , and transcriptional regulators. , , These findings suggest the use of a proteomic panel to stage Dupuytren activity.
Collagen fibrils are trimers of three collagen molecule chains linked in a triple helix. In this configuration, collagen molecule proteolytic substrate sites face inward, block enzyme access, and provide resistance to enzymatic degradation. Mechanical stress causes conformational changes that dynamically affect this resistance. Low tensile loads (e.g., normal hand use) suppress enzymatic degradation. In contrast, high tensile loads (e.g., external skeletal traction) facilitate enzymatic degradation. High tensile loads also stimulate both collagen synthesis and collagenase production by fibroblasts. ,
Dupuytren patients have abnormal increases in three collagen components: α1(I) homotrimer collagen fibrils, type III collagen fibrils, and collagen crosslinks.
Homotrimer type I collagen: The predominant Dupuytren collagen is heterotrimer type I collagen, assembled from two α1(I) collagen chains and one α2(I) chain. The less common homotrimer type I collagen has three α1(I) chains. Dupuytren-affected tissues have a higher percentage of type I collagen homotrimers than fascia from unaffected patients. Homotrimer type I collagen is more resistant to collagenases than heterotrimer type I collagen at rest but, under mechanical stress, is more susceptible to enzymatic degradation than heterotrimer type I collagen.
Type III collagen is a homotrimer of three α1(III) collagen chains. In patients without Dupuytren, palmar fascia is predominantly type I collagen with less than 5% type III collagen. , Type III collagen levels increase in Dupuytren affected tissues, contributing 30% to 40% of nodule collagen , and 20% to 30% of cord collagen. Fibroblast-related contraction is more rapid for type III collagen than type I collagen. Persistent elevation of type III collagen may be unique to Dupuytren biology. Clinically unaffected palmar fascia in DC patients contains 10% to 15% type III collagen, and this high percentage persists through end-stage disease. , This profile is unlike normal wound healing biology in which type III collagen levels rise rapidly after wounding but drop rapidly to preinjury levels with scar maturation. , This indefinite elevation of type III collagen is not typical of other chronic fibrotic diseases, including pulmonary fibrosis, scleroderma, liver fibrosis, arteriosclerosis, keloid, or hypertrophic scar.
Crosslinks between collagen fibrils increase tensile strength and stiffness. Dupuytren-affected palmar fascia has 5 to 10 times the collagen crosslinks than normal palmar fascia, and additional hydroxylation and glycosylation stabilizing these crosslinks. This effect is similar to the increased collagen stiffness, reduced elasticity, and protease resistance from glycosylation and advanced glycation end-products from diabetes.
Fibroblasts are normal resident cells in the palmar fascia and retaining ligaments and septal fibers of the palm, and are the dominant cell type in Dupuytren nodules. Fibroblasts cultured from Dupuytren-affected tissues contract more rapidly than fibroblasts from the unaffected palmar fascia in the same patient. Other cell types overrepresented in local Dupuytren pathology include myofibroblasts, macrophages, lymphocytes, and pericytes.
Myofibroblast persistence is a feature of DD. This contrasts with the temporary presence in other scenarios such as wound healing, in which myofibroblast presence rapidly ends with the loss of mechanical and cytokine stimulation.
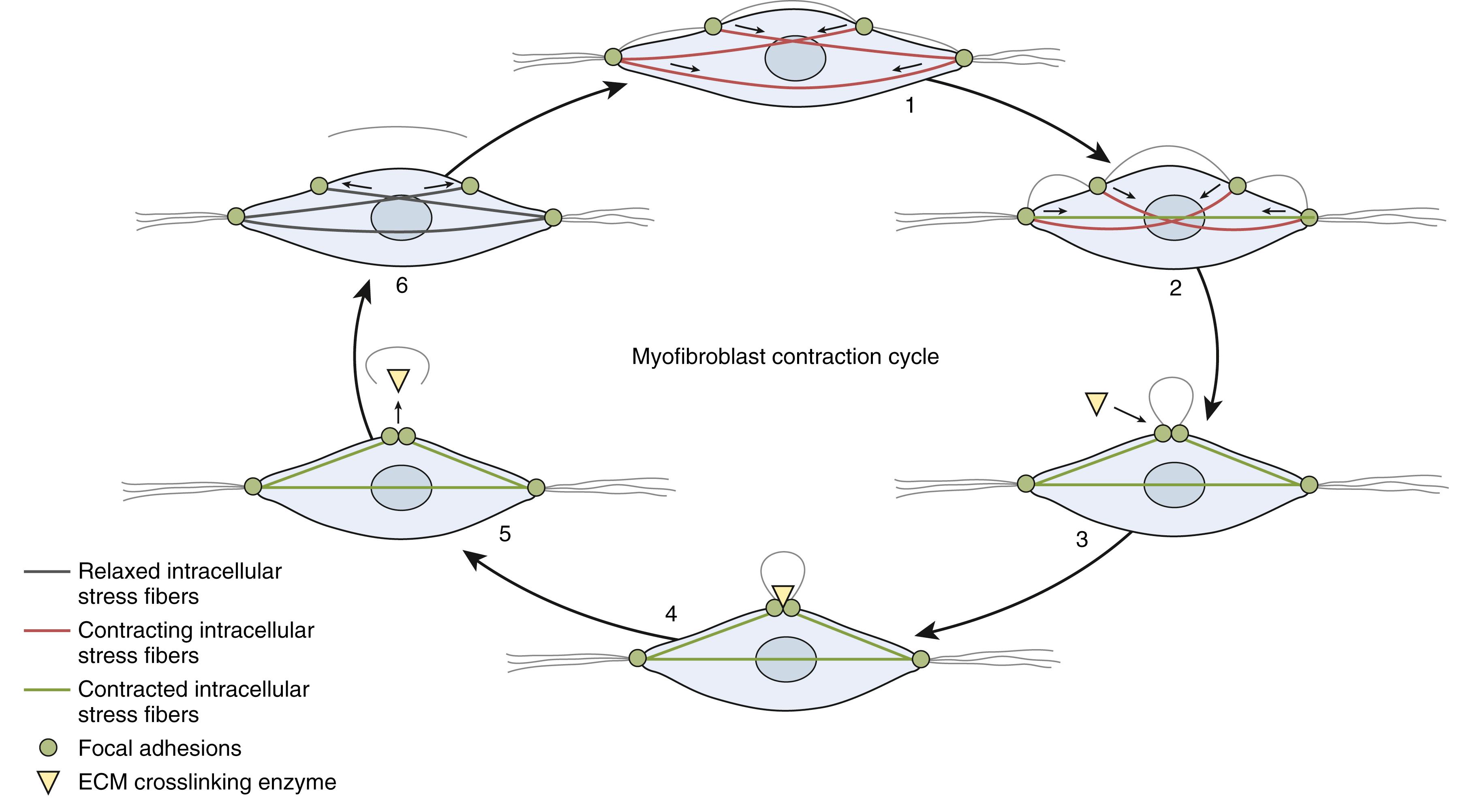
Fibroblasts and myofibroblasts populate Dupuytren nodules in a 3:1 ratio. Myofibroblast collagen deposition and matrix remodeling are responsible for creating and shortening Dupuytren cords. Myofibroblasts are produced by epithelial/endothelial-mesenchymal transition (EMT/EndMT) of local cells or circulating bone marrow–derived fibrocytes. Myofibroblasts resemble fibroblasts under light microscopy but have ultrastructural differences: stress fibers, cell membrane adhesion complexes, fibronectin fibers, cytoplasmic actin, smooth muscle α-actin, and other characteristics. They appear in response to tension and dedifferentiate in response to a loss of tension. Fibroblasts differentiate into proto-myofibroblasts under mechanical stress, expressing some myofibroblast-like ultrastructural changes. , The full myofibroblast phenotype arises from to the combined stimulation of mechanical tension and growth factors such as TGFβ. Myofibroblasts vary in phenotype depending on cell type origin and local conditions and can express a range of biophysical properties within the same surgical specimen. Normal-appearing fibroblasts derived from dedifferentiated Dupuytren myofibroblasts are more responsive to TGFβ stimulation than fibroblasts from unaffected transverse carpal ligament tissue.
Myofibroblasts demonstrate the direct relationship between mechanical forces and gene expression in DD. Changes in soft tissue tension are physically linked from matrix collagen to pericellular fibronectin to cell membrane-bound integrins to cytoplasmic stress fibers to other cytoskeletal proteins to chromosomes within the cell nucleus, where they trigger gene expression changes. These connections let myofibroblasts sense and alter extracellular matrix tension and remodel the matrix through coordinated calcium-mediated and calcium-independent cytoskeletal contraction mechanisms. Myofibroblast can contract as a coordinated syncytium-like group via intercellular junctions. Myofibroblasts also secrete cytokines, growth factors, enzymes, and other factors involved in Dupuytren pathobiology.
Macrophages appear in Dupuytren nodules and perivascular locations at the periphery of nodules. In Dupuytren nodules, macrophages accompany myofibroblasts, and their density correlates with myofibroblast density. An important macrophage classification is M1 and M2 phenotypes. M1 macrophages are involved in acute inflammation. They secrete inflammatory cytokines, such as tumor necrosis factor alpha (TNF-α) and interleukin one beta (IL-1β), that promote myofibroblast activation and recruitment. As the inflammation subsides, these change to the M2 phenotype that secretes antiinflammatory cytokines and profibrotic growth factors, including TGFβ1 and IL-10. Mechanical tissue strain increases the M1 phenotype, a possible connection between strenuous hand activities and Dupuytren incidence. Corticosteroids trigger a transition from M1 to M2 phenotype, a possible factor in nodules’ response to corticosteroid injection. ,
Lymphocytes: Both T and B lymphocytes accumulate in perivascular locations at the periphery of Dupuytren nodules. , , Circulating DR+ T-cell levels are increased and circulating CD5+ B-cell levels are decreased in Dupuytren patients. , These peripheral blood findings are more pronounced in people with DD and Ledderhose disease than in those with Dupuytren alone and those with active versus end-stage disease. Corticosteroids lower local lymphocyte density and cytokine expression, possibly contributing to clinical response to corticosteroid nodule injection. Mechanical tissue strain stimulates T-cell dependent fibrogenic pathways, perhaps playing a role in the influence of manual labor on Dupuytren incidence. Lymphocytes are involved in a range of fibrotic pathologies, but it is unclear whether they play a primary or secondary role in DD.
Pericyte proliferation, occluded microvessels, and basal lamina thickening in cords, nodules, and perinodular areas occur, accompanied by perivascular myofibroblast, macrophage, and lymphocyte accumulation. , , ,
Early DD is underdiagnosed and underreported. Noticeable early changes may go unnoticed by the patient. The earliest signs are skin tightness (exaggerated blanching with finger extension), contour changes (skin crease deformation, dimples), nodules, cords without contractures, or prominence of the palmar monticuli, as shown in Fig. 4.3 .
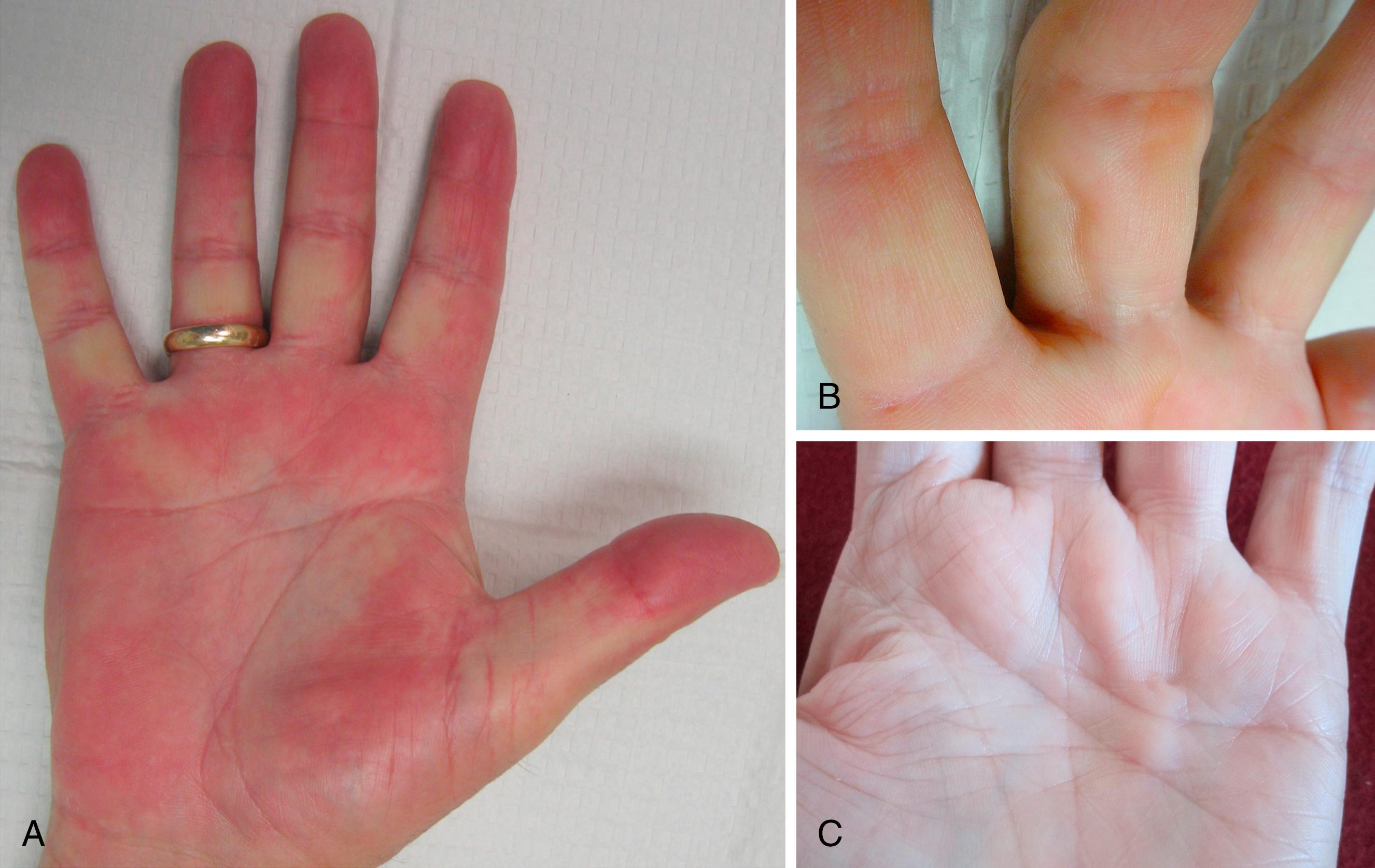
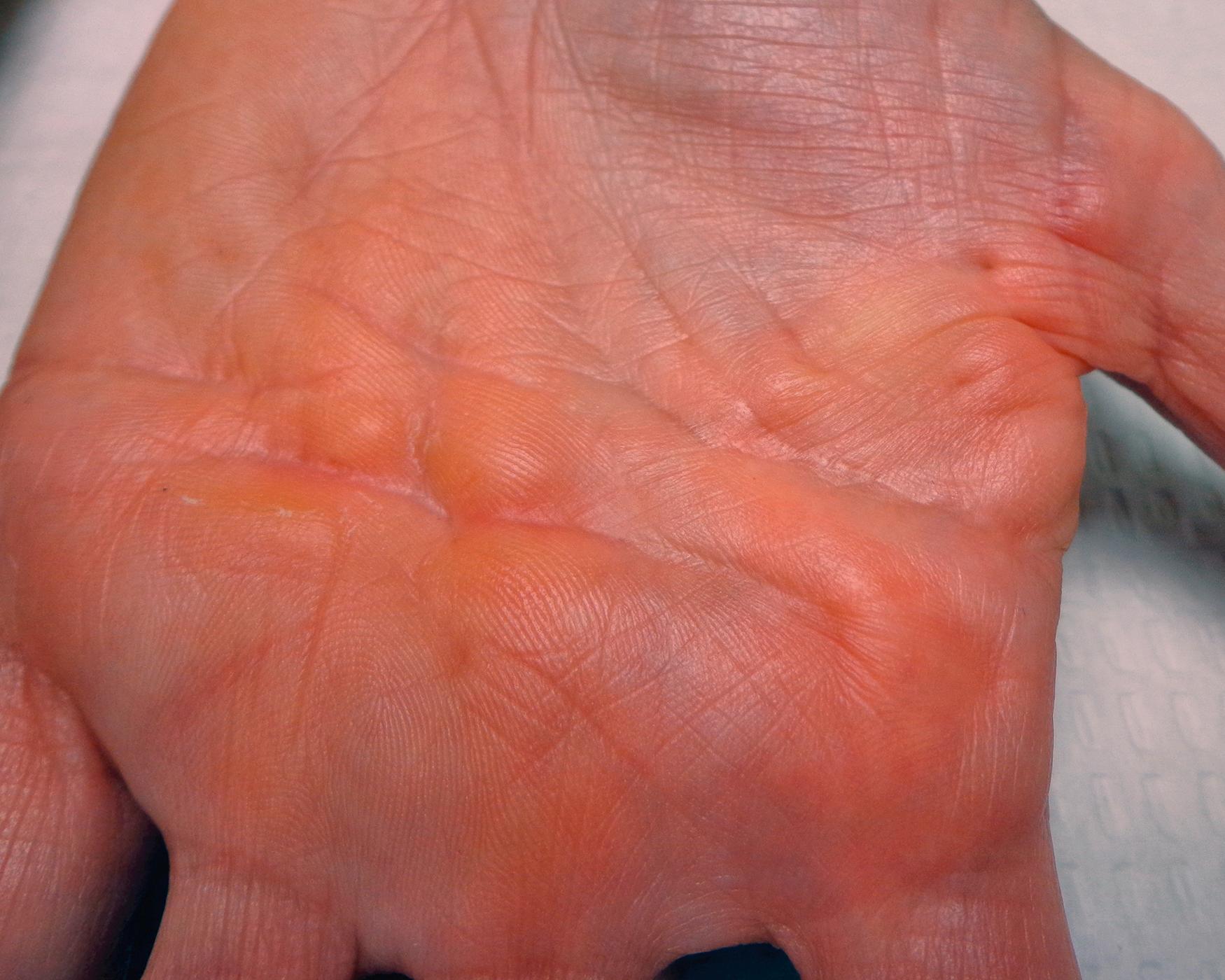
Palmar nodules are the first change noticed by 6 out of 10 patients. Nodules are firm subcutaneous masses, flattened round or oval with indistinct margins, 0.5 to 1.5 cm in diameter, usually fixed to the dermis and, if present, the underlying fascia. The dermal papillae overlying nodules may be prominent or longitudinally compressed, different from the type of papillary ridge stretching and flattening seen with other slowly growing tumors ( Fig. 4.4 ). One in four patients reports pink, tender, or itchy nodules on the first presentation. Skin crease deformation or dimples are the first changes in about 1 out of 10 patients.
Cords feel like strings beneath the skin and are secondary to the linear shortening of palmar soft tissues. They may be broad or narrow, single, multiple, or branched. Palmar nodules and cords usually align as beads on a string. Cords are strips of dense acellular collagen, and nodules are cellular tumor-like clusters, but overlap is common. , Cords with little nodularity are only visible and palpable under tension and are only attached to the skin at dimples or flexion creases, if at all. Nodular cord areas are palpable even when relaxed, and the overlying skin may be tethered or have nodule-type changes. Tendons may be mistaken for cords if they bowstring from flexor pulley incompetence due to trauma, prior surgery, spasticity, or rheumatoid. Tendon contours can become more prominent and cord-like with age-related loss of palmar subcutaneous fat.
Dorsal Dupuytren nodules (DDNs), also called Garrod pads or knuckle pads, are firm masses on the digital joints’ extensor aspect ( Fig. 4.5 ). Histologically, these resemble palmar nodules. Most DDNs involve finger PIP joints. DDNs are less common on distal interphalangeal (DIP) joints, MCP joints, interphalangeal (IP) joint of the thumb, and corresponding toe joints, and rarely involve the digital extensor mechanism. DDNs are fixed to the extensor mechanism’s superficial paratenon and may involve overlying subcutaneous tissue, digital retinacular fibers, or skin. A DDN may be confused with a dorsal cutaneous pad: local dorsal joint skin thickening and hyperkeratosis that only involves the skin. One in five DD patients has DDNs. These may precede palmar DD and are associated with a greater risk of palmar contracture progression. , In contrast, dorsal cutaneous pads are not associated with DD and are equally common with and without DD. Occasionally, the earliest sign of a DDN is a tethered depression of the extensor skin creases (see Fig. 4.5 ).
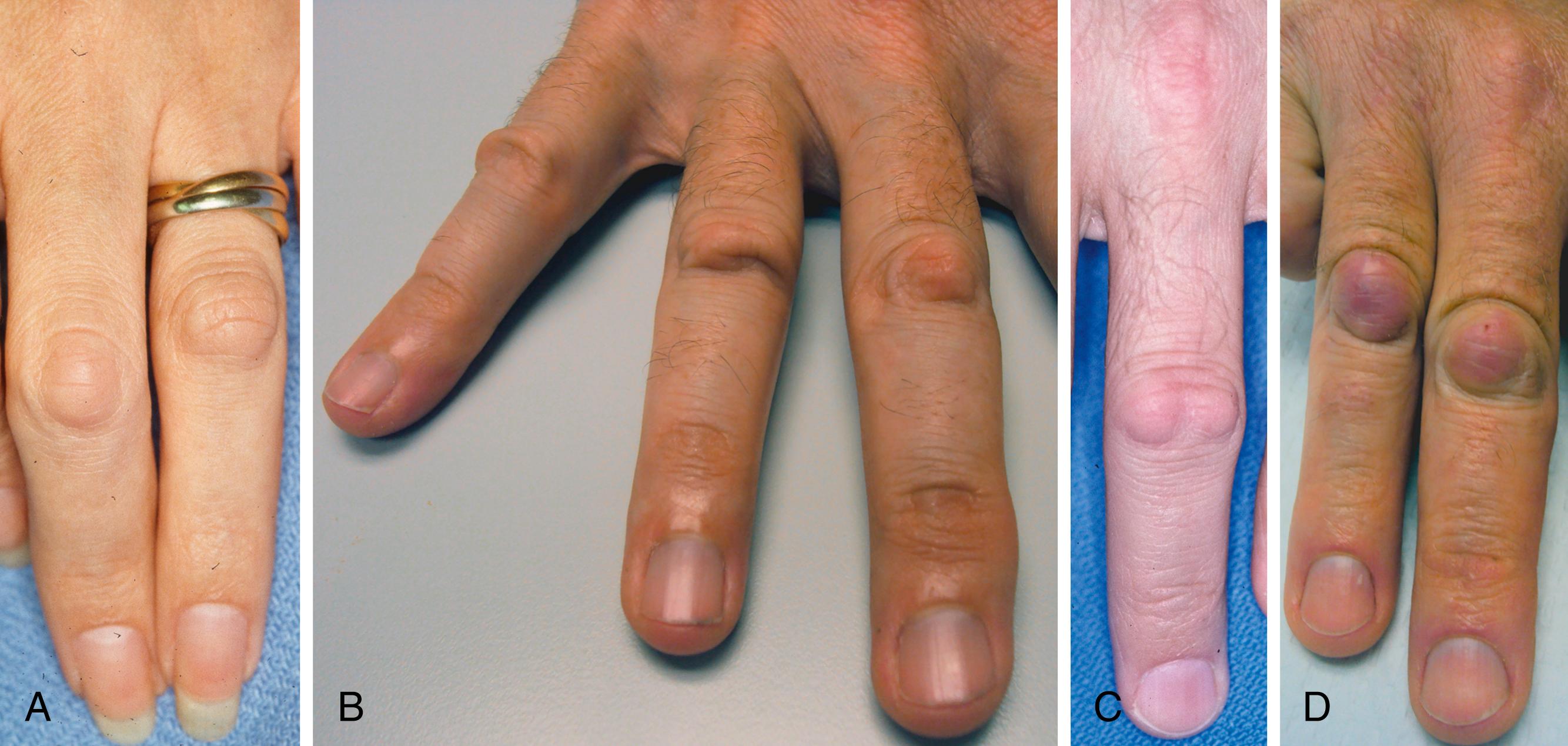
The differential diagnosis of palmar nodules includes fibrosarcoma, fibrous histiocytoma, giant cell tumor, synovial sarcoma, calcifying aponeurotic fibroma, epithelioid sarcoma, and other less common tumors. Because the diagnosis is based entirely on examination, consider scheduling a follow-up after the initial diagnosis to confirm the mass does not undergo unexpected changes.
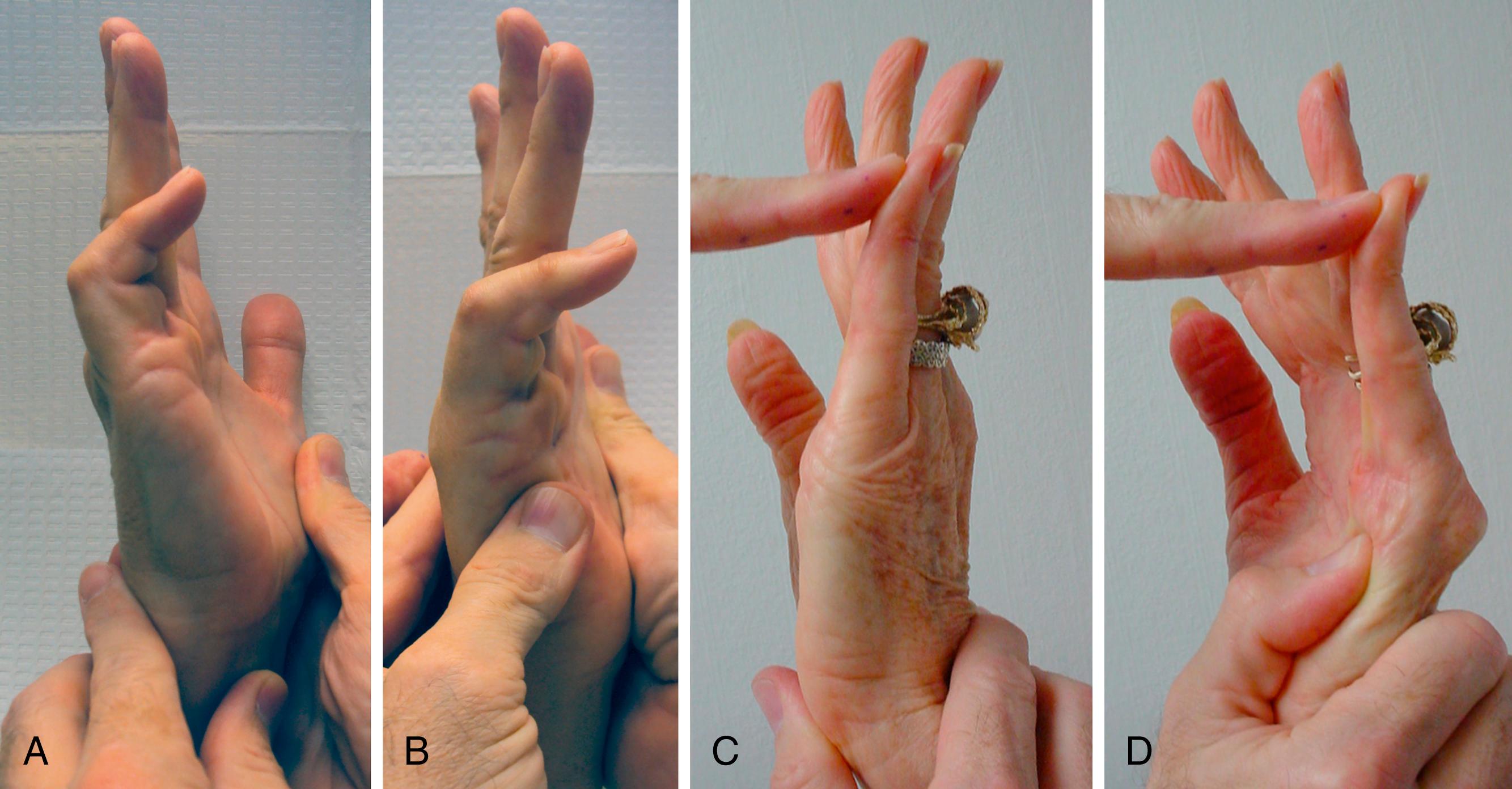
The earliest appearance of DC is passive extension deficit due to a contracted cord, most often affecting the MCP and PIP joints of the fingers. Almost all cord locations are palpable with fingertip pressure during passive extension. Because nonnodular cords feel firm only when under tension, the key to cord palpation is feeling a change from soft to firm as the relaxed finger is passively ranged from flexion to extension. Most cords develop along the lines of mechanical tension and relaxation produced by passive extension or abduction. Patients with aggressive Dupuytren biology or who have had prior treatment are more likely to vary from these patterns.
Cords may cause isolated flexion contractures of the MCP, PIP, DIP joints, or combinations of these. Thumb involvement may result in carpometacarpal (CMC), MCP, or IP flexion contractures and radial or palmar adduction contractures. The adducting effect of MCP joint contractures may mask natatory ligament contractures. Contractures on the ulnar border of the palm may produce little finger MCP flexion/abduction contractures.
The most common Dupuytren staging metric is the extension deficit. Other quantitative staging schemes include functional tests, , disease location, , linear distance from a flat surface, , patient-reported disability, and combinations. , , Range-of-motion measurements may use incompatible benchmarks such as individual versus multiple joints, active versus passive measurements, and other author-defined measures. Range-of-motion measurements alone do not correlate well with Disabilities of the Arm, Shoulder, and Hand (DASH) or other surveys of perceived hand function in DD. , , , ,
Dupuytren diathesis is not a useful quantitative staging tool. Diathesis factors influence the likelihood of disease progression after a corrective procedure but not treatment-related range-of-motion changes, treatment complications, or function.
The complexity of staging Dupuytren has more in common with hand burn contractures (for which no universal classification exists) , than contractures due to a primary joint, tendon, or neurologic disease. Granting “all models are wrong, and some are useful,” the most common classifications are Luck (clinical assessment of biology), Tubiana (composite contracture), and single-joint extension deficits.
Luck described a progression of three histologic stages: proliferative, involutional, and residual, roughly corresponding to nodules (early disease), nodular cords (active contracture progression), and nonnodular cords (late disease), respectively. The proliferative cellular stage is mitotic histology with randomly oriented fibroblasts, myofibroblasts, and sparse randomly oriented collagen fibrils. Involutional fibrocellular specimens are less cellular with no mitoses and some parallel orientation of cells and collagen fibrils. The residual fibrotic stage histology is relatively acellular with flattened cells in dense parallel collagen bundles.
Luck stage correlates with collagen type. The ratio of type III to type I collagen is highest (>35%) in the proliferative stage. This ratio decreases to the range of 20% to 35% in involutional specimens and less than 20% in residual disease. Luck stage also correlates with recontracture: 8 years after fasciectomy, patients with proliferative histology had twice the recontracture rate than those with involutional histology and more than three times the recontracture rate of those with residual histology.
The Tubiana stage groups MCP + PIP joint flexion contracture of each ray into 45-degree increments:
Stage 0: no contracture
Stage 1: 0 to 45 degrees
Stage 2: 45 to 90 degrees
Stage 3: 90 to 135 degrees
Stage 4: greater than 135 degrees
These stages exclude DIP joint measurements. Tubiana modifications include notations for the presence of nodules, DIP hyperextension for each ray, and a summary number for the entire hand, as well as other notes. The advantage of Tubiana staging is that it is a simple and concise summary. It complements but does not replace individual joint measurements.
Range-of-motion measurement of individual joints using a goniometer might seem to be the most objective documentation. However, Dupuytren pathoanatomy is more complicated than isolated joint contractures. Dupuytren causes soft tissue contractures adjacent to multiple sequential joints. An affected joint’s angular contracture varies independently with cord shortening, joint dimensions, and cord location relative to the joint. Cords are inelastic but are often anchored to elastic soft tissue structures, allowing for passive extension examiner bias. Cords link the extension deficit angles between sequential joints: PIP to MCP joints and in the ring and small finger rays also to CMC motion ( Fig. 4.6 ). This linkage impacts the interpretation of procedure outcomes: one-third of isolated MCP releases for combined MCP-PIP contractures also improve PIP extension.
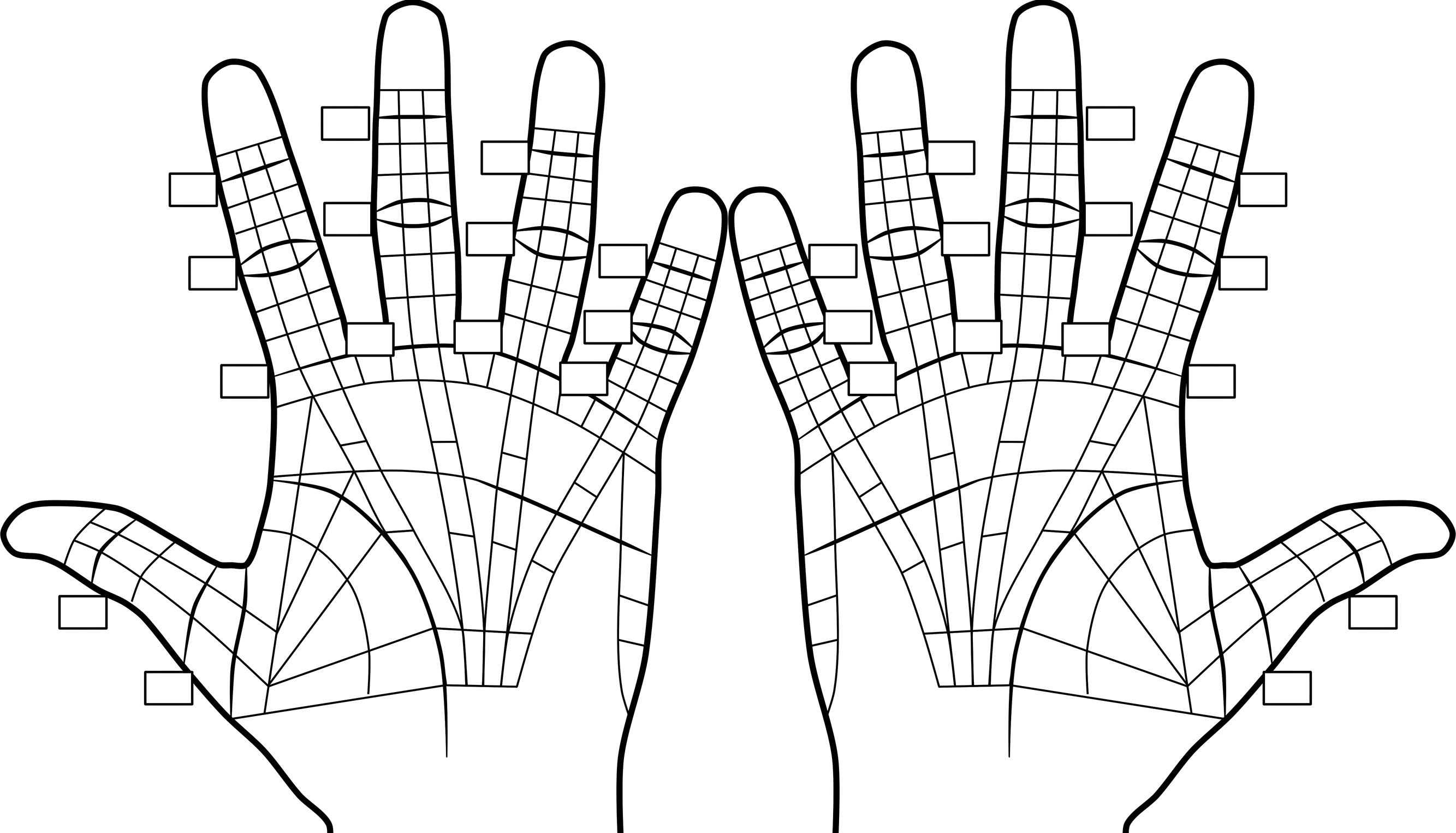
Dupuytren cords arise via fibrous transformation of palmar subcutaneous tissues and, where present, the adjacent palmar fascia’s superficial surfaces. Nodule and cord locations only loosely correspond to normal palmar fascia anatomy. The author developed a standard map to document cord and nodule locations ( Fig. 4.7 ). Forms designed to simplify documentation of initial evaluation disease location, joint measurements, and treatment details include this diagram and are available at http://dupforms.com .
Dupuytren severity broadly refers to the likelihood of having a poor outcome from Dupuytren and its treatment. Severity is multidimensional, involving biology, anatomy, correction, and recontracture. Anatomic and biologic severity separately affect short- and long-term treatment expectations. Anatomic severity (angular contracture severity) determines how likely the finger is to be straight early after treatment. Biologic severity (disease activity persistence) determines how likely fingers are to be straight late after treatment. Historic severity affects both: prior recontracture is a risk factor for future recontracture.
Initial angular correction depends on pretreatment angular contracture severity, not biology. Greater pretreatment angular contracture severity lowers the chance a corrective procedure will restore full extension. , , Failure to achieve complete correction increases the likelihood of some initial correction loss within a year of treatment. , , Greater angular contracture also correlates with reduced grip strength, which is not improved by contracture release.
In addition to joint measurements, documentation of DD biologic severity should include a family history of DD in siblings or parents, gender, the age of onset of DD, current age, the age of first treatment, bilaterality of DD, the number of digits involved, thumb involvement, the presence of palm nodules, DDNs, Ledderhose disease, Peyronie disease, and history of frozen shoulder.
Recontracture is the critical unsolved issue of Dupuytren care. Recontracture and retreatment complications are the prime drivers of poor long-term Dupuytren outcomes. Biologic and mechanical abnormalities are widespread in healthy appearing tissues before treatment , and persist after corrective procedures. For this reason, the terms “recurrence” or “extension” after a procedure are misrepresentative. More precise descriptors are progression (new soft tissue changes with or without contracture), remission (no new soft tissue changes), primary contracture (no prior procedure), and recontracture (after a corrective procedure). Primary contracture is due to Dupuytren-related soft tissue shortening. Recontracture may represent Dupuytren-related soft tissue shortening, scar contracture, tendon imbalance, or combinations of these factors, discussed later.
Recontracture is a rate (i.e., percent per year), not an event. Different definitions of recontracture include the need for repeat treatment, loss of a defined percent of initial correction, or loss of a specified number of correction degrees. Early collagenase studies also used early posttreatment outcomes to redefine recontracture. These metrics aren’t interchangeable, so always check the definition(s) of recontracture to avoid comparing studies using incompatible criteria. Pretreatment anatomy, individual biology, and procedure each influence the risk and timeline of recontracture.
Not all contracture recurrence is Dupuytren recontracture. There are three common patterns of recontracture: early, progressive, and late ( Table 4.1 ).
| Type | Begins | Course | Cause |
|---|---|---|---|
| Early | 1–2 weeks posttreatment | Plateau after 6–12 weeks | Residual secondary pathology |
| Progressive | 6–12 weeks posttreatment | Progressive | Residual primary pathology |
| Late | After posttreatment plateau lasting 12 months or longer | Progressive | New disease activity (true recurrence) |
Early recontracture develops during the first three postoperative months, followed by a plateau. Early recontracture is due to residual secondary effects of a chronic flexed posture on tendons, ligaments, skin, and other soft tissues. Although these changes may not prevent passive extension, they cause persistent soft tissue imbalance promoting flexion in a healing finger. Greater angular severity produces more of these secondary effects. Incomplete correction and early recontracture are more likely for PIP contractures greater than 40 degrees to 60 degrees, MCP contractures greater than 50 degrees, and composite contractures greater than 110 degrees, regardless of treatment technique. These residual secondary changes are static influences, and their effects soon plateau.
Progressive recontracture is a steady worsening of contracture that continues to progress after the first 3 months postoperative. Progressive recontracture is due to residual active disease. PIP contractures, severe contractures, and diffuse disease are more likely to have multiple cords affecting the same joint, some of which may escape treatment. Residual intact cord fibers with soft tissue attachments may stretch without becoming discontinuous and remain biologically active and contractile during the early postoperative period. Myofibroblast-rich cellular histology at the time of a corrective procedure correlates with progressive recontracture. , ,
Late recontracture begins after an initial period of stability lasting a year or more. Like disease extension, late recontracture represents new disease activity in previously quiescent tissues and is true “recurrent Dupuytren contracture.” Biologic factors lead to late recontracture.
Dupuytren is a clinical diagnosis that currently lacks a pathognomonic laboratory or imaging profile. Plain x-rays may reveal degenerative joint changes or heterotopic ossification of cords. Ultrasound imaging or Doppler ultrasound examination may help identify a spiral neurovascular bundle (discussed in Secondary Pathology). Dupuytren may resemble schwannoma or other tumors on magnetic resonance imaging (MRI). Ultrasound or MRI can assess disease cellularity and, in theory, the Luck stage, but have not correlated with early progression.
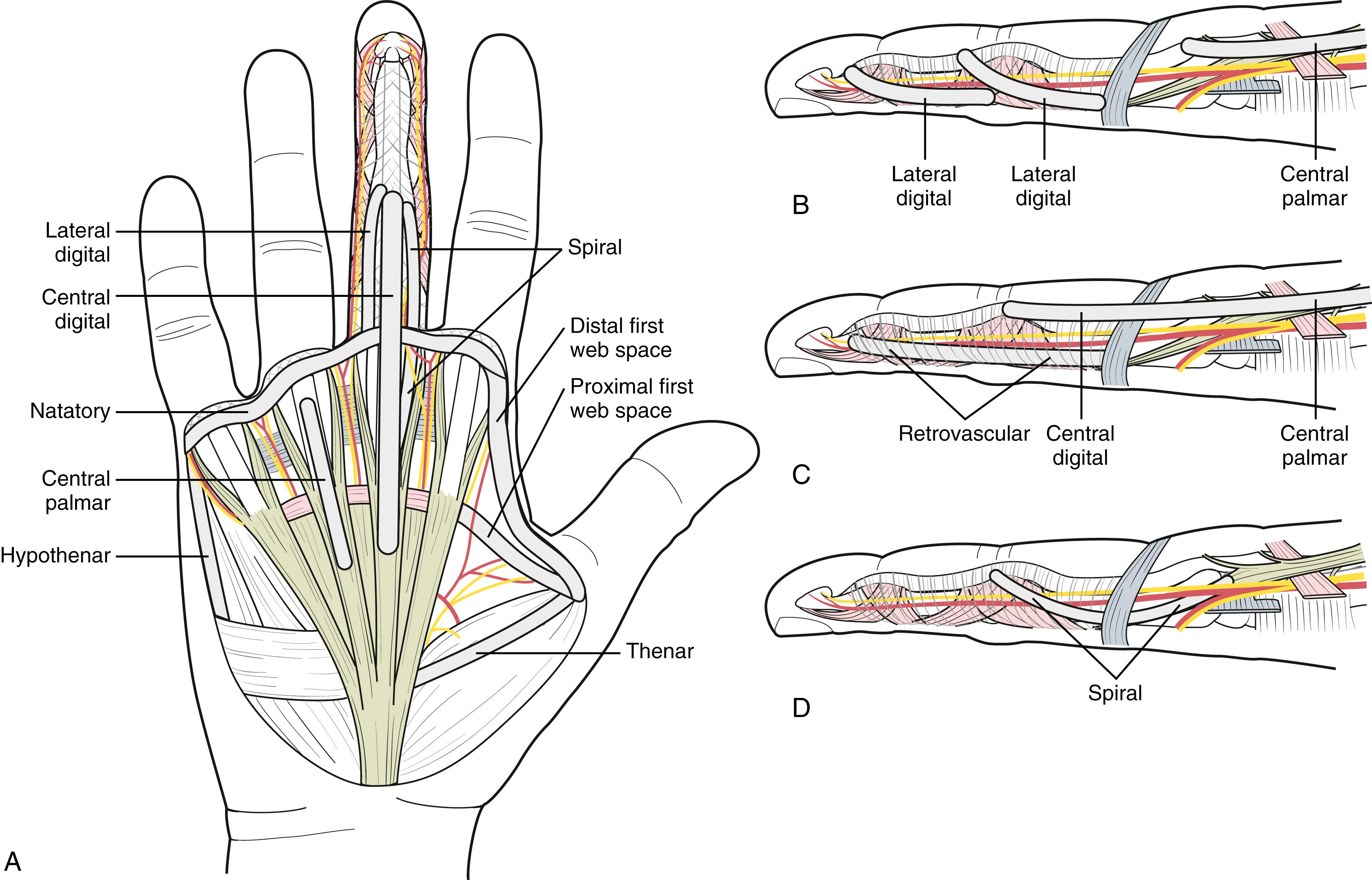
The superficial palmar fascia lies in a coronal plane beneath the palmar subcutaneous tissue. It covers a triangular area of the central palm, the proximal corner facing directly proximal. The palmaris longus tendon, when present, terminates in continuity with the fibers of this proximal corner. From this common point, four central bands of fascia extend distally toward each of the fingers. There is no central band for the thumb. Confluent proximally, the central bands separate and diverge just distal to the transverse retinacular ligament, each following the underlying ray. At the distal palmar crease level, the central bands are bridged transversely by the superficial transverse palmar ligament. Although the superficial transverse palmar ligament appears to lie deep beneath the central bands, fibers from the central bands pass above, below, and through it. At this level, each central band branches in the following three directions.
Superficial fibers stay superficial and merge with vertical retinacular fibers at the dermis’s undersurface in the distal palm in areas between skin flexion creases. Because these are subcutaneous, they are more often involved with nodules than the other two groups.
Intermediate fibers split transversely into two sections, which extend toward the lateral border of the digit base. These fibers are the spiral band that tracks around the neurovascular bundle: Proximally, it is central and superficial to the bundle; distally, it is lateral and deep beneath it. Spiral neurovascular bundles develop when spiral bands shorten.
Deep fibers continue dorsally to merge with sagittal interosseous fascia fibers and pierce the transverse deep intermetacarpal ligament to merge with fibers of the sagittal bands of the extensor mechanism. These fibers are rarely involved in contractures.
The digital neurovascular structures are surrounded by a diffuse network of thin transverse oblique fibers, as shown in Fig. 4.8 . Fibers dorsal to the neurovascular bundle are collectively called Cleland ligament, and those palmar to the neurovascular bundle are called Grayson ligament. Undisturbed, these “ligaments” are a mesh of nonparallel individual fibers. The appearance of a ligament is a dissection artifact from releasing skin attachments. These fibers have a common origin in continuity with the fingers’ retinacular ligaments, the floor of the flexor tendon sheath, and retinacular fibers attaching the palmar digital skin to deeper structures. On the small finger’s ulnar border, lateral fascial attachments are in continuity with the abductor digiti minimi fascia and tendon.
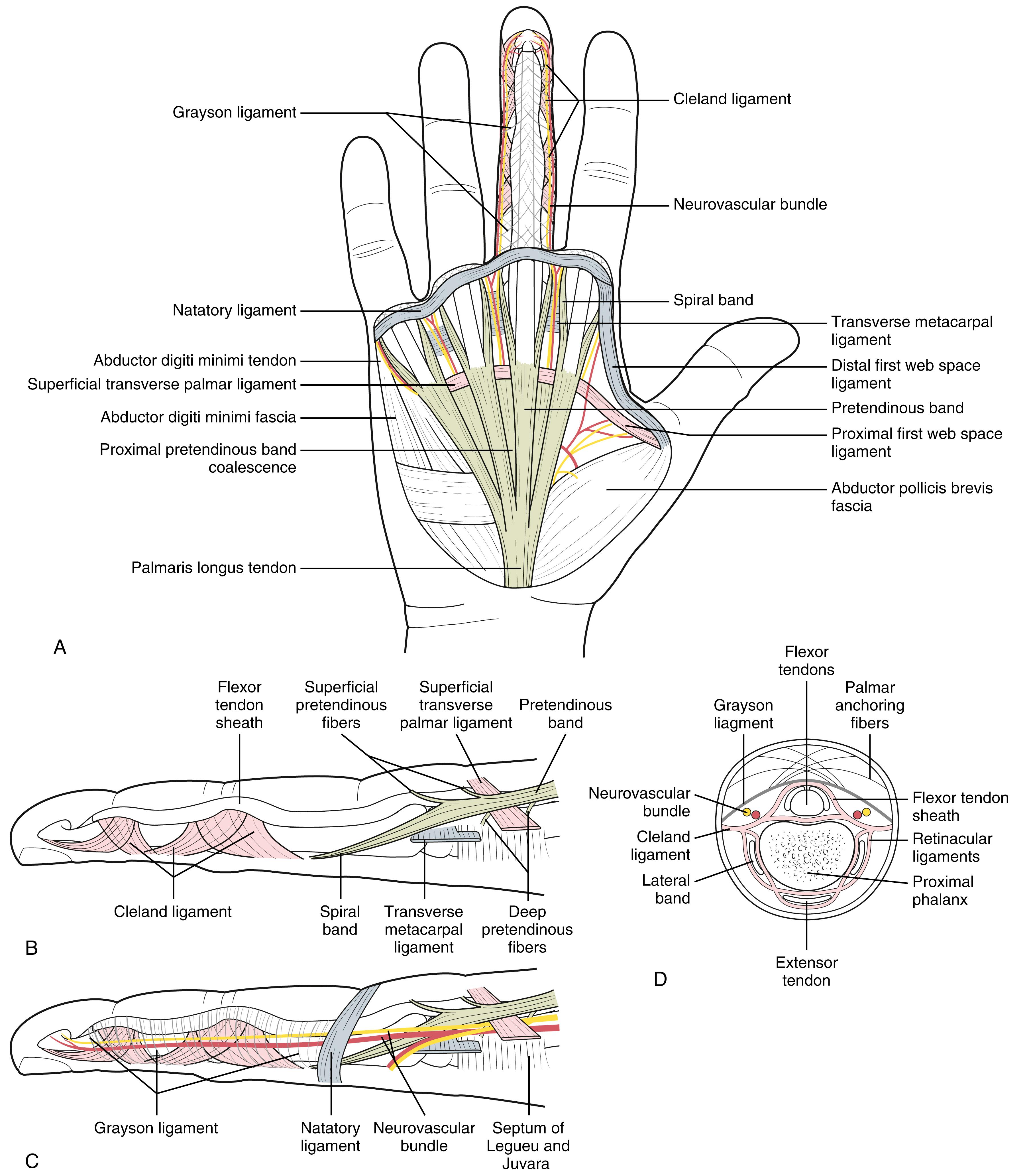
A subdermal fascial layer borders the periphery of the web spaces from roughly the radial thumb sesamoid to the ulnar side of the small finger’s proximal flexion crease. These border fibers form the distal first web space ligament between thumb and index in continuity with the natatory ligament that spans the remaining web spaces. Fibers from the natatory ligament extend distally at each finger’s lateral base in continuity with Grayson ligament fibers and the lateral digit dermis.
Dupuytren-related abnormalities widely affect palmar tissues before any visible tissue changes occur. Abnormalities in clinically normal palmar tissues include increased myofibroblasts, , inflammatory cells, , overall collagen, type III collagen, , , collagen crosslinks, , crosslink hydroxylation and glycosylation, laminin and other glycoproteins, , reduced elastin, abnormal mechanical stress-strain curves, and similar gene expression profile. , , Clinically unaffected subcutaneous tissues adjacent to nodules show Dupuytren-related gene expression changes. Mesenchymal stem cells accumulate in perinodular fat and the skin overlying Dupuytren nodules. Based on these findings and the observation that Dupuytren cords can arise in areas without fascia and recur in the same location after removing the fascia, Dupuytren is not a disease of the fascia. It is a regional, not a tissue-specific disease process.
Nodules are usually the first visible and most active sites of Dupuytren pathobiology. Nodules arise on the palmar fascia’s superficial surface, where retinacular fibers anchoring the dermis to fascia resist shear forces from gripping. Nodules appear in areas of mechanical stress on palmar tissues ( Fig. 4.9A ).
Cords develop and orient along the lines of mechanical tension and relaxation in susceptible tissues. Because nodules and cords arise in response to different biomechanical triggers, their most common locations differ (see Fig. 4.9B ). Matrix stiffness guides fibroblast alignment. Myofibroblast contraction folds individual collagen strands, and extracellular matrix enzymes crosslink across these folds ( Fig. 4.10 ). This process progressively shortens and stiffens the extracellular matrix through collagen crosslinking. Stiff matrix transmits mechanical forces to adjacent tissues, which then undergo the same process.
Patterns of contracture may have more to do with collagen crosslinking at rest than with myofibroblast contraction. Some locations (i.e., distal palm, palmar digit) produce clinical contractures. Other common sites (i.e., DDN, Ledderhose) very rarely develop contractures even though biopsy tissue from each of these areas contracts similarly in vitro. A possible explanation is that Dupuytren biology fixes tissues in their resting position. The biology of collagen deposition and remodeling activates from mechanical stress but then continues during periods of rest. At rest, activated myofibroblasts and collagen crosslinking enzymes in the extracellular matrix shorten redundant collagen fibrils. This shortens tissues to their resting length, similar to a PIP volar plate contracture after injury and prolonged flexion. This would result in flexion and adduction contractures resembling the hand’s resting posture, so typical of DC. It would also explain why knuckle pads and Ledderhose rarely develop contractures because the IP joints rest in flexion and the metatarsophalangeal joints of the toes rest in extension.
Stress shielding downregulates Dupuytren activity. It protects some structures from clinical disease: Cleland ligament (shielded by the adjacent phalanx), longitudinal fibers deep beneath the transverse superficial palmar ligament (shielded by the central band), transverse superficial palmar ligament (shielded by the transverse metacarpal ligament), and the septa of Legueu and Juvara (shielded by the adjacent metacarpal). Stress shielding also provides an endpoint for disease progression. As contractures progress, stress shielding from lack of use or secondary capsuloligamentous joint contractures eventually removes mechanical stress from the cord. This allows myofibroblast apoptosis and progression to a final, involutional, static disease stage.
Fig. 4.11 illustrates common cord patterns. Cords may arise in the palm, the digit, or span both. Central palm cords are central palmar, spiral, and proximal first web. Border palm cords are natatory, distal first web, hypothenar, and thenar. Thenar and hypothenar cords are uncommon unless associated with diffuse disease. The majority of MCP joint contractures involve an isolated central cord. In contrast, most PIP contractures have multiple cords: central digital (most common), retrovascular, and spiral or lateral (least common). Multiple cords can exist at the same level in either palm or digit.
Over time, flexed posture results in anatomic changes of joints and tendons independent of the causative cord. These issues are more likely to develop with more severe contractures. The PIP joint is particularly vulnerable. Accessory collateral ligament contractures are more common if PIP joint contractures are greater than 45 degrees. Central slip attenuation contributes to extension deficit for PIP contractures greater than 60 degrees, which are less likely to achieve full correction than those with less severe contractures. Boutonnière, sagittal band rupture, or mallet deformity may develop secondary to chronic contractures. Sagittal band rupture can be difficult to diagnose in the context of a fixed MCP flexion contracture. A clue is MCP abduction–supination in the absence of a responsible palmar cord. Chronic rotational or lateral deformities from digital cords often persist after treatment of the primary disease.
Mild postural abnormalities from a prior injury, chronic flexor tendinitis, osteoarthritis, peripheral neuropathy, intrinsic weakness, or mild spasticity are common in the senior demographic population. They must be kept in mind in evaluation because they affect outcome expectations, and the patient may be unaware of them. This author has seen all of these conditions concurrent with DC, often not previously diagnosed.
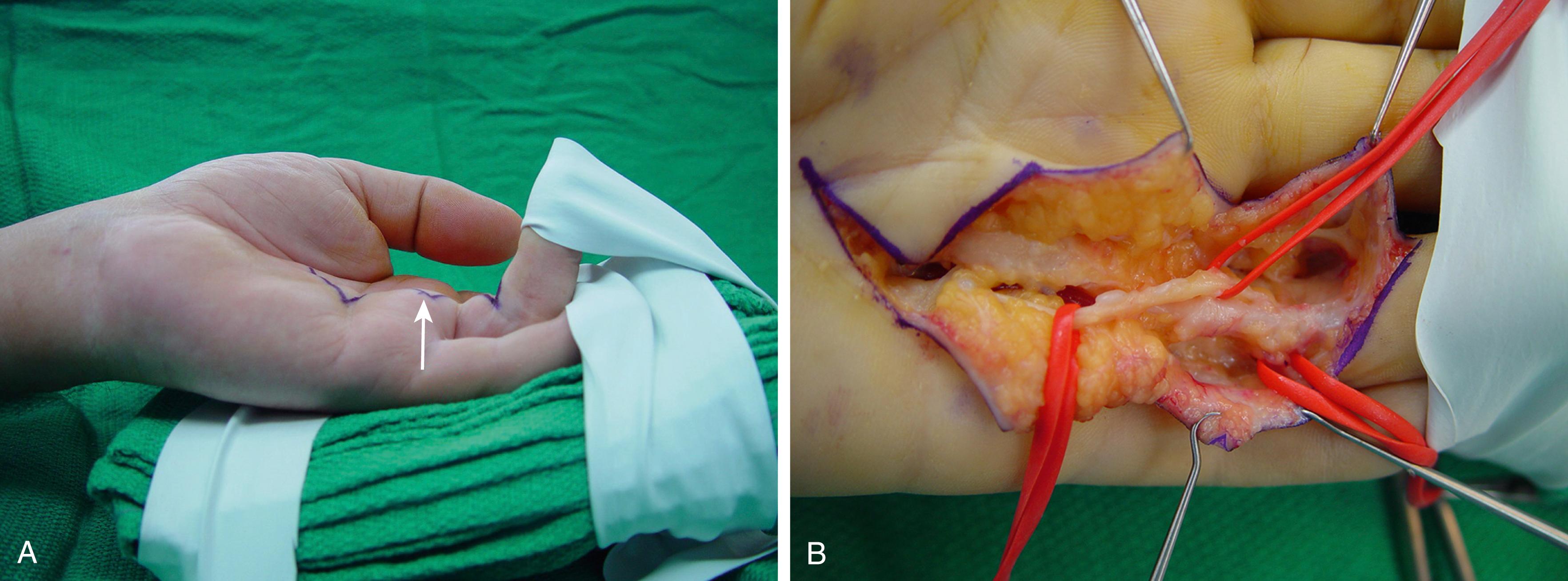
If tissues that form a spiral longitudinal path around a neurovascular bundle tighten into a linear cord, they displace the neurovascular structures into a spiral path or spiral neurovascular bundle. As many as one-half of operated Dupuytren hands have spiral neurovascular bundles, , which can arise anywhere between the distal palmar crease and the DIP flexion crease ( Fig. 4.12 ). The altered anatomy places a short length of the bundle superficial to the cord and into harm’s way. Variations occur, including cords that pass between the digital nerve and a dorsal branch, with double spirals in the same bundle, or involving both neurovascular bundles of the same digit. They are more likely where a fleshy prominence covers a well-defined cord ( Fig. 4.13 ). A preoperative Doppler stethoscope or ultrasonography may identify spiral neurovascular bundles in suggestive areas. Alternatively, passively extend the finger to hold the cord taut while firmly compressing the overlying tissue prominence against the cord with a fingertip. Pain or paresthesias from this maneuver suggests a neurovascular bundle path superficial to the cord at this location. Spiral bundles are increasingly likely with greater PIP contracture angles and can occur without a soft tissue prominence. ,

Small finger PIP joint contractures have a worse prognosis and higher recontracture rate than other PIP joints, independent of surgical technique. Abductor digiti minimi (ADM) tendon involvement, common in small finger contractures, is not an independent risk factor for either inferior outcome or recontracture. Similar intrinsic tendon involvement can occur in any digit. ADM fascia involvement may cause a loss of small finger supination.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here