Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Drug distribution and clearance determine the concentration of drug that will be attained at the site of drug action. Drug targets include cell surface receptors, intracellular receptors, enzymes, transcriptional mechanisms, ion channels, and molecular transport systems. These targets may be within the circulatory system, in well-perfused tissues, in less well-perfused tissues, or behind specialized endothelial or epithelial barriers. The fetus lies behind one of these circulatory barriers—the placenta. The placenta is the interface between the maternal and fetal circulations, keeping them separate but bringing them into close apposition for transport of nutritional needs and removal of waste products. In addition, this interface is the major route of drug delivery to and elimination from the fetus. The fetus also has specialized circulatory arrangements designed for intrauterine life that require additional considerations in the understanding of fetal drug distribution. Furthermore, developmental differences in body composition, drug metabolism, renal clearance, and specialized barriers make fetal drug distribution distinct from that in the infant, child, and adult.
Most drug action is predicted on the basis of plasma drug concentrations. It is not easy, even in experimental models, to measure tissue concentrations, particularly when the extracellular versus intracellular concentration warrants consideration. Hence major emphasis is placed on the determinants of fetal plasma concentration. An appreciation of pharmacokinetics requires an understanding of physicochemical properties of drugs, placental transfer of drugs, and fetal clearance of drugs. Many of these concepts also pertain to tissue distribution. Drug delivery to the central nervous system of the fetus is of particular interest and further illustrates concepts relevant to tissue distribution. In considering developmental issues relevant to fetal disposition of drugs, an important point is that drug targets also have complex developmental trajectories. Understanding fetal drug distribution may allow prediction of drug concentration at the site of drug action, but prediction of drug action, which is the true goal, also requires understanding the interaction between the drug and its target.
The plasma concentration of a drug in the mother is the main factor determining the plasma concentration of drug in the fetus. Fig. 19.1 shows the linear relationship between concentrations of zidovudine measured simultaneously in fetal plasma to those measured in maternal plasma under steady-state conditions in a nonhuman primate. Steady state is defined as the condition in which the amount of drug in a compartment does not change with time—that is, the amount of drug being added to the system is the same as that leaving the system.
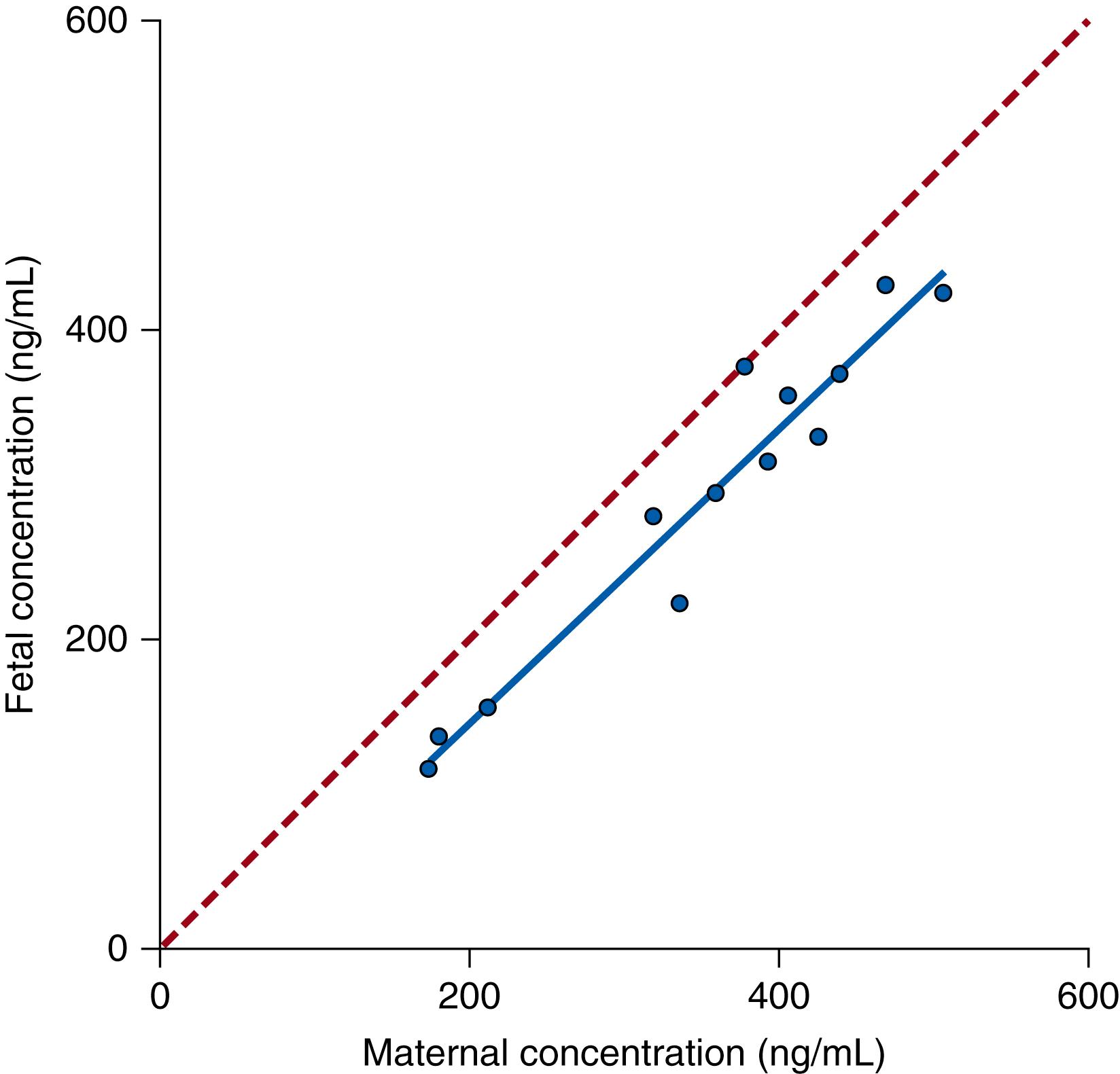
This linear relationship is the hallmark of first-order kinetics, with the implication that a doubling of the maternal concentration will double the fetal concentration. In this example of zidovudine infusion to pregnant baboons, the fetal concentration of zidovudine is slightly less than the maternal concentration. This observation is common for many drugs and indicates that other factors also influence the fetal plasma concentration. The focus of this chapter is to review how placental permeability, fetal drug elimination, drug ionization and protein binding, and volumes of distribution affect fetal drug levels.
Once the maternal concentration is known, fetal distribution can be divided into three phases: transfer across the placenta, modification of the fetal plasma concentration, and tissue distribution. An integrated pharmacokinetic approach with graphic representations is used throughout to describe how differences in these various contributors affect fetal drug levels (be it plasma, extracellular, or intracellular).
The maternal plasma concentration is the driving force for drug delivery to the fetus. For many drugs, physiologic changes of pregnancy lead to altered drug absorption, distribution, and clearance in the mother, and thus plasma concentrations are different from those seen in the nonpregnant state. , Generally, plasma drug concentrations tend to be lower in pregnancy. There is an increase in the volume of distribution resulting from an increased plasma volume and increased fat deposition, as well as addition of the fetal compartment. Maternal renal clearance is enhanced owing to increased cardiac output and renal blood flow, or to pregnancy-related changes in renal transporters. Hepatic clearance also may be enhanced as a consequence of increased hepatic blood flow or hormonal stimulation of drug-metabolizing enzymes. In some cases, however, pregnancy hormones may inhibit drug-metabolizing enzymes. Increasingly, comparative data are becoming available for drugs used in pregnancy. For fetal considerations, the physiologic changes of pregnancy that alter maternal drug distribution can be bypassed by measuring the concentration of the drug in maternal plasma.
The placenta is the specialized interface between mother and fetus across which drug distribution occurs. Most drugs are believed to cross the placenta by passive diffusion; accordingly, the surface area provided by the placenta and the nature of the interface, together with drug characteristics, determine placental permeability. Placental transporters are now recognized as an important contributor to fetal drug disposition.
The following discussion is a synopsis of placental development highlighting the aspects relevant to drug transport, with a focus on the relationship among the maternal and fetal circulations, the surface area of exchange, and the nature of the diffusional barrier.
During implantation, the trophoblastic tissue invades and becomes surrounded by decidua. The placenta develops at the embryonic pole while the trophoblast in contact with the rest of the decidua gradually breaks down. Spaces develop within the expanding trophoblastic tissue to form the lacunae that lie between the villous structures. The uterine spiral arteries supplying the decidua and the veins draining the decidua are invaded by trophoblasts in such a manner that these maternal vessels open directly into the lacunae, and maternal blood bathes the villous structures. Anchoring villi extend the full thickness of the trophoblast layer, whereas other villi project like trees into the villous space ( Fig. 19.2A ). As pregnancy advances, the placental surface area increases by increasing the number of villi and the number of branches. As with most epithelial transport surfaces, the luminal plasma membrane of the villus trophoblast has microvilli that further increase surface area (see Fig. 19.2C ). Later in gestation, the diffusional capacity of the placenta increases mostly by thinning of the trophoblast layer where it overlies fetal vessels within the villi. The villus itself consists of a stromal core to support the fetal blood vessels and is surrounded by a single layer of syncytial trophoblast attached to a basement membrane (see Fig. 19.2B ). Cytotrophoblasts and some Hofbauer cells (placental tissue macrophages) lie between the two. The syncytial trophoblast is a multinucleate cellular structure formed by the fusion of trophoblastic cells to form a syncytium. Underlying cytotrophoblastic cells add to the syncytiotrophoblast by fusion, and by term, few cytotrophoblasts are present within the villus. The fetal arterioles branch into a capillary bed, also surrounded by a basement membrane. The capillaries are nonfenestrated and have variably spaced tight junctions between endothelial cells. In the mature placenta, the contact zones between the syncytiotrophoblast and endothelial cells are free of nuclei and are thinner than other regions (see Fig. 19.2B ). The layers between maternal and fetal blood over which diffusion occurs are shown in Fig. 19.2C . In addition to the microvillus surface, the luminal membrane of the syncytiotrophoblast (that is in contact with maternal blood) contains clefts. The abluminal membrane also has infoldings. The syncytial nature of the syncytiotrophoblast precludes intercellular spaces through which transport can occur. By contrast, the endothelium does allow some paracellular transport of low-molecular-weight hydrophilic substances. Placental capillaries are less permeable than most other capillaries present in tissues with continuous or nonfenestrated capillaries; however, they are still more permeable, by two orders of magnitude, than those present in the brain.
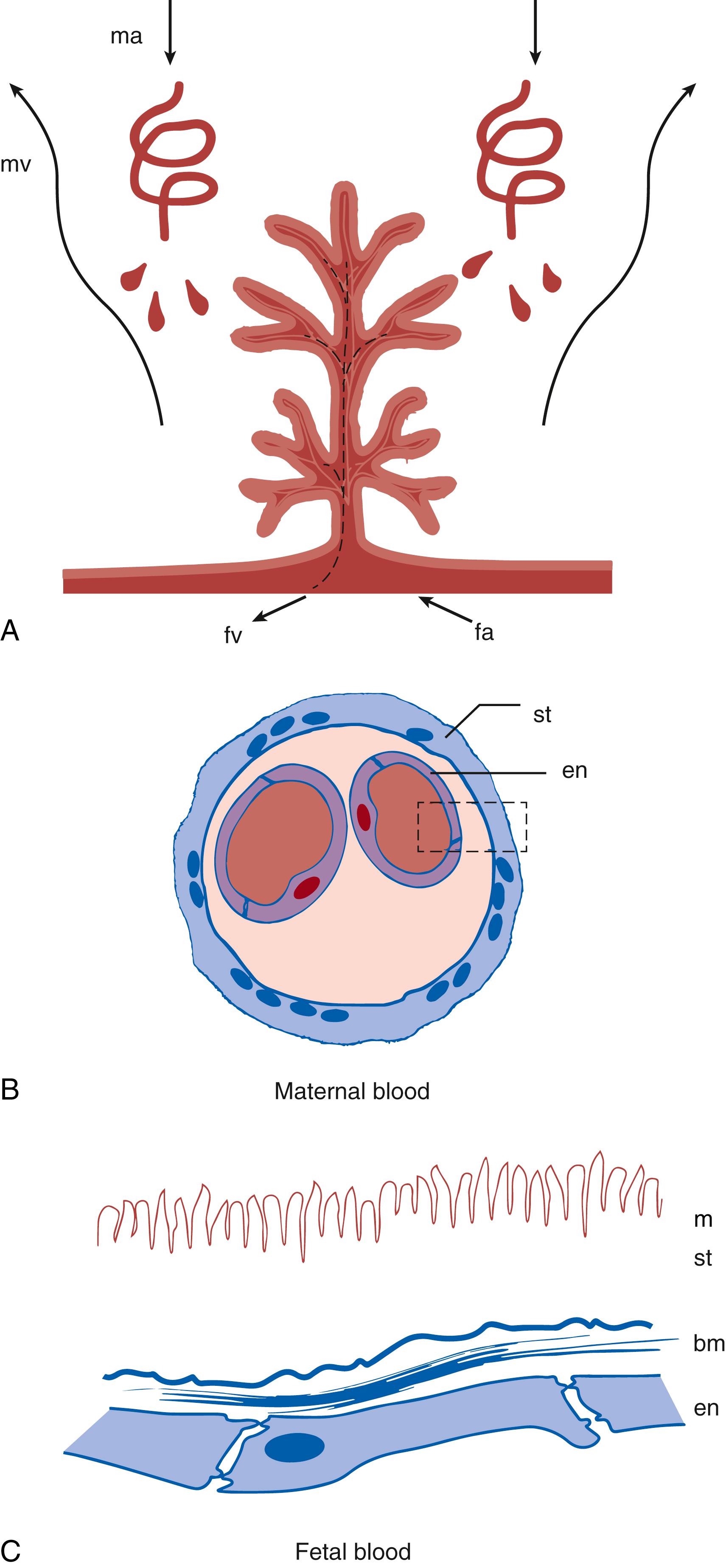
From an anatomic perspective, many placental characteristics are important in the transfer of drug to the fetus. The most striking features are the very large exchange surface and the very thin syncytial-endothelial barrier between the maternal and fetal circulations, supporting passive transfer of substances (see Fig. 19.2 ). Visualization of the human placental structure suggests a crosscurrent exchange interface; however, experimental data at best support a concurrent model (detailed in Chapter 10 ).
The simplest and perhaps most illustrative way to view the maternal-fetal dyad with respect to drug distribution is as a two-compartment model ( Fig. 19.3 ). , This model differs from the standard peripheral compartment model in that the fetal compartment includes an elimination route independent of the placenta. In addition, at least in experimental models, this compartment can be sampled. Rate equations for the model describe how the amount of drug in each compartment changes with time and are determined by considering how much drug is entering and leaving each compartment. The amount of drug (D) that leaves in a given time period (mass/time) equals the clearance (Cl) (volume/time) multiplied by the mean concentration ( c , mass/volume) for that time period as follows (see parameter descriptions in Fig. 19.3 ).
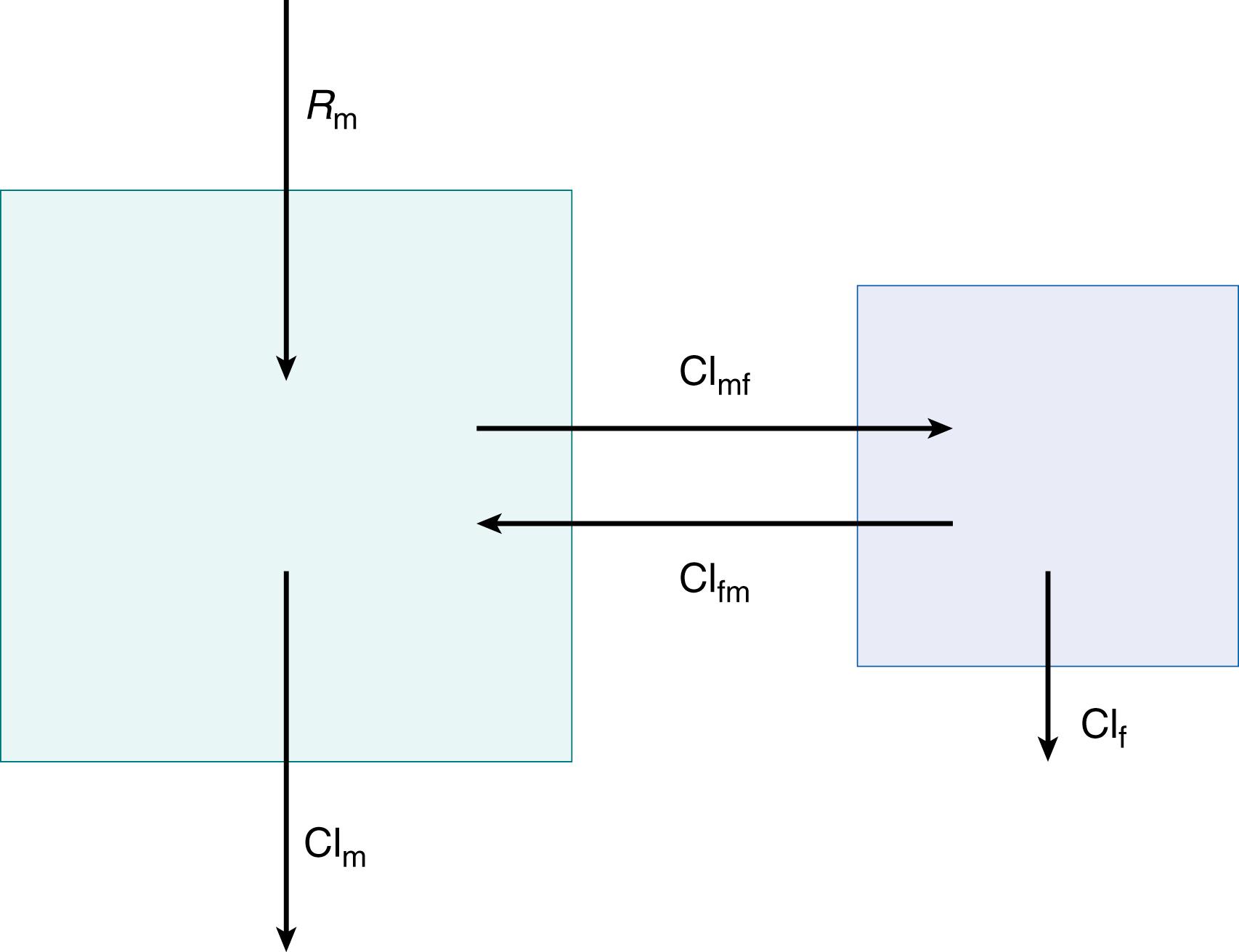
Rate equation for maternal compartment:
Rate equation for fetal compartment:
Solutions of these rate equations are used to generate concentration-time plots for each compartment to illustrate the effects of placental permeability and nonplacental fetal elimination on fetal drug distribution, as shown in Figs. 19.4 and 19.5 . Fig. 19.4 describes drug administered by an oral bolus, and Fig. 19.5 shows drug administered by continuous infusion to steady state and then stopped. The assumptions for these simplified examples are that drug concentrations are not affected by protein binding or pH effects, and that drug crosses the placenta by passive diffusion such that placental clearance is the same in both directions. In panel A of Figs. 19.4 and 19.5 , no direct fetal clearance occurs—that is, fetal nonplacental clearance is set at zero. Moving down the panel shows the effect of decreasing placental clearance on the fetal drug concentration-time curve. Placental permeability will affect peak fetal drug concentrations when administered by the bolus (see Fig. 19.4A ), but total fetal drug exposure as measured by the area under the concentration-time curve is the same in each case. With continuous infusion of drug to steady state when there is no direct fetal clearance (see Fig. 19.5A ), fetal drug levels will be as high for drugs with low placental permeability as for those that are highly permeable if continued for a sufficient period of time.
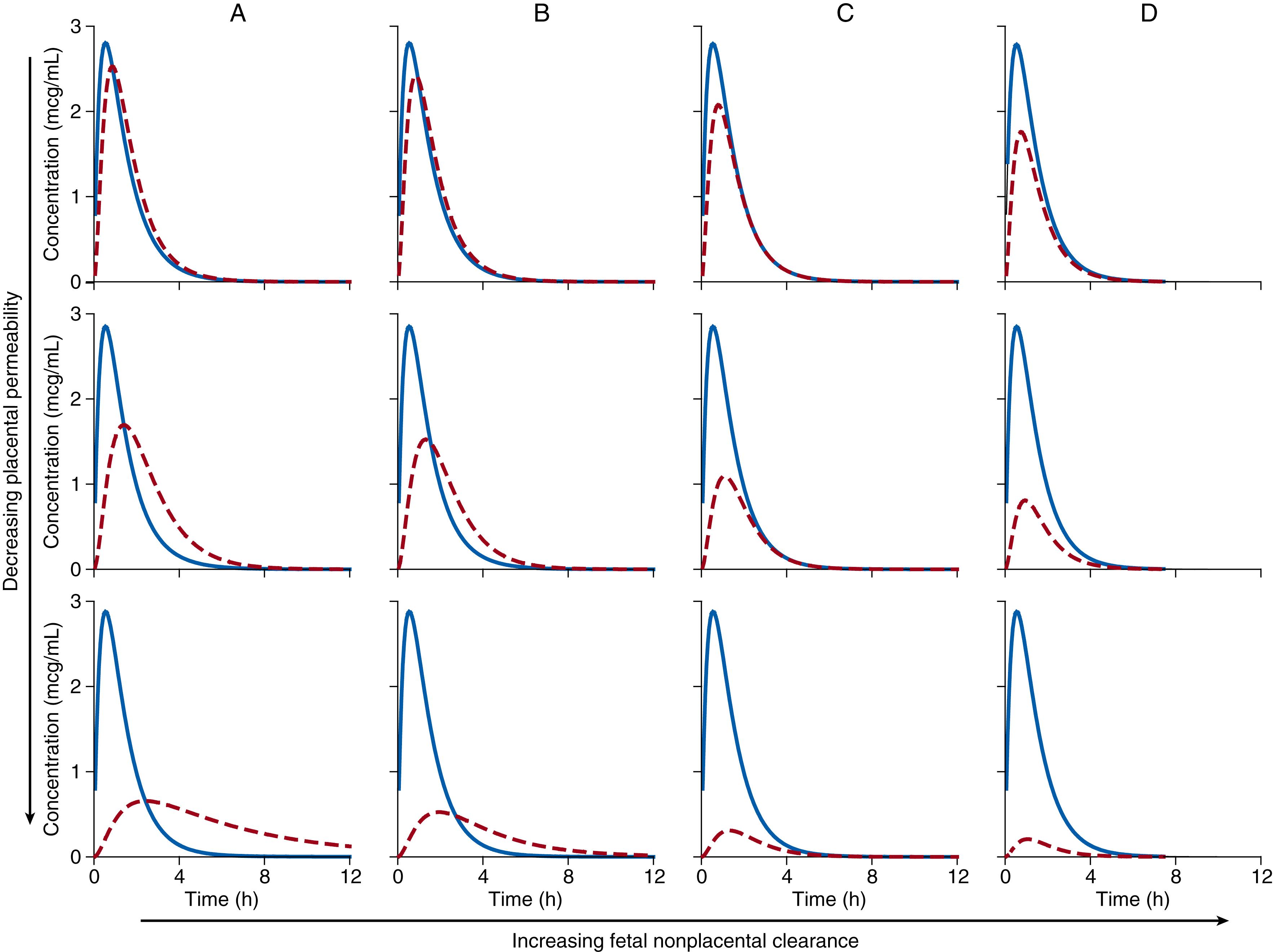
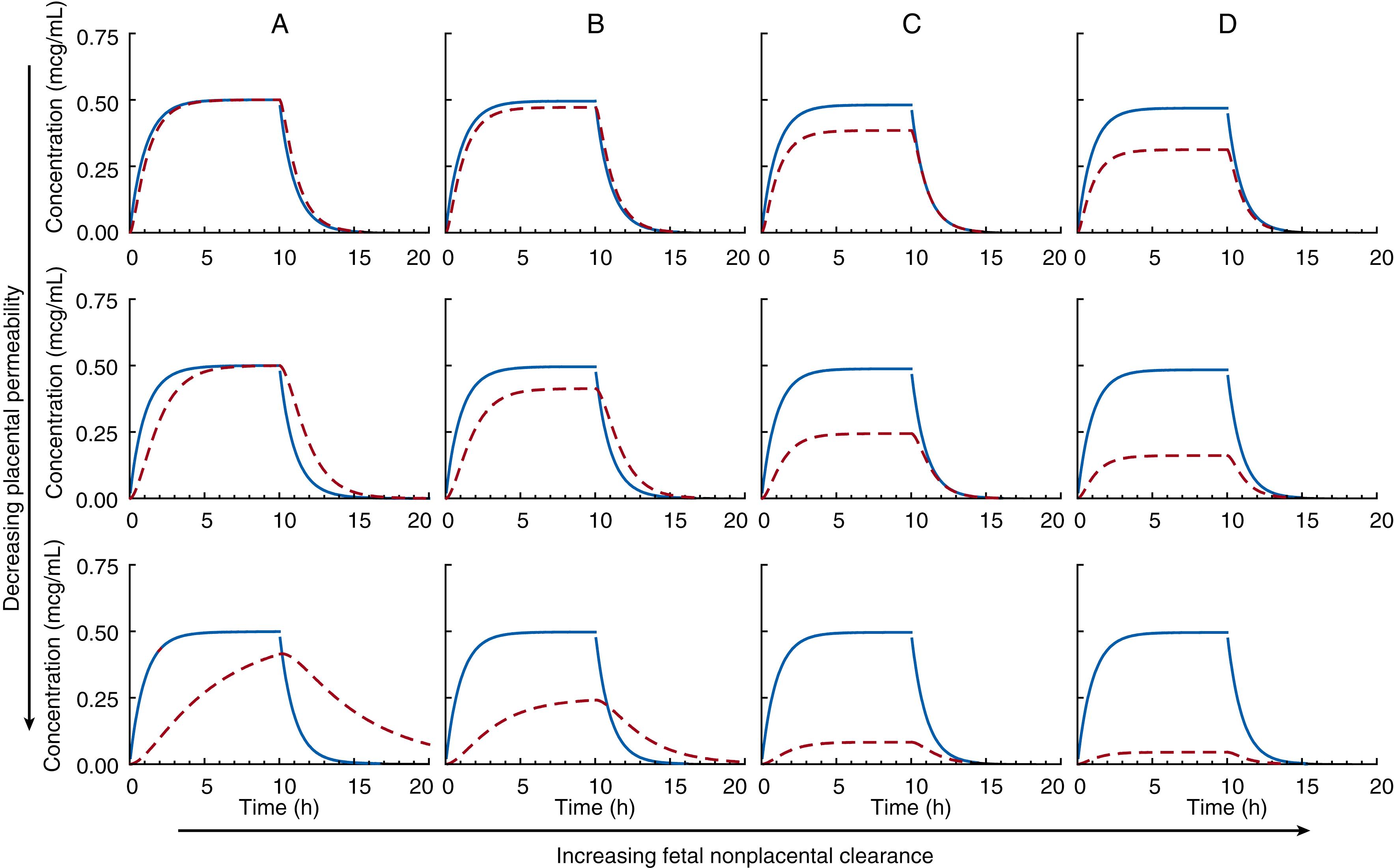
A much-debated question was whether fetal drug concentrations could exceed those in the mother with passive placental transfer. During the elimination phase after bolus administration, fetal concentrations are higher than maternal—but the peak fetal concentration will not exceed the peak maternal concentration (see Fig. 19.4A ). Even for rapidly transferred substances, the peak concentration will be blunted. During continuous infusion (see Fig. 19.5A ), mean steady-state concentrations in the fetus will not exceed maternal concentrations; however, during the elimination phase, fetal concentrations may exceed maternal levels. Moreover, total drug exposure in the fetus will not exceed that in the mother. In Figs. 19.4A and 19.5A , when there is no direct fetal clearance, not only is the area under the curve (measure of total drug exposure) the same in each fetus, but the area under the curve is the same in the mother as in the fetus. In the absence of direct fetal elimination, mean steady-state concentrations (or areas under the concentration time curves) in the fetus are equal to those in the mother. This is an important concept to grasp, because single maternal-fetal drug determinations after bolus drug administration have caused considerable confusion in the understanding of fetal drug exposure. In certain situations, mean active drug concentrations in the fetus can exceed maternal concentrations—for example, in the presence of active transport from the maternal to fetal circulation and after prodrug administration, when active drug metabolite concentrations can be higher in the fetus than in the mother. These circumstances are explored later.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here