Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The radiologist’s most obvious “product” is the interpretation rendered for an imaging study. Although this visible document is a key component of the radiologist’s job, it is only one of many responsibilities under the modern radiologist’s purview. Many radiologists also oversee departmental practice functions, including, but not limited to, licensing and certification requirements, regulatory compliance, quality improvement, patient and staff safety, equipment purchasing and maintenance, patient scheduling, technologist training, study optimization, communication of results, and billing. Foremost among these responsibilities is ensuring patient safety. Since the early 2010s, regulatory agencies and national societies have placed a new focus on a poorly understood but critical component of patient safety: radiation dose awareness and optimization.
Radiation biology, or radiobiology, is a field that examines the interaction of ionizing radiation on living things down to their DNA. It is constantly evolving and continues to offer new perspectives into the effects of radiation within living cells. The study of past radiation events can offer insight into the long-term carcinogenic risks of ionizing radiation. However, to achieve these goals, a study population must be extremely large to offer an accurate risk estimate because of a multitude of uncontrolled geographical and environmental variables, in addition to the challenges posed by a very small absolute cancer risk at low levels of radiation.
The most widely cited estimates of cancer risk come from long-term studies of atomic bomb survivors. These data have been parsed in numerous ways, most visibly in the . More recent retrospective cohort studies in patients actually exposed to medical radiation have supported the conclusion that medical radiation is indeed a risk factor for secondary malignancies, although the methods used in these studies are controversial. For example, in a Lancet article published by , the authors note an increased risk for brain cancer and leukemia for children undergoing head computed tomography (CT). However, the estimated radiation doses in this study were taken from national surveys and not from individual patients. Second, because the absolute risk for development of these cancers is extremely small to begin with, any increase (albeit small and uncertain) will result in a relatively large excess risk.
As a matter of practicality, most radiologists and medical physicists have come to accept a linear no-threshold (LNT) model for radiation dose and stochastic effects, such as the development of secondary malignancies. Although some may argue with to what degree the effects exist, there is near-universal agreement that the prudent approach is to reduce radiation dose when possible, particularly in children.
It is important to understand that there are many limitations to our current understanding of risk associated with medical imaging. If an “estimate” of radiation risk is discussed in such a way that causes alarm to a patient or parent, this creates a real risk if that clinically indicated examination is then delayed or refused. It is imperative to explain the medical need for the examination, along with the speculative and unknown risk for ionizing radiation.
Because of the rapidly dividing nature of children’s cells and their proportionally longer life span, a child’s risk for secondary malignancy is thought to be even greater than adults. Although the risks remain small overall, they must be constantly and meticulously weighed against the diagnostic yield of imaging studies that do, and do not, require the use of ionizing radiation. When the risks are gauged and justify a radiation-based imaging study, the radiologist must remember the ethical imperative codified in the Hippocratic Oath to “first, do no harm.” That is, the radiologist in collaboration with a technologist and medical physicist must optimize the ordered imaging study such that the lowest radiation dose possible is used to answer the clinical question. This is especially true in children. This mantra is championed by the Image Gently ® campaign, founded by the American College of Radiology, the Society for Pediatric Radiology, the American Association of Physicists in Medicine, and the American Society of Radiologic Technologists. The Image Gently campaign has succeeded in raising awareness in the radiology community of the potential risks of ionizing radiation. In this chapter, we will offer an overview of basic principles related to CT and radiation risk, along with practical steps that the radiologist can use to improve the safety and consistency of medical imaging.
Radiation damage occurs at a cellular level. At energies in the diagnostic range (10–150 keV), radiation interacts with matter in two fundamental ways: via Compton scatter and the photoelectric effect. In both cases the result is the production of a free electron. Although injury to cellular components may occur by direct injury from the incident photon/electron, the vast majority of the initial energy will be conferred to other electrons via an exponential cascade of ionization and excitation; hence the phrase ionizing radiation. The end result of this cascade is the disruption of ionic bonds and the formation of free radicals, which are the dominant mechanism by which cellular components are ultimately damaged. The most important cellular component damaged in this fashion is DNA, which is required for the replication and survival of the cell and, if damaged, can lead to genetic mutations. Cells can repair DNA damage, but only to a certain point—at a certain threshold level, damage may be irreversible. Deterministic (nonstochastic) effects such as radiation burns and skin necrosis occur when a sufficient dose has been delivered to result in cell death. Stochastic (or probabilistic) effects such as induction of malignancy occur when DNA damage is sufficiently severe to alter tumor suppressor pathways, but not so severe as to result in immediate cell death.
DNA is more likely to become damaged when it is in an unwound state, which occurs before, during, and immediately after cell division. Therefore, as the frequency and duration of cell division increases, so does the possibility of DNA damage. In addition, the frequency by which a mutation will be expressed is directly related to cellular activity and cell division. As a result, more rapidly dividing cells have a higher probability of expressing and replicating DNA errors than a dormant cell.
These factors help explain why rapidly dividing cells are more susceptible to radiation damage than other cells. The most susceptible cell lines include lymphocytes and hematopoietic stem cells, because of their high rate of division. Similarly, cells in a child’s body generally divide more rapidly than those in an adult, as a consequence of normal growth and development. For this reason, it is estimated that children may be at least two times more sensitive than adults to the effects of ionizing radiation. With decreasing age, the degree of radiosensitivity is thought to increase.
In addition to a greater degree of radiosensitivity, children are also more vulnerable due to a proportionately longer life span and longer lag time during which mutations can manifest. Even if the biological sensitivity of a neonate and a 90-year-old were exactly the same, the radiation damage sustained by the neonate would have a much longer period to manifest, compared with the relatively shorter expected life span of the 90-year-old.
For the reasons outlined earlier, we must take radiation safety seriously in children and strive to keep exposures as low as reasonably achievable (ALARA) when using ionizing radiation.
The most widely studied radiation-exposed population is the survivors of the atomic bombs detonated over Japan to end World War II. This effort has been termed the Life Span Study. This study is the major source of data with respect to radiation-induced solid malignancies and leukemia. The most comprehensive report summarizing the characteristics of this cohort is the BEIR VII report. This report was published by the National Academy of Sciences, the National Academy of Engineering, the Institute of Medicine, and the National Research Council. Notably, this committee-based study sought to quantify risk estimates of radiation exposure in the medical range, which represents the “low-level” range.
Needless to say, this is an extremely challenging task. The first requirement of the BEIR studies, as with any other studies looking retrospectively at the consequences of radiation exposure, is an estimate of the initial dose received above background radiation. Since 1957, dose estimates have been produced by a joint Japanese–United States organization called the Radiation Effects Research Foundation in an attempt to quantify survivors’ doses at the time of detonation. These models became increasingly more complex, using real data from exposed materials within the atomic bomb radius in combination with survivor descriptions of their specific locations and relative adjacent shielding (or lack thereof) at the time of exposure. The BEIR VII report improved on prior studies by using the most accurate, computer-aided dose estimates to date. BEIR VII also used interim published data of radiation-exposed nuclear workers and even some early studies of patients exposed to radiation in the medical setting.
In summary, BEIR VII concluded that the stochastic effects of radiation (such as development of a secondary malignancy) are best represented by a LNT model. That is, even at very small doses, an increased risk is conferred. The BEIR VII report also presents a methodology for calculating lifetime attributable risk from data in tables 12 D-1 and 12D-2. These tables present values for cancer incidence and mortality per 100,000 persons exposed to 100 mGy and at various ages of exposure. It should be noted that these tables have been used to create sensationalistic numbers of potential cancer deaths by dividing an “average” or “typical” dose absorbed for a CT study by 100 mGy and multiplying the result by the number of patients undergoing CT studies, divided by 100,000 persons. The result is a speculative number predicting future cancer risk. This calculation is inappropriate for two reasons. First, effective dose (E dose), as defined by the International Commission on Radiological Protection, is not intended as an epidemiological tool for risk assessment. This is discussed further in the upcoming section. Second, the LNT model is a “preferred” model, but a large degree of uncertainty is present at doses less than 100 mSv, with error bars that include zero excess risk. To date, there has been no direct evidence of increased cancer risk to patients exposed to a level less than 100 mSv.
In direct response to media reports in the popular press regarding the risks of radiation, the American Association of Physicists in Medicine ( ) released a public position statement that contains the following statement: “Risks of medical imaging at E doses below 50 mSv for single procedures or 100 mSv for multiple procedures over short periods of time are too low to be detectable and may be nonexistent. Predictions of hypothetical cancer incidence and deaths in patient populations exposed to such low doses are highly speculative and should be discouraged.”
Before we can answer this common question, we need to understand the terminology used to describe radiation dose in CT. Typically when a patient, parent, or clinician asks, “How much radiation is used for a CT study?” they are indirectly asking, “What is the risk incurred by the patient by undergoing this study?” Approaches to answering this question are provided in the Communicating Risks section at the end of this chapter. In this section, we lay the groundwork by defining the various metrics used to describe radiation dose in CT examinations.
The term radiation dose in the context of CT can be split into three categories:
the radiation output of the CT scanner, quantified by the Computed Tomography Dose Index (CTDI; mGy) and the dose length product (DLP; mGy∗cm);
the radiation absorbed by the patient, quantified by absorbed organ dose (mGy) and E dose (mSv); and
carcinogenic risk to the patient, typically estimated in terms of lifetime attributable risk from the BEIR report VII or calculated as an equivalent surrogate examination (i.e., number of chest radiographs).
The absolute and relative risks to the patient are the most difficult questions to answer, because insufficient data exist at the low dose levels of radiation used in diagnostic CT to allow us to accurately predict cancer risk with any degree of certainty. The definitions of each quantity mentioned earlier are explained later.
It is instructive for any radiologist to review the definition of CTDI VOL , the most widely used descriptor of “dose.” This value and the DLP are exported by the scanner via a Radiation Dose Structured Report (RDSR) on modern scanners that can be included in the patient’s medical record. It is also available in the form of a secondary capture dose screen, typically viewable on a PACS (picture archiving and communication system) workstation. CTDI VOL is also measured on an annual basis for typical examinations (e.g., pediatric head, pediatric abdomen, adult head, and adult abdomen) by a Qualified Medical Physicist (QMP) as part of compliance testing and American College of Radiology accreditation requirements.
CTDI VOL is a calculated value based on a measured quantity, termed CTDI 100 . The quantity CTDI 100 is the dose measured by placing a 100-mm ionization chamber in the peripheral and central holes of a standard 16- or 32-cm CTDI phantom during one axial rotation ( Fig. 22.1 ).
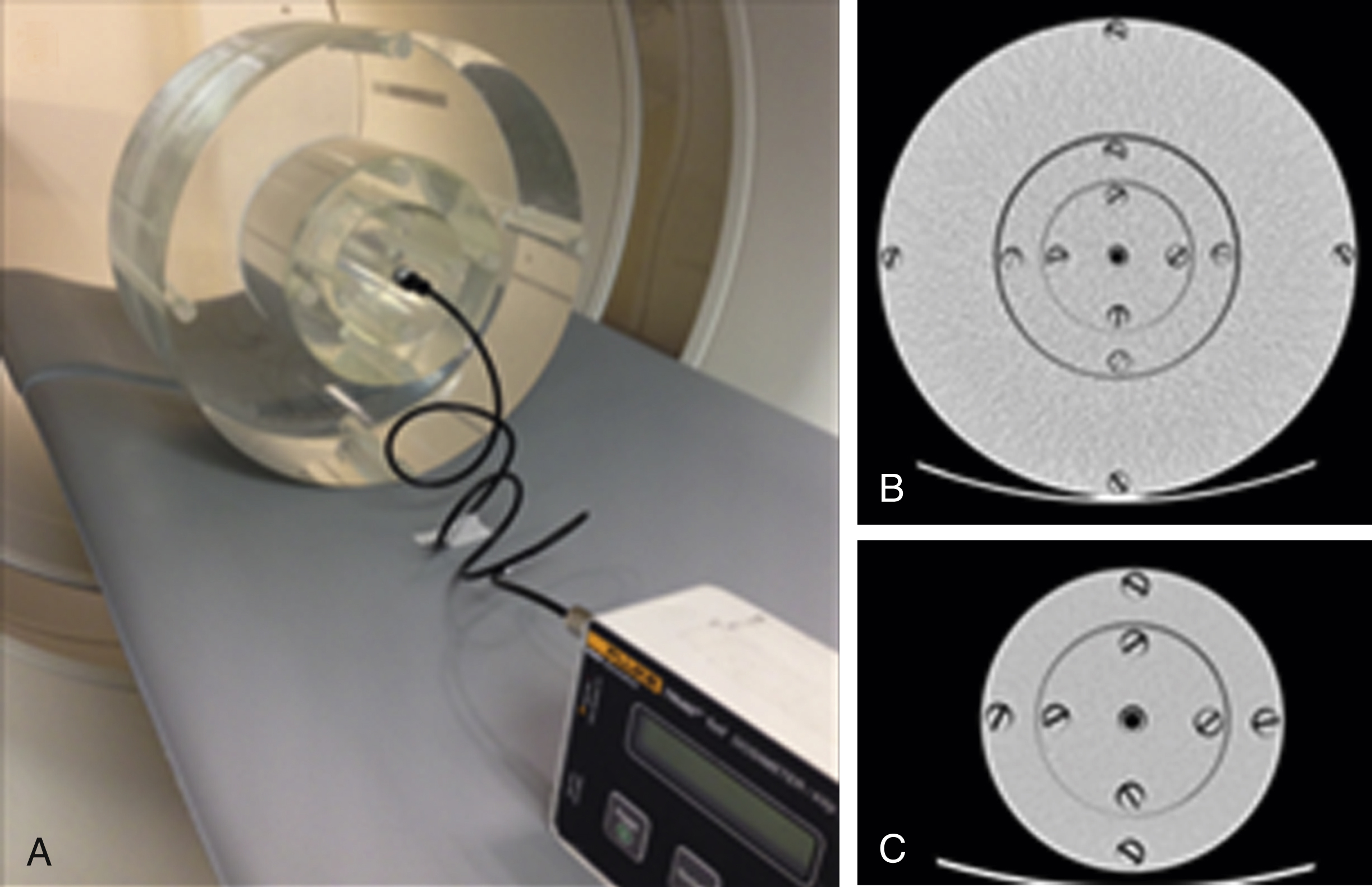
The quantity CTDI w (CTDI weighted) is calculated from measurements of CTDI 100 at the periphery and center to estimate the average dose across the radial cross section:
The quantity CTDI VOL includes the effect of pitch and is calculated by the following equation:
Pitch will be discussed further in the Advanced Topics section. Historically, the 16-cm CTDI phantom was intended to represent the approximate size of an adult head or pediatric abdomen, while the 32-cm CTDI phantom was intended to represent an adult abdomen. However, the use of these phantom sizes is not consistent across CT manufacturers. For example, Siemens calculates CTDI using the 32-cm phantom for all body protocols and the 16-cm phantom for all head protocols, while GE uses the 32-cm phantom only for adult body protocols and the 16-cm phantom for pediatric body and all head protocols. The CTDI VOL value should always be displayed with the size of the CTDI phantom (16 or 32 cm) used for the calculation and is available on the dose capture screen as shown in Fig. 22.2 . Using identical scanning techniques, the CTDI VOL measured with the 16-cm phantom will be approximately twice as high compared with the CTDI VOL measured with the 32-cm phantom, with some deviation from this rule at lower kilovoltage (kV) (80 and 100) where the ratio is slightly greater than 2. CTDI VOL is typically used as the metric for protocol design and optimization because it eliminates the variable of scan length (unlike DLP), which can vary significantly with patient height.
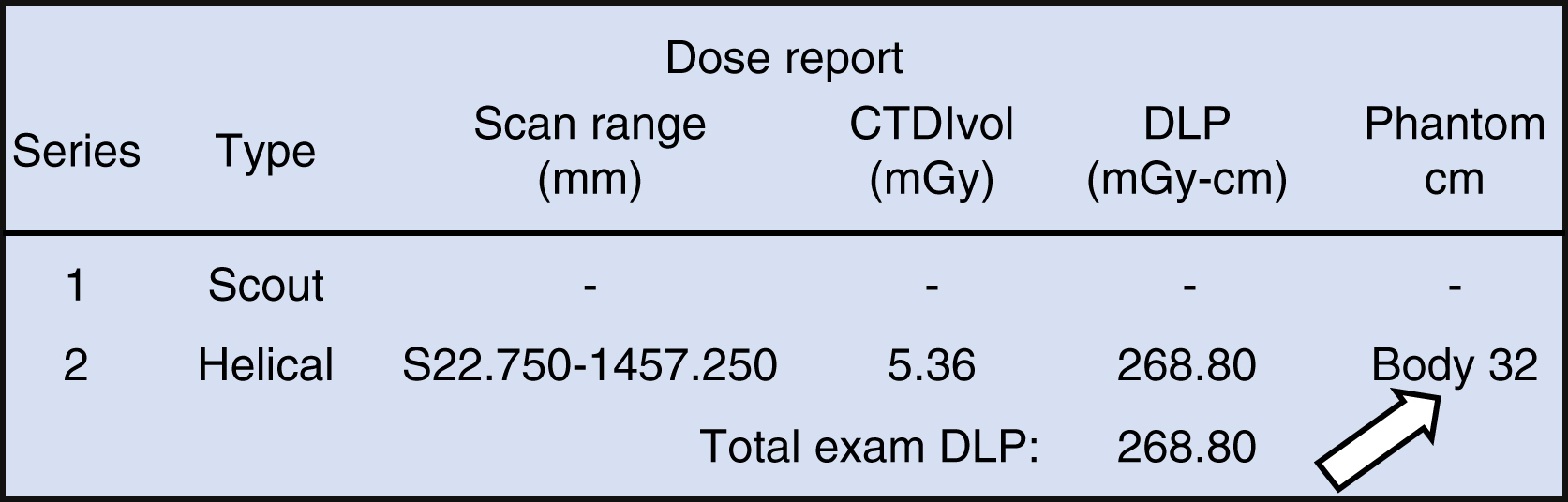
The DLP is the product of CTDI VOL and scan length (in units of mGy∗cm). It describes the CT scanner’s radiation output to the CTDI phantom along the entire scan length:
DLP also includes overscanning that occurs in helical scanning protocols. Overscanning is required in helical scanning to obtain sufficient projection data to reconstruct images at the beginning and end of the scan range. The actual irradiated length can be obtained by calculating the DLP/CTDI VOL ratio. As such, DLP is the most comprehensive value for total radiation output of the scanner. DLP is typically used to estimate E dose by using DLP-ED conversion factors with units mSv/mGy∗cm. This will be discussed further in the Effective Dose section.
The size-specific dose estimate (SSDE) is a quantity derived from CTDI VOL to estimate the absorbed dose to the patient instead of a standard plastic cylinder. SSDE was developed by the American Association of Physicists in Medicine (AAPM) Task Group (TG) 204. The TG report published conversion factors that could be used to calculate SSDE from CTDI by measuring the patient anteroposterior (AP) and/or lateral dimensions. The TG 204 conversion factors applied only to body parts of solid soft tissue. To account for body parts with heterogeneous density (e.g., thorax), TG 204 was supplemented with TG 220, which defines water equivalent diameter as the standard for estimating patient size. In theory, each z-axis position along the scan length has an associated SSDE. In an effort to simplify the calculation of a single SSDE, it has been shown that using the scan-average output (i.e., CTDI VOL from the dose report) and measuring water equivalent diameter at the center of the scan range results in acceptable error.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here