Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Transcranial Doppler (TCD) can be performed, with or without imaging. Four windows are available after closure of the anterior fontanelle—temporal, orbital, transforaminal, and submandibular.
Physiologic variables including age, gender, hematocrit, viscosity, carbon dioxide, temperature, blood pressure, and mental or motor activity can impact flow velocity.
There are several established clinical applications for TCD and many potential applications for the assessment of cerebral blood flow in the pediatric and adult age groups.
TCD can be used as a screening test to assess for stroke risk in children with sickle cell disease. Those children with time-averaged maximum mean velocities higher than 200 cm/sec or peak systolic velocities greater than 250 cm/sec in the middle cerebral artery (MCA) or distal internal carotid artery are at highest risk for stroke.
TCD can be used to assess for vasospasm and help guide therapy. A peak systolic velocity greater than 200 cm/sec is considered abnormal in the appropriate setting. The accuracy of TCD in the diagnosis of vasospasm can be increased by using the Lindegaard ratio (MCA/cervical internal carotid artery ratio). A ratio of higher than 6 suggests severe vasospasm; a ratio between 3 and 6 is associated with mild to moderate vasospasm, and less than 3 in patients with elevated flow velocities is likely secondary generalized hyperemia.
Duplex Doppler sonography with color flow imaging through the anterior fontanelle is simple and has proved useful in evaluating abnormalities of cerebral blood flow in the neonate and young child. Once the fontanelle closes, transcranial Doppler (TCD) sonography can still be performed noninvasively using a low MHz (2- to 2.5-MHz) pulsed wave Doppler transducer through the thin temporal bone, the orbits, and the foramen magnum. This technique can be used to measure the velocity and pulsatility of blood flow within the intracranial arteries of the circle of Willis and the vertebrobasilar system. TCD sonography has become a key tool in the management of children with sickle cell disease (SCD) and has proved to be a valuable adjunct in the evaluation of various intracranial pathologies in children, including vasospasm, vascular malformations, and brain death, as well as the assessment of cerebral hemodynamics after trauma, asphyxia, migraine, and stroke.
Two types of TCD sonographic equipment are available: nonimaging (TCD) and imaging (TCDI). The nonimaging technique uses continuous wave and pulsed wave Doppler to insonate specific vessels using strict criteria for vessel identification based on the depth and direction of flow for intracranial vessels through the temporal bone. This blinded technique requires meticulous skill in obtaining the highest achievable audible Doppler frequency and the ability to maintain the mental image of the circle of Willis. Advantages of nonimaging TCD include the fact that the units designed for this exam are small, portable, and inexpensive; have good Doppler sensitivity; and have superior window maneuverability due to small transducer size. Limitations include the need for intensive training, difficulty in finding specific vessels by sound, and lack of unit availability in radiology departments. Duplex Doppler sonography with color imaging using low MHz transducers via the temporal bone has increased the utility of TCD sonography. Advantages of TCDI include rapid vessel identification, a shorter learning curve, and availability of units in radiology departments. This imaging technique allows more confident vessel identification, resulting in easier, more reliable, and reproducible information. However, with training and experience, both techniques have been shown to be reliable and reproducible.
The anterior fontanelle typically remains open through the first year of life. Once closed, three cranial windows (in addition to burr holes and surgical defects) can be used routinely to insonate the intracranial circulation: the transtemporal window, orbital window, and the suboccipital window. The submandibular window can also be used to complete the examination of the cervical segment of the internal carotid artery (ICA).
The transtemporal approach is through the thin suprazygomatic portion of the temporal bone using a low MHz (2- to 2.5-MHz) transducer. The transtemporal acoustic window is usually found on the temporal bone just cephalad to the zygomatic arch and anterior to the ear. The intracranial anatomic landmark in this plane by gray-scale imaging is the heart-shaped cerebral peduncles ( Fig. 47.1A ). Just anterior to the peduncles is the star-shaped, echogenic interpeduncular or suprasellar cistern. Anteriorly and laterally from this basilar cistern lies the echogenic fissure for the middle cerebral artery (MCA). Color Doppler sonographic imaging ( Fig. 47.1B , ) and spectral analysis ( Figs. 47.1C and 47.2A , ) of this vessel show flow toward the transducer. Insonating the vessel deeper toward the midline directs the operator into the bifurcation of the A1 segment of the anterior cerebral artery (ACA) and the MCA ( ). Spectral analysis at this bifurcation landmark will show bidirectional flow, that is, flow toward the transducer in the MCA and flow in the ACA away from the transducer ( Fig. 47.2B ). As the Doppler gate is moved more medial anteriorly, flow is seen entirely in the ACA away from the transducer ( Fig. 47.2C ). The MCA should be studied from its most peripheral location to the point of bifurcation, and the ACA studied as medially as possible. The distal internal carotid artery (DICA) is just inferior to the bifurcation. The flow of the DICA may be dampened with a harsher sound from the increased angle of insonation, with flow typically directed toward the transducer ( Fig. 47.2D ). The posterior cerebral artery (PCA) can be visualized as it circles around the cerebral peduncles. Flow in this vessel may be away or toward the transducer depending on curser placement ( Fig. 47.2E ). At times, the MCA, the ACA, and the PCA on the opposite side may also be visualized. Ideally, each side should be studied through the ipsilateral window whenever possible.
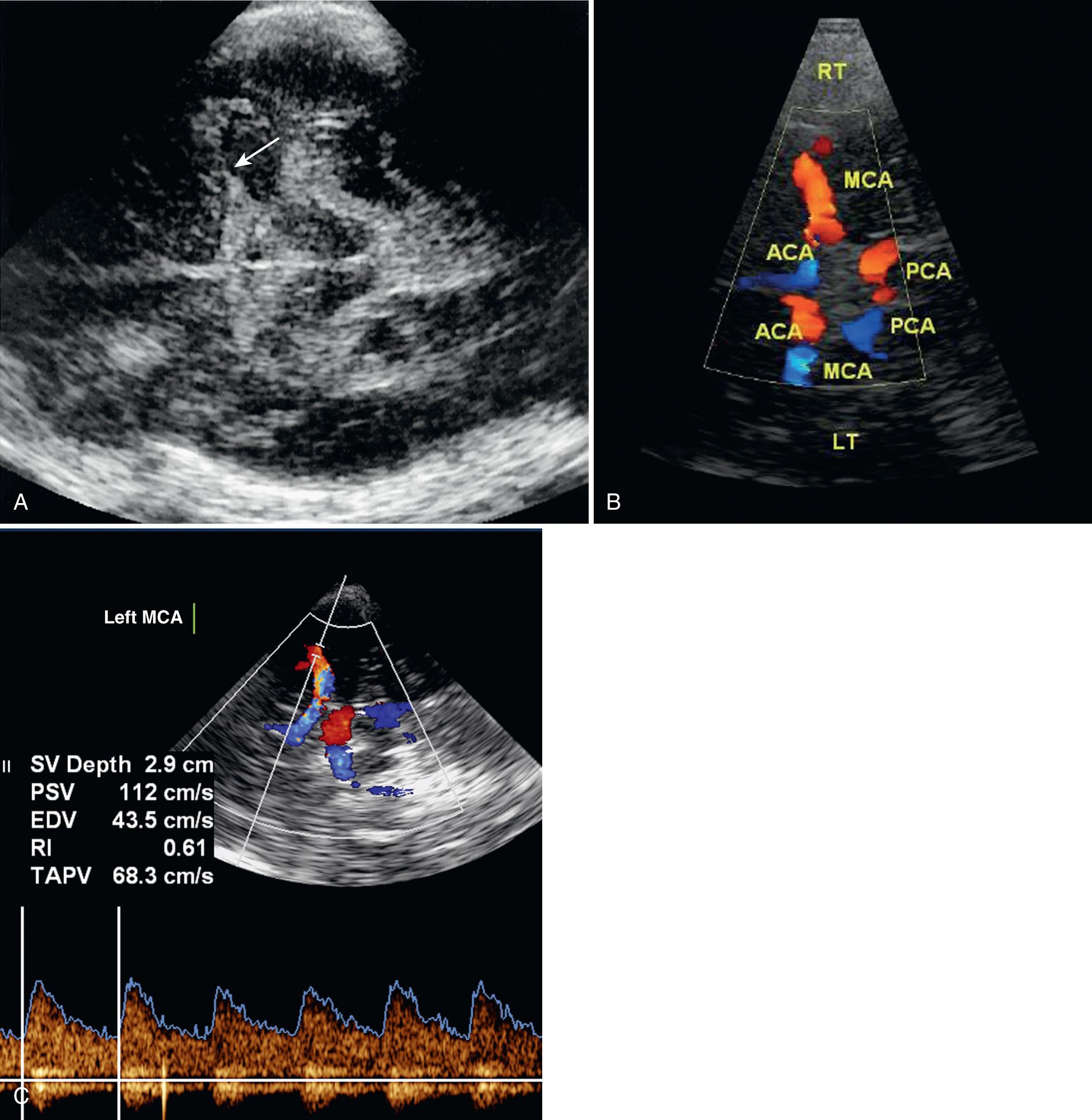
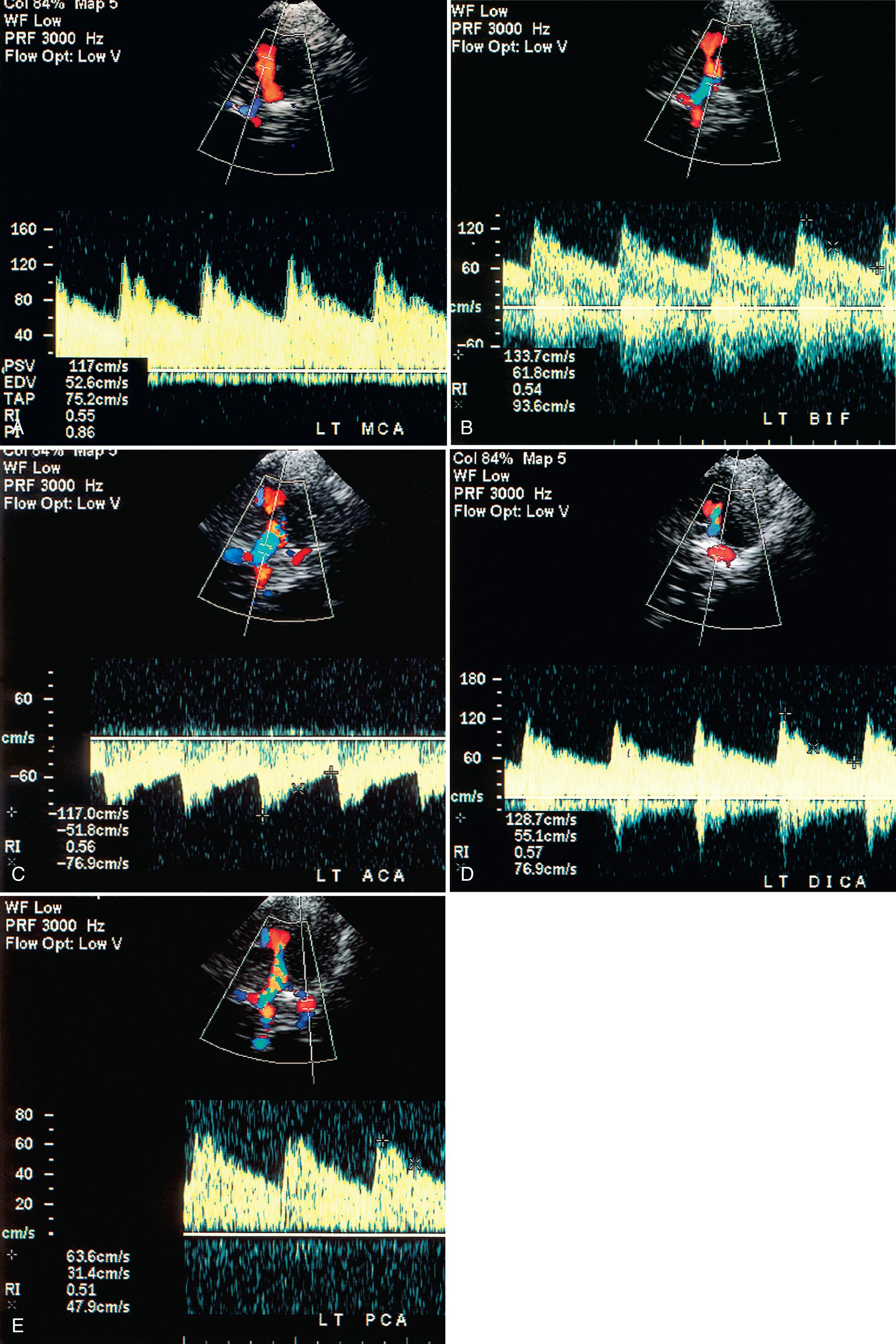
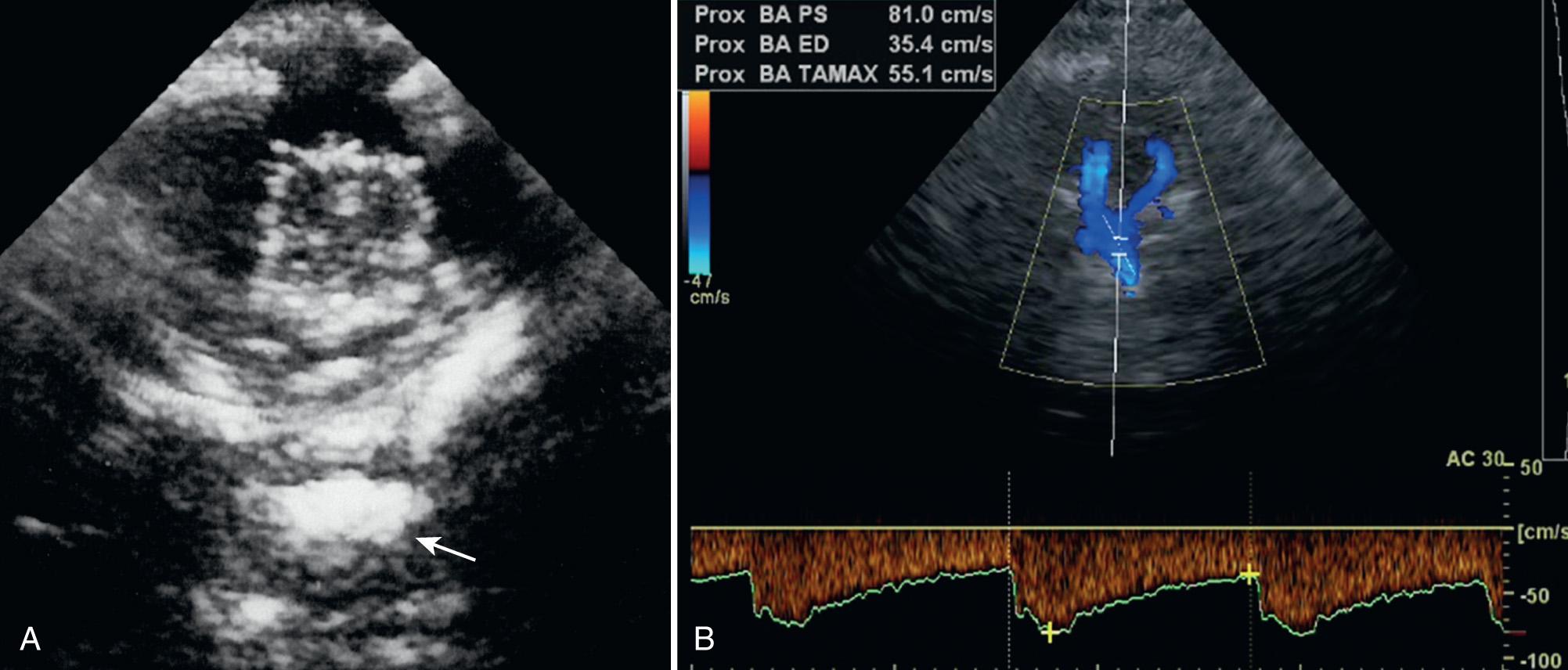
The vertebral and basilar arteries can be studied through the suboccipital window via the foramen magnum with a low MHz transducer. The patient lies on one side or prone, and the head is bowed slightly so the chin touches the chest, causing a gap between the cranium and the atlas to enlarge. The transducer is placed midline in the nape of the neck and angled through the foramen magnum toward the orbits. The normal landmark is the rounded hypoechoic medulla just anterior to the echogenic clivus ( Fig. 47.3A ). The vertebral arteries appear in a V-shaped manner as they rise to the medullopontine junction to form the basilar artery between the hypoechoic medulla-pons junction and the echogenic clivus ( Fig. 47.3B ). From this posterior view, flow in the vertebral and basilar arteries should be directed away from the transducer ( Fig. 47.3C ).
The transorbital window allows for evaluation of the ophthalmic artery (OA), the retinal artery, and the carotid siphon. This evaluation is performed with the eyes closed using a higher MHz (5- to 7.5-MHz) transducer on its lowest power setting ( Fig. 47.4 ). Unlike the temporal approach in which the bone attenuates a majority of sound thus requiring a low MHz transducer, the fluid-filled globe only minimally attenuates the sound waves. Although no bioeffects from the ultrasound evaluation of the eye are known, ocular damage can potentially occur as ultrasound energy can create cavitation and heat. The U.S. Food and Drug Administration (FDA) guidelines suggest limiting spatial peak temporal average to 17 mW/cm 2 for orbital imaging and limit the mechanical index to 0.28. The time of insonation must also be as minimal as possible to decrease risk of damage to soft tissues. The FDA has approved certain imaging transducers on various manufacturer equipment for the evaluation of the orbit. It is important for operators to determine which transducer is approved for orbital imaging for their system.
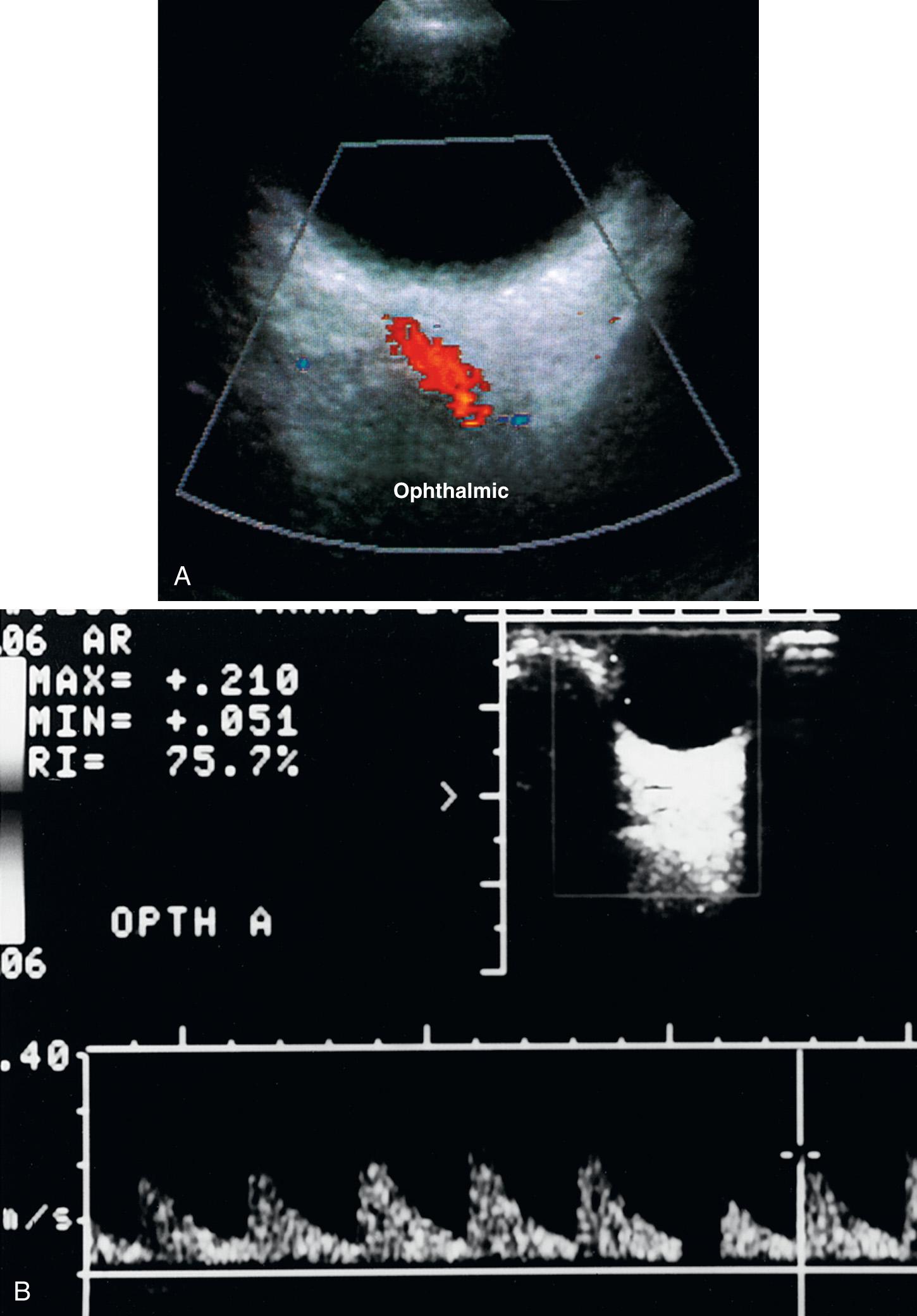
A liberal amount of gel is needed on the eyelid with only light pressure utilized on the globe. The orientation marker should be pointed medially toward the nose. Flow in the OA should be toward the transducer. The OA enters the optic foramen and lies lateral and slightly inferior to the optic nerve. It then usually crosses superior to the optic nerve and proceeds anteriorly on the medial side of the orbit. The color pulse repetition frequency should be decreased to visualize the OA. The mean velocity of the OA is 21 + 5 cm/sec with high pulsatility. The central retinal artery branch of the OA is the easiest branch to interrogate with color flow Doppler imaging, just posterior to the retina. Because visualization of this central retinal artery entails directing sound waves through the lens, the lowest power setting must be used to minimize the risk of lens injury and subluxation. However, a large OA branch proceeds along the nasal or medial wall of the orbit. Because interrogating this vessel does not involve directing the sound beam through the lens, a higher power setting may be used for this branch. In the carotid siphon, flow direction varies depending on the segment: toward the probe in the infraclinoid segment, bidirectional in the genu, and away from the probe in the supraclinoid segment. The mean velocity of the carotid siphon is 47 + 14 cm/sec with a low resistance signal.
The submandibular window, located at the angle of the jaw, allows for examination of the extracranial ICA, including morphology, flow direction, and velocities. Once the vessels have been identified, different information can be obtained through evaluation of the spectral waveform. Flow direction is one of the first parameters obtained with evaluations (by convention, flow toward the transducer is coded red and above the baseline, whereas flow away from the transducer is coded blue and below the baseline). A large sample volume (4-6 mm) allows for good signal to noise ratio. Velocities, such as peak systolic velocity (PSV), end diastolic velocity (EDV), and mean flow velocity, and indices reflecting vascular resistance including pulsatility index and resistive index are evaluated.
On spectral analysis of the waveform, the maximum, minimum, and mean velocities can be measured in centimeters per second. It is important to remember which velocity measures are used for various applications. For the STOP study assessing stroke risk in the sickle cell population, the time-averaged mean of the maximum (TAMM) velocity is followed, and is sometimes called the time-averaged peak velocity. This is not the same value as the mean velocity. PSV is the value followed for vasospasm. Terminology of the various mean and TAMM velocities can be vendor specific and need to be clarified dependent on the application TCD is being used for. At least two tracings should be made for each vessel. The highest velocity obtained is likely the most accurate, because it is believed to be the velocity obtained at the best insonating angle to the vessel.
Angle correction cannot be performed with the nonimaging technique, and a 0-degree angle is assumed. Although angle correction is possible with the imaging technique because of visualization of the vessel course, published velocities typically have not been angle-corrected. In clinical applications such as assessing stroke risk in children with sickle cell anemia, guidelines regarding normal versus abnormal/conditional velocities were validated in large clinical trials using nonduplex equipment that was not angle-corrected. Angle correction can significantly increase velocities in vessels that are not coursing directly toward the transducer. Although angle correction has been suggested as a way to correct for variations between the imaging and nonimaging examinations, the lack of published data for angle-corrected velocities currently limits this approach. Audible optimization for curser placement is key in obtaining similar values with TCD and TCDI without angle correcting. Placing a cursor on a two-dimensional color image may not result in the most optimized tracing because the third dimension of the vessel may be out of the image plane. For this reason, curser placement based solely on a color image may result in a lower velocity than curser placement utilizing the highest achievable audible Doppler frequency. Because the MCA and OA usually course almost directly toward or away from the transducer using the technique described here, angle correction is less of an issue in these vessels. ACA and PCA velocities are more variable because of their tortuous course.
An index of pulsatility, either the pulsatility index (PI) (PSV − DV [diastolic velocity]/mean velocity) or resistive index (RI) (PSV − DV/PSV), can also be evaluated. Both of these indices are ratios and therefore minimize the effect of vessel angulation.
Physiologic variables including age, gender, hematocrit, viscosity, carbon dioxide, temperature, blood pressure, and mental or motor activity can impact flow velocity. Age-dependent reference values are available for velocities of the various intracranial vessels. Early in life, velocities increase with age, demonstrating a rapid increase after birth and a slower increase until 6 to 8 years of age. Afterward and through adolescence, a slow decrease is seen until 70% of the maximal velocity is reached around 18 years of age. Regarding gender, slightly higher velocities are observed in pubertal girls when compared to boys. An inverse relationship is present between hematocrit and viscosity and flow velocity; a decrease in 30% to 40% of the hematocrit will result in an increase in velocities around 20%. If anemia is the cause of elevated velocities, the changes should be detected in all of the intracranial arteries. A focal velocity increase suggests a focal cause.
Changes in arterial carbon dioxide (CO 2 ) affect cerebral blood flow and arterial velocities. Hyperventilation causes a decrease in the MCA mean velocity and an increase in the PI. Hypoventilation causes an increase in MCA mean velocity and decrease in PI. If a child is sleeping or in pain and is hyperventilating, TCD results may be affected.
Heart rate also influences intracranial arterial velocities. The sonographer may need to adjust Doppler display sweep time in extreme cases of bradycardia or tachycardia. If autoregulation is intact, cardiac output should not have an affect on TCD velocities.
Velocities can demonstrate daily and interobserver variability. Studies evaluating these variations suggest that normal daily changes should be less than 10 cm/sec and interobserver variability can be 7.5% (measured at the same time) to 13% (daily variation). They suggest that a variation of more than 14% be considered abnormal.
Normal mean velocity in the MCA in adults range from 50 to 80 cm/sec; in the ACA, 35 to 60 cm/sec; in the PCA, 30 to 50 cm/sec; and in the basilar artery, 25 to 50 cm/sec. PSVs up to 150 cm/sec have been described in patients with SCD secondary to anemia. The velocity in the OA is normally about one-fourth the velocity in the MCA. The velocity in the PCA and vertebral and basilar arteries should be about one-half the velocity in the MCA.
The normal PI ranges from 0.7 to 1.1 and the normal RI ranges from 0.43 to 0.58 after fontanelle closure. The OA has a higher RI, usually between 0.70 and 0.79, and less diastolic flow because it supplies a muscular bed (see Fig. 47.4B ). An increase in diastolic flow will result in a decrease in the RI, whereas a decrease in diastolic flow will result in an increase in the RI. As intracranial pressure increases above mean arterial pressure, diastolic flow may become reversed, demonstrating an RI of greater than 1.
The Lindegaard ratio is a ratio between the velocity of the MCA or ACA and the ipsilateral extracranial ICA and can help differentiate changes caused by generalized hyperemia from vasospasm. A similar ratio can also be obtained between the basilar artery and the extracranial vertebral artery.
The American Institute of Ultrasound and Medicine and the U.S. federal guidelines of the spatial peak temporal average intensity (I SPTA ) for the pediatric head recommend not to exceed 94 mW/cm 2 . The limit decreases to 17mW/cm 2 when evaluating vessels in the eye. A maximum mechanical index of 0.28 is also recommended with a limit in the time OA of insonation. The power settings of transducers of various manufacturers are different for each piece of equipment and each probe. Depending on the manufacturer and transducer, energy levels may be within the guidelines only on the low power setting.
When the transtemporal approach is used, at least 65% (and likely more) of the energy is attenuated by the skull. Lower MHz transducers are thus needed for adequate penetration of the skull, and higher settings may therefore be used. It is important when moving to OA insonation that a different transducer with lower setting be used. At any time, the “as low as reasonably achievable” (ALARA) principle should be followed by limiting the exposure time and increasing gain to improve signal and avoiding an increase in power output.
TCD and TCDI are inexpensive, noninvasive, and widely available, but they are highly operator dependent. Thorough training and knowledge are required to adequately perform this type of examination. Finding the best transtemporal window can be difficult. The transtemporal window varies in size and location. Anywhere from 10% to 37% of patients do not have a good acoustic window. The inability to penetrate the temporal bone is influenced by age, sex, and race. Hyperostosis of the skull is most common in African Americans, Asians, and older females. Contrast agents have been used in an attempt to improve vessel visualization.
There are numerous pitfalls to performing a TCD sonographic examination in children ( Table 47.1 ). Low wall filter adjustments, high Doppler ultrasound frequency, and critical assessment of velocity spectral analysis are crucial for accurate interpretation of the data. If the examiner becomes lost, it is best to start over, using a systematic search for a better window. First, with TCDI, identify the cerebral peduncles on gray scale. Then add color, optimizing color control settings, angling the transducer, and locating the MCA-ACA bifurcation. Then follow the exam protocol. Remember that although the clinician can assume a patent circle of Willis in children, one artery should not be used to represent the entire cerebral circulation.
| Issue | Suggested Tips |
|---|---|
| Child too active | Put the child at ease and answer all questions before starting the exam. |
| Use distractors such as TV. | |
| Let younger children sit on the mother's lap. | |
| Doppler signal is weak | Search for a better window. |
| Change the angle of the transducer. | |
| Change the sample volume depth. | |
| Use maximum power if needed and adjust color gain/PRF setting. | |
| Use headphones to hear subtle Doppler signals. | |
| Multiple Doppler signals are noted | Decrease sample volume size. |
| Search for a better window. | |
| Background noise is high | Adjust gain. |
| Motion artifact | Check for patient motion or transducer motion. |
| Adjust sample volume. | |
| Doppler signal aliasing | Increase PRF setting. |
| Lower baseline. |
Insufficient temporal bone windows, unfavorable insonation angles, and low flow velocities can result in nonvisualization of normal or pathologic flow with TCD sonography. In adult studies, echocontrast agents such as galactose microbubble suspensions and sulfur hexafluoride microbubbles have been used in an attempt to increase the applications of transcranial studies. These contrast agents have been shown to facilitate visualization of vessel patency, stenosis, occlusion, and collateral flow. Small-caliber arteries and vessels that run at unfavorable angles may be identified. Velocities obtained using this method provide reliable data regarding stenosis and occlusion and comparisons with digital subtraction angiography have been favorable.
Indications for echo-enhancing agents include depiction of vessel anatomy of tumors, vascular malformations, and stenosis. In the evaluation of brain death, use of contrast may improve the level of confidence of documenting low flow. Limitations of this technique include short duration of some of the contrast agents, blooming artifact, and limited availability for pediatric studies in the United States.
Contrast-enhanced three-dimensional power Doppler sonography has also been shown to improve identification of vessels. Using this technique in adults with inadequate windows, Postert et al. noted clear three-dimensional visualization of most major intracranial vascular segments. The addition of contrast resulted in more sensitive sonographic imaging than unenhanced three-dimensional reconstructions. These studies were found to be easy to perform and interpret, increasing the level of operator diagnostic confidence.
Detection and follow-up of stenosis or occlusion in major intracranial arteries
Monitoring of thrombolytic therapy
Detection of cerebral vasculopathy and evaluation of collateral intracranial flow
Detection and monitoring vasospasm
Detection right-to-left shunts and/or circulating cerebral microemboli
Assessment of cerebral vasomotor reactivity
Adjunct in evaluation of cerebral death
Intraoperative evaluation of embolization, thrombosis, and hyper/hypoperfusion
Assessment of arteriovenous malformations and intracranial aneurysms
Evaluation of positional vertigo or syncope
Evaluation of stroke risk in sickle cell disease
Detection and monitoring vasospasm
Assessment of intracranial pressure and hydrocephalus
Assessment of hypoxic ischemic encephalopathy
Assessment of cerebral vasomotor reactivity
Adjunct in evaluation of cerebral death
Assessment of dural venous patency and arteriovenous fistula
Intraoperative monitoring
Neurologic manifestations are common in SCD and include overt infarction or stroke, silent cerebral infarcts, and hemorrhage (rare in children, more often seen secondary to aneurysm rupture in adults). Stroke results in neurologic deficits, and silent cerebral infarcts can result in academic and behavioral difficulties and increase the risk of having a future stroke. Patients present with vasculopathy involving the large intracranial arteries, most frequently the distal internal carotid artery and proximal middle and anterior cerebral arteries. The vasculopathy can progress for months to years before symptoms develop and can result in formation of numerous small collateral vessels, consistent with moyamoya syndrome. This occlusive vasculopathy is characterized by endothelial damage with intimal thickening, muscular proliferation, and inflammatory changes with or without in situ thrombosis. Hemodynamic abnormalities in sickle cell anemia (including hyperemia, hypervolemia, and cerebral vascular dilation) play a role in the development of neurologic injuries, especially in the pathogenesis of silent cerebral infarcts.
The incidence of stroke varies depending on the origin of the patient and haplotype of SCD; for example, the incidence is 4.1% in Mediterranean patients, 6.7% in first-generation African patients living in France, and 10% in African Americans younger than 20 years of age. Silent cerebral infarcts are the more common type of cerebral injury in patients with SCD. They are described as a 3 mm or larger lesion seen on at least two T2-weighted magnetic resonance imaging (MRI) sequences with no history or clinical evidence of focal neurologic deficit. Silent cerebral infarcts can be seen in patients younger than 1 year of age with reported incidences of 13% by 13.7 months, 27% in patients younger than 6 years and 37% in 14-year-olds. First and recurrent strokes can be prevented, but not completely suppressed, by transfusion therapy in patients at risk. With transfusion treatment the anemia is partially corrected and cerebrovascular reserve is improved, resulting in a decrease in TCD velocities and a reduction in the risk of stroke to less than 1%, although patients may still develop new silent cerebral infarcts (18% at 1-year follow-up and 28% at 2-year follow-up). Bone marrow transplantation has proved curative in young patients with symptomatic SCD and has led to stabilization of nervous system vasculopathy.
TCD sonography has proved to be a safe, reliable, and cost-effective screening method for children at risk of stroke. A sensitivity of 90% and specificity of 100% detecting arteriopathy after stroke when compared to angiography has been described. No significant association has been identified between silent infarcts and TCD velocities or magnetic resonance angiography (MRA).
Adams et al. first showed the effectiveness of nonimaging Doppler sonographic studies in screening for cerebrovascular disease in patients with SCD. Using the transtemporal and suboccipital approach, Adams et al. screened 190 asymptomatic SCD patients and found in clinical follow-up that a TAMM velocity in the MCA of 170 cm/sec or greater indicated a patient at risk for development of cerebrovascular accident (CVA, stroke).
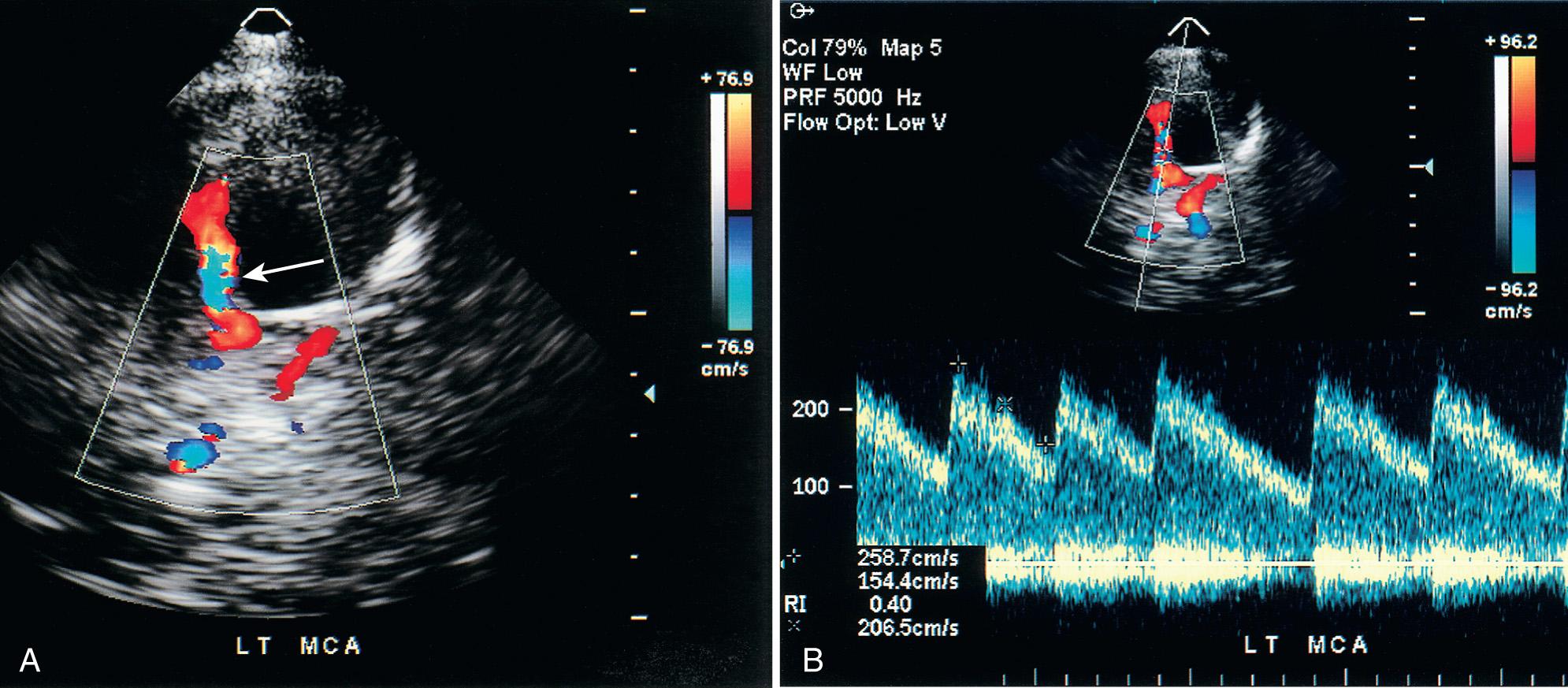
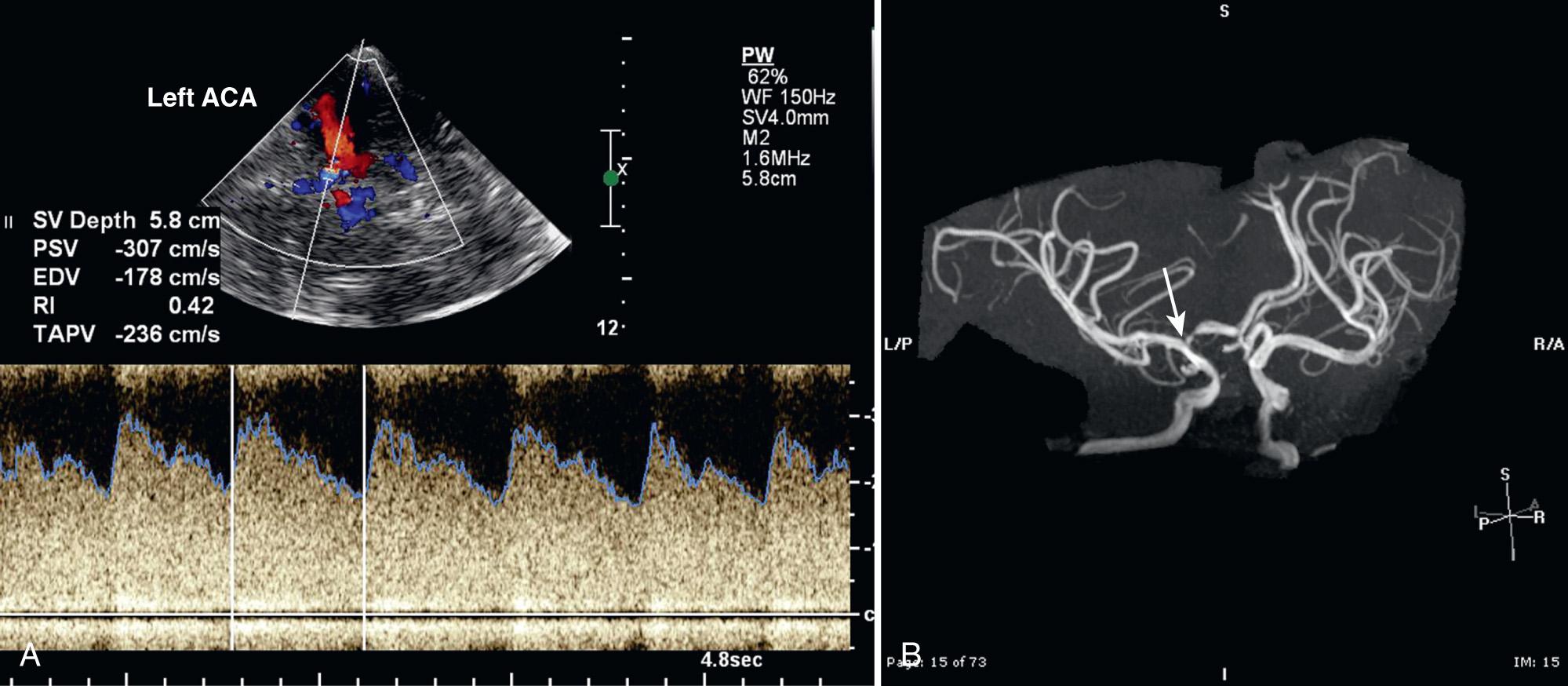
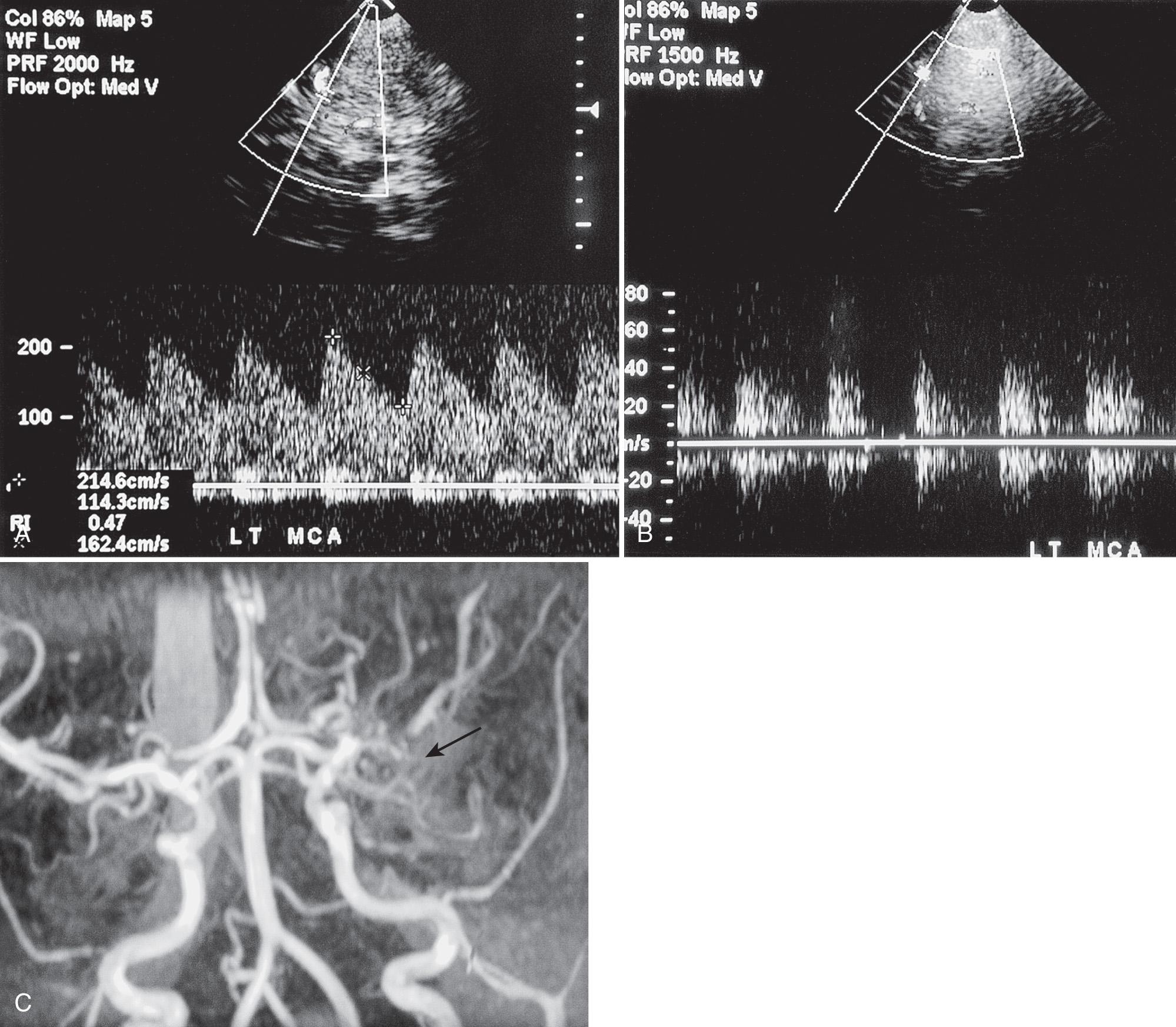
Using duplex Doppler imaging, MRA, and MRI, Seibert et al. described five indicators of cerebrovascular disease in patients with SCD: (1) maximum velocity in the OA of more than 35 cm/sec ( Fig. 47.5 ), (2) mean velocity in the MCA of more than 170 cm/sec ( Fig. 47.6 ), (3) RI in the OA less than 0.6 ( Fig. 47.7 ), (4) velocity in the OA greater than that of the ipsilateral MCA, and (5) maximum velocity in the PCA or the vertebral or basilar arteries greater than maximum velocity in the MCA. An 8-year follow-up of 27 neurologically symptomatic and 90 asymptomatic sickle cell patients showed all five original TCD indicators of disease were still significant. Four additional factors were also significant: (1) turbulence, (2) PCA or ACA visualized without seeing the MCA ( Figs. 47.8, 47.9, 47.10 ), (3) any RI less than 0.3, and (4) maximum MCA velocity greater than 200 cm/sec ( Fig. 47.11 ). The sensitivity of Doppler ultrasound as a predictor of stroke was 94% with a specificity of 51%.
Maximum velocity, OA ≥ 35 cm/sec
TAMM velocity, MCA ≥ 170 cm/sec
RI in OA ≤ 0.6
Velocity in OA greater than velocity in ipsilateral MCA
Maximum velocity in PCA, vertebral, or basilar arteries greater than or equal to MCA velocity
Turbulence
Visualization of PCA or ACA without visualization of MCA
Any RI < 0.3
Maximum MCA PSV ≥ 200 cm/sec
ACA, Anterior cerebral artery; MCA, middle cerebral artery; OA, ophthalmic artery; PCA, posterior cerebral artery; PSV, peak systolic velocity; RI, resistive index, TAMM, time-averaged mean of the maximum.
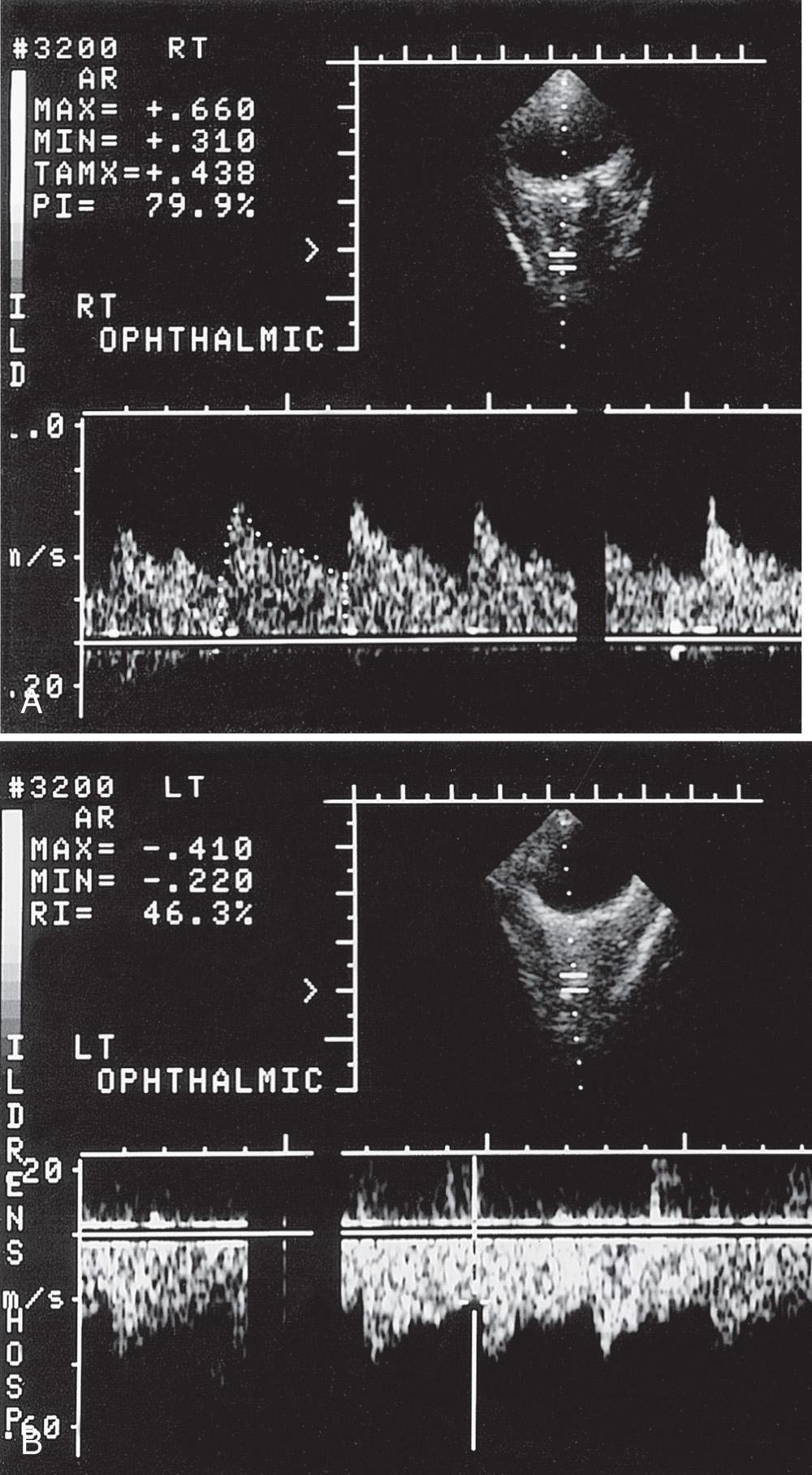
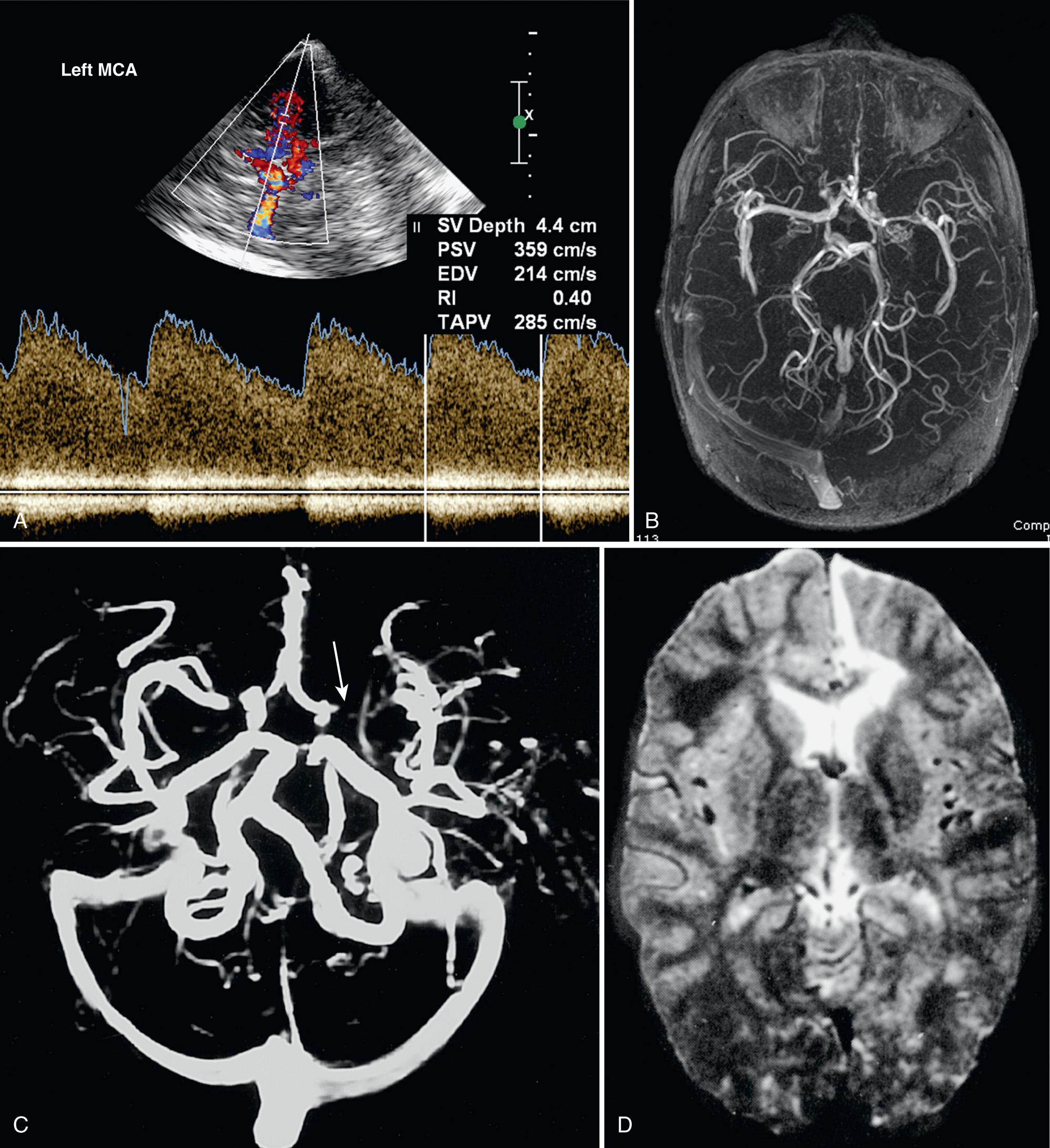
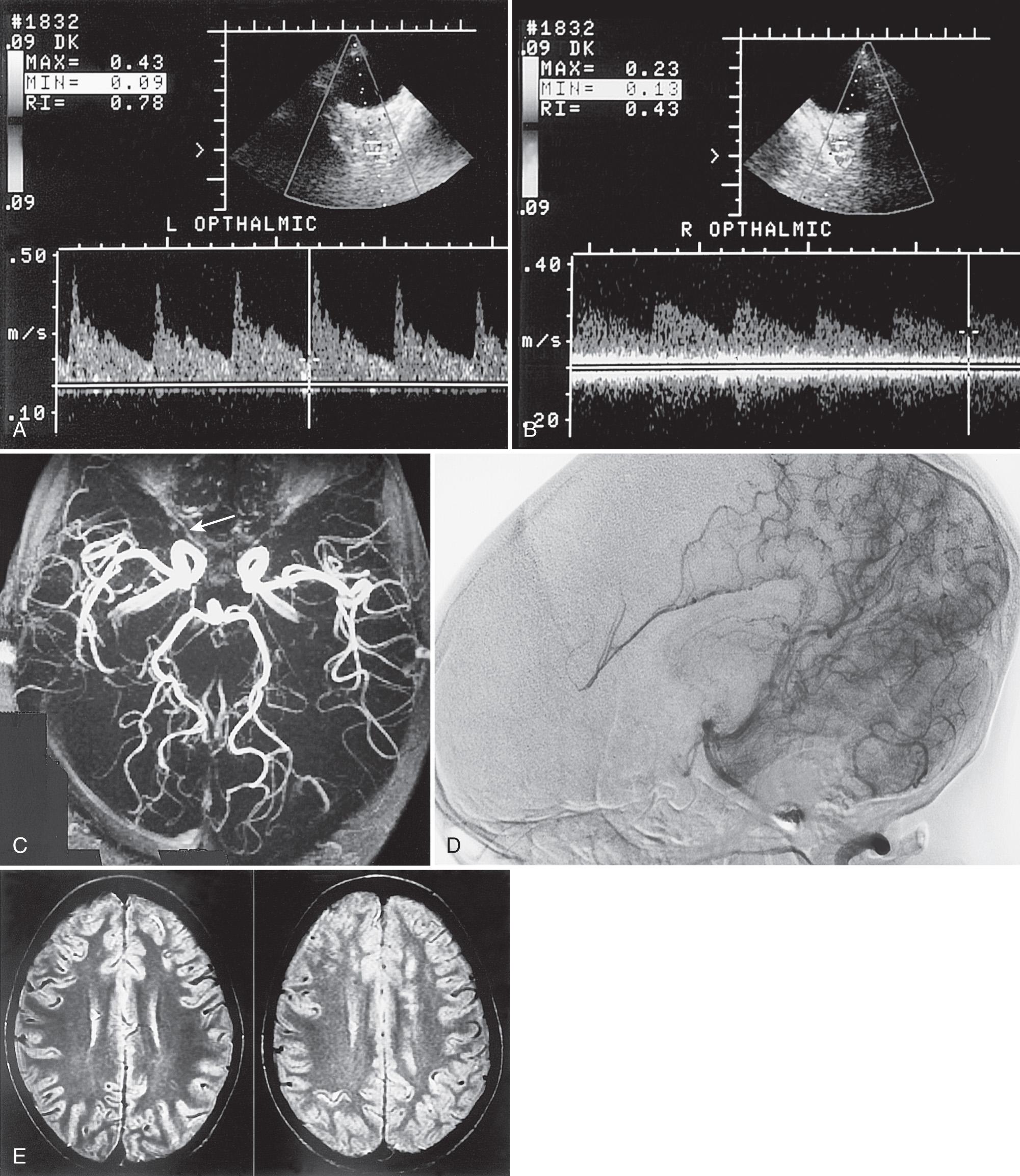
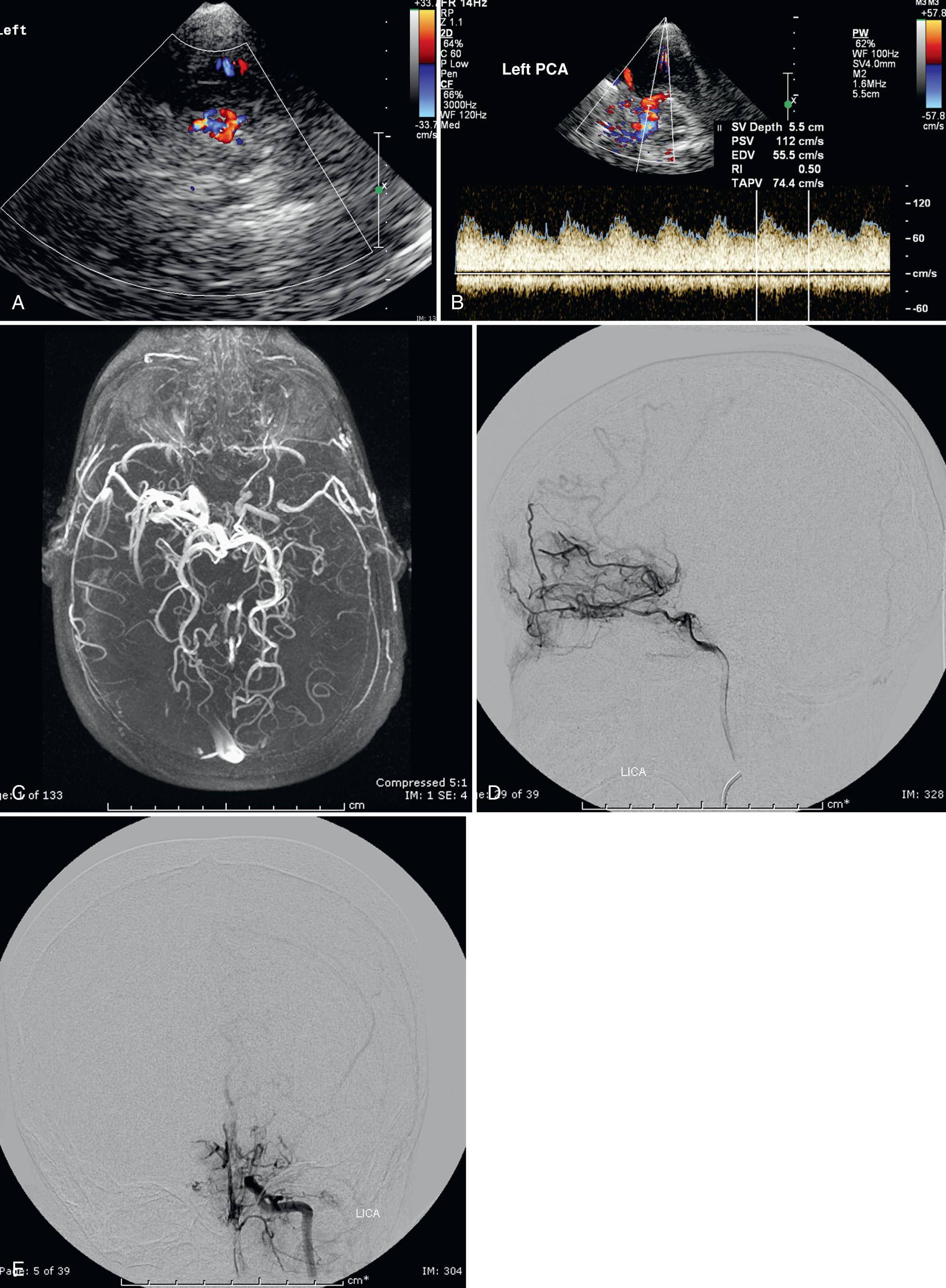
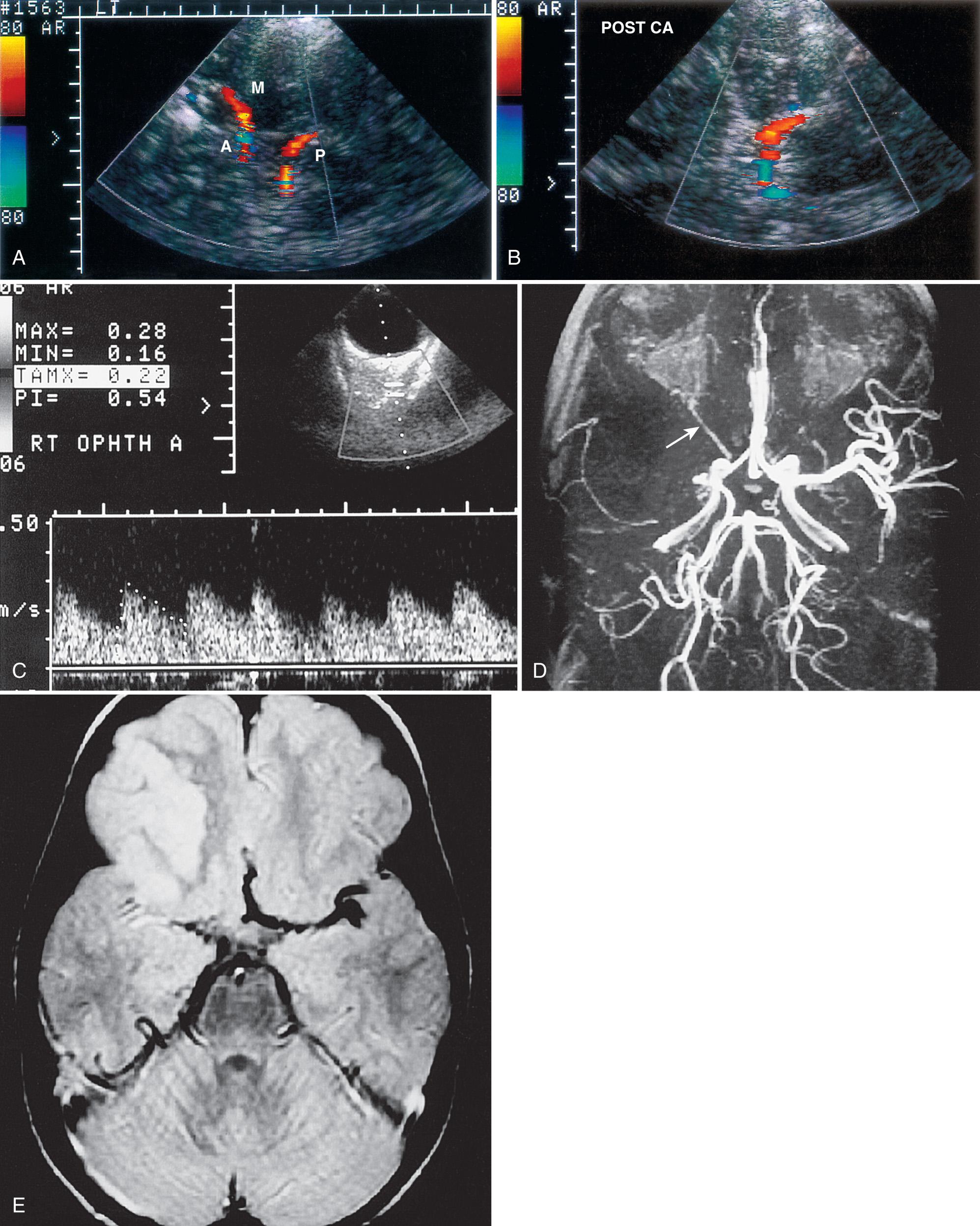

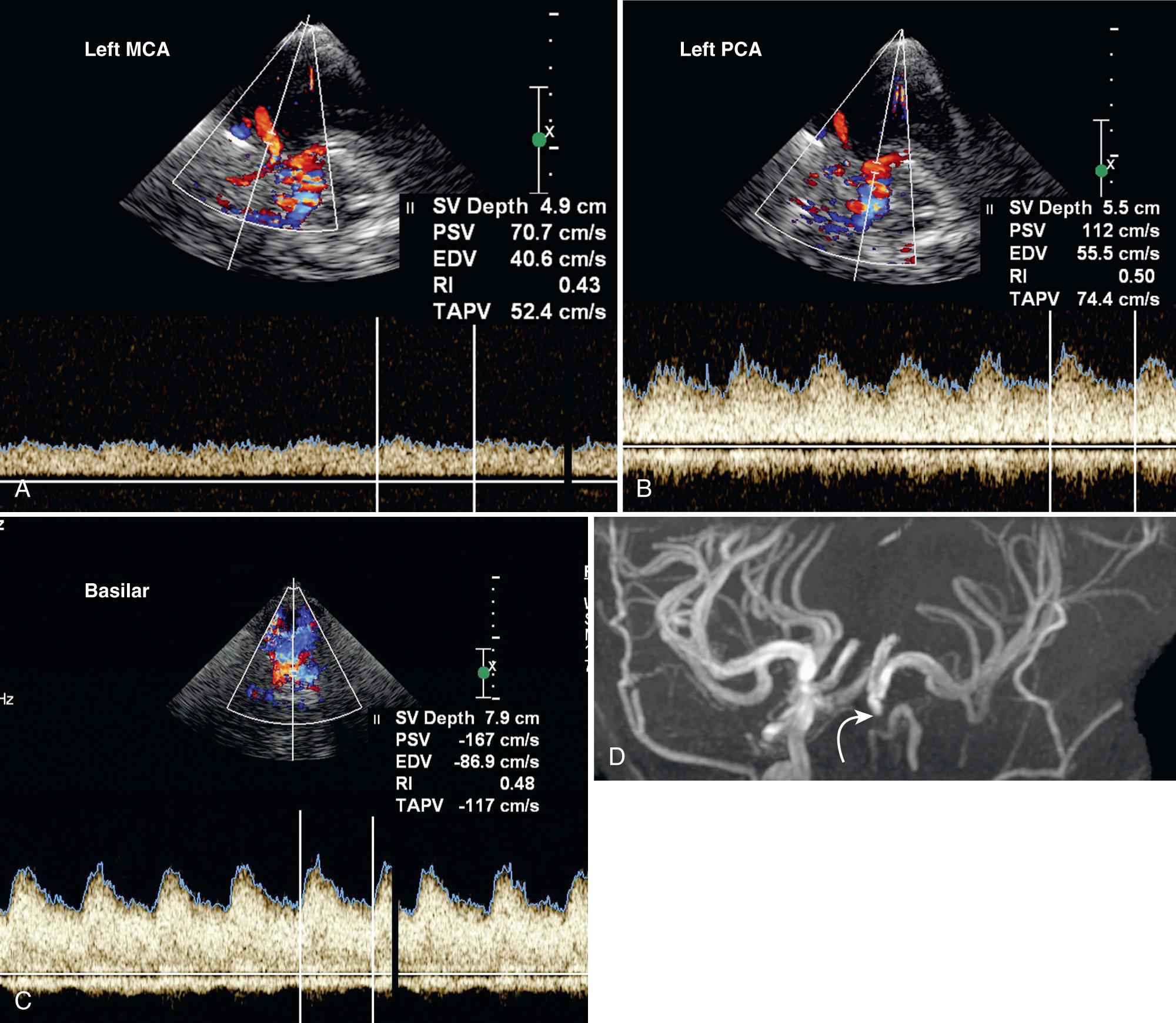
Siegel et al. compared transtemporal TCD to neurologic examination and also found a maximum flow in the MCA of more than 200 cm/sec or less than 100 cm/sec (including no flow) as significant for disease. Verlhac et al. studied SCD with duplex Doppler imaging with a 3-MHz transducer transtemporally and suboccipitally, as well as with MRA and MRI. Arteriography was performed in patients with suspected stenosis on TCD sonography. They found that patients with a mean velocity greater than 190 cm/sec had stenosis on arteriography. Kogutt et al. evaluated symptomatic SCD patients and found 91% sensitivity and 22% specificity of TCD using MRA as the reference standard. Abnormal TCD values were (1) maximum and mean velocity (V max and V mean ) greater than 2 standard deviations (SD) from normal values reported by Adams et al. in SCD: V max MCA > 168 ± −38 cm/sec and V mean MCA 115 ± −31 cm/sec, and V max ACA 138 ± 34 cm/sec and V mean ACA 94 ± 28 cm/sec; (2) RI < 0.40; and (3) V max MCA < V max ACA ( Fig. 47.12 ).
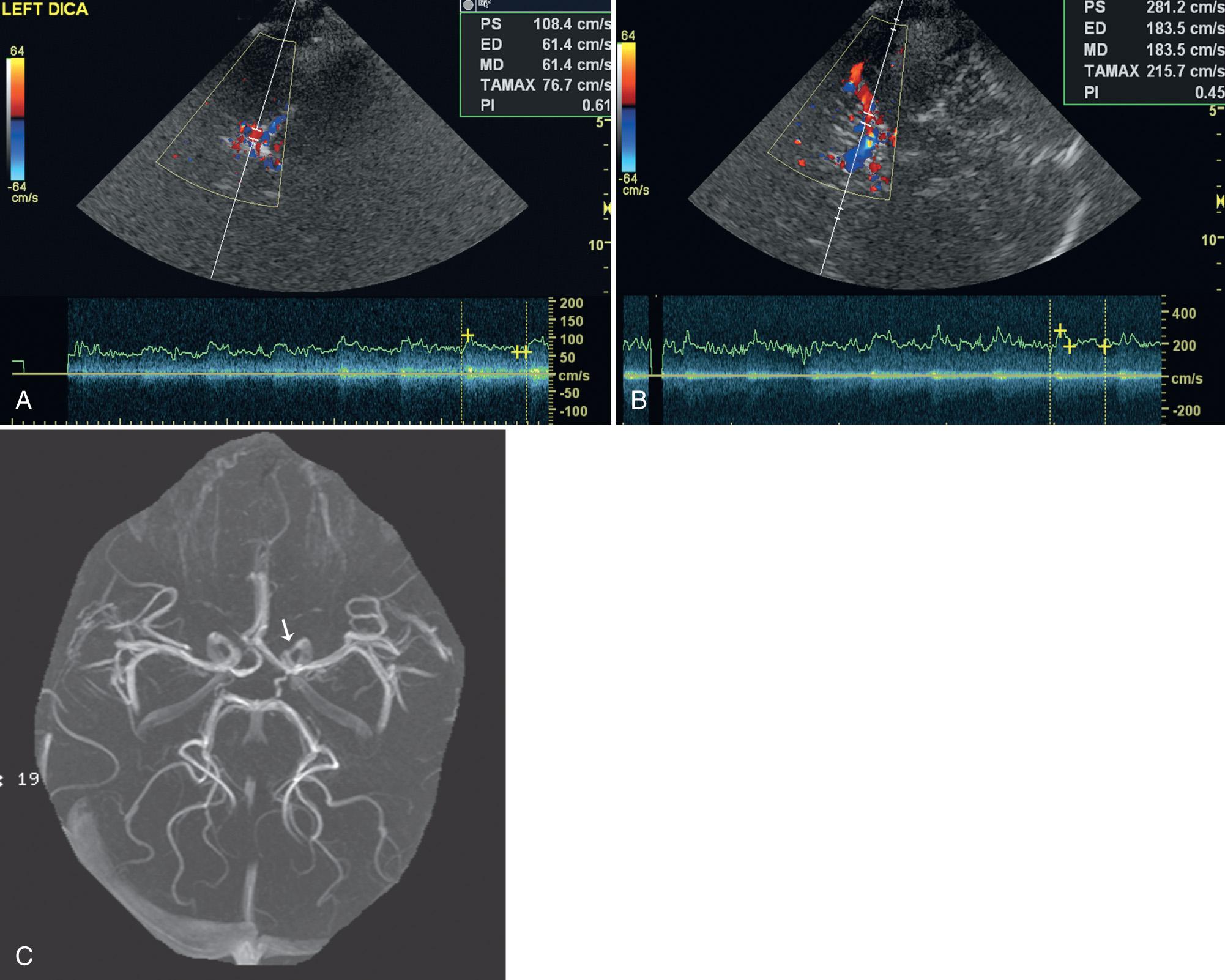
Screening sickle cell patients with the nonimaging pulsed Doppler ultrasound technique involves scanning the patient transtemporally to evaluate the MCA, bifurcation, DICA, ACA, and PCA. The OA can be evaluated through the orbit. The basilar and vertebral arteries can be evaluated from the occipital approach. The PSV, EDV, TAMM velocity, and RI of each of these vessels should be measured at least twice to obtain the most accurate measurement. The MCA should be tracked from the peripheral branch to the bifurcation with velocities obtained every 2 mm. Optimizing the tracing is crucial in identifying regions of stenosis. Color Doppler flow imaging (TCDI) can also be used to screen SCD patients. This technique is faster to learn, allows for quicker vessel identification, and improves gate placement. Because image-based probes tend to be larger than nonimaging probes, however, optimizing the tracing may be more difficult and can result in slightly lower velocities than those obtained by nonimaging methods.
The Stroke Prevention Trial in Sickle Cell Anemia (STOP) study led by Robert Adams showed that regular transfusion of children at risk for stroke (based on Doppler sonography) could prevent CVA. Adams studied children age 2 to 16 years, from 14 medical centers, who had MCA mean velocities higher than 200 cm/sec and were at risk of developing stroke. Half received blood transfusions every 3 to 4 weeks, enough to lower sickle cell hemoglobin to below 30%. After 1 year, the transfusions lowered the stroke risk by 90%. Although only one transfusion patient had a stroke (1.6%), 11 (16%) nontransfusion children sustained a CVA ( Table 47.2 ).
| Category | Definition |
|---|---|
| Low | <170 cm/sec TAMM velocity |
| Conditional | 170-199 cm/sec TAMM velocity |
| Abnormal | ≥200 cm/sec TAMM velocity |
| or >250 cm/sec PSV |
After early termination of the study, patients were offered to start or continue transfusion treatment, and an extended evaluation was performed confirming the efficacy of TCD in prediction of stroke risk (even during transfusion treatment) and the benefit of transfusion treatment reducing this risk. This observation period also suggested that the risk of stroke decreases with age progression, even in the absence of transfusion treatment. Reviewing the STOP data, Jones et al. reported that a PSV greater than 250 cm/sec may be useful for assessing stroke risk in SCD patients.
The SIT trial, a multicenter clinical study aimed at evaluating the usefulness of transfusion therapy in the prevention of recurrence of silent clinical infarcts demonstrated that children with silent clinical infarcts and normal TCD who undergo transfusion therapy have a 58% relative risk reduction of infarct recurrence. The results were positive although not as significant as those seen with STOP (92% relative risk reduction).
Verlhac et al. describe the use of evaluation of the extracranial ICA in patients with SCD and suggest that tortuosities of the ICA increase the risk of developing stenosis. They also report that increased extracranial ICA velocities (>160 cm/sec), even in the absence of abnormal intracranial velocities, should be a considered risk factor for cerebrovascular complications.
TCD is recommended as a screening test for all 2- through 16-year-old children with SCD. Optimal timing and frequency vary depending on the presence of velocities used as thresholds (TAMM velocity range cutoff of 155-170 cm/sec) and age. It has been suggested that SCD patients with a normal TCD can be followed every 12 months, but closer follow-up should be performed in patients with results at or near the threshold (“conditional” velocities, at 3-6 months). Patients with a TAMM velocity of greater than 200 cm/sec or a PSV greater than 250 cm/sec in the MCA on two examinations are at high risk of stroke and are recommended for transfusion therapy.
All patients with abnormal TCD have vasculopathy, but some patients with vasculopathy and silent stroke can have normal TCD examinations. These patients have more than two times the risk of developing a stroke compared to the unscreened population. In a study comparing the use of PSV versus TAMM velocity in predicting MRI/MRA abnormalities performed by Naffaa et al., PSV had a higher sensitivity (73% vs. 41%) and TAMM velocity had a higher specificity (100% vs. 81%). They suggest using the PSV as the measurement of choice for TCD screening and lowering the TAMM velocity threshold to 165 cm/sec to obtain a higher sensitivity in the prediction of vasculopathy apparent on MRI (92%). Correlation with MRI and MRA findings are useful because an association of abnormal TCD and MRI findings increases the risk for developing a new silent infarct or stroke when compared to those whose initial MRI is normal. In the STOP trial, the rate of stroke was higher for patients with presence of silent cerebral ischemia and abnormal TCD (52%) compared to patients with abnormal TCD but no evidence of silent ischemia on MRI (21%).
Low mean velocities may also be a marker for a high risk of stroke in the SCD population if the stenosis is so tight that minimal flow courses through the vessel ( Fig. 47.13 ). Buchanan et al. described cerebral insults in five patients with low TCD velocities (<70 cm/sec) and suggest these abnormal results also require further evaluation with MRI/MRA and closer follow-up examinations.
Elevated ACA velocity, although not part of the STOP criteria, also suggests an increased stroke risk ( Fig. 47.14 ). Kwiatkowski et al. found that an elevated ACA TAMM velocity higher than 170 cm/sec was associated with an increased risk of stroke. In subjects with normal ICA-MCA velocities and an elevated ACA velocity, the risk of stroke was greater than 10-fold, whereas the risk more than doubled with those who had both elevated ACA and elevated ICA-MCA velocities.
Although the STOP recommendations are for yearly exams from age 2 to 16, patients younger than 2 years of age can also benefit from TCD evaluation. The BABY HUG study demonstrated that infants with lower hemoglobin levels at baseline have higher TCD velocities and a higher incidence of clinical events. Hydroxyurea treatment has been shown to reduce both the rise in TCD velocities and the presence of clinical events. Further evaluation of the BABY HUG trial data may help determine whether early TCD evaluation can identify infants at risk of having a stroke during childhood.
When assigning the risk of stroke based on TCD velocities, it is important to consider how velocities may differ with the overall health of the patient, patient ethnicity, and the specific equipment used compared to those obtained with nonduplex equipment in the initial STOP studies. When ill, comorbidities including hypoxia, fever, hypoglycemia, and worsening anemia can increase the cerebral blood flow velocities, making the results invalid if conditional or abnormal. In these cases, repeating the study when the patient is no longer ill will help verify the results. Because different ethnicities and haplotypes of the disease have different average velocities, risk of stroke, and prognosis, it is possible that different criteria have to be determined to assess risk for a specific ethnicity.
Regarding the equipment used, studies have evaluated the differences in nonimaging TCD versus imaging TCD techniques. In studies performed in the early 2000s, nonangle-corrected velocities obtained with Acuson and ATL TCDI equipment were approximately 10% lower in the MCA than those obtained with Nicolet (Vascular, Madison, WI) nonimaging equipment. Later studies showed no significant difference in TAMM velocity measurements obtained with General Electric TCDI equipment compared with Nicolet nonimaging equipment. The reasons for these differing results are likely multifactorial. Early imaging probes were bulkier, making it more difficult to optimize Doppler tracings. Padayachee et al. discussed how the TAMM velocity is quantified using the Doppler equation. Optimizing the waveform without angle correction can result in similar results compared to the nonimaging TCD technique negating the need for decreasing the threshold for TCDI cutoffs. A 10% lower cutoff point for TAMM velocities has been suggested depending on the protocol and machine used. Besides appropriate curser placement, instrument settings (volume size, gain, waveform display) should be optimized. Close attention to technique can reduce the differences between velocity data acquired with different ultrasound machines. Centers should be aware of these potential differences and perform their own comparison studies when using various imaging equipment before changing velocity thresholds for TCDI.
Although angle correction with TCDI may be a way to correct for lower velocities obtained by TCDI compared with nonimaging TCD, this technique has not yet been validated and may overestimate stroke risk in children with SCD. Therefore angle correction currently is not recommended when performing and interpreting TCDI for stroke risk assessment in pediatric SCD.
The natural history of TCD velocities in sickle cell patients continues to be studied. The STOP 2 trail was performed to assess when discontinuation of transfusion therapy was appropriate. After 30 months of transfusion treatment and normalization of TCD velocities, patients were randomized to continue or suspend transfusion therapy. STOP 2 demonstrated that patients in whom transfusion therapy was suspended after normalization of TCD have a return to abnormal TCD velocities with a higher occurrence of stroke, and are more likely to develop silent clinical infarcts, indicating the need to continue transfusion treatment.
Optimal management for children with abnormal velocities remains problematic, with no consensus regarding long-term therapy in children with persistently abnormal velocities. The side effects of prolonged transfusion therapy have clinicians searching for other methods of improving stroke risk, including the use of hydroxycarbamide (hydroxyurea) therapy and bone marrow transplant. Hydroxyurea with phlebotomy proved to be useful in the prevention of stroke recurrence during the SWiTCH protocol, but not as good as transfusion therapy with chelation. Other studies (TWiTCH trial) are evaluating the use of hydroxyurea in the prevention of primary stroke in patients with abnormal TCD velocities.
The management of children with conditional velocities is also being studied. Many remain in the conditional range for years or eventually normalize, but up to 23% of this cohort convert over time to abnormal velocities ( Fig. 47.15 ). Protocols using less invasive therapies such as hydroxycarbamide (e.g., hydroxyurea therapy) in children with conditional velocities show promise and suggest that following these patients with TCD may be useful to assess treatment response.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here