Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
I thank Omer Berenfeld, David Filgueiras-Rama, Raphael Martins, Yoshio Takemoto, Rafael Ramirez, Jiang Jiang, and all other members of the Center for Arrhythmia Research of the University of Michigan who participated in the studies that are the focus of this chapter. The successful accomplishment of all the studies would have been impossible without their commitment to the projects on AF progression in the sheep model of tachypacing-induced remodeling. I also thank the National Heart Lung and Blood Institute, the American Heart Association, and the Leducq Foundation for many years of generous support.
Atrial fibrillation (AF) is the most common arrhythmia seen by clinicians in their practice and the most common cause of embolic stroke. Estimates suggest that AF affects more than 40 million people worldwide and that one in three people will develop AF throughout life, increasing the risk for stroke or thromboembolism. , The risk for AF is high in patients with comorbidities such as diabetes or high blood pressure, in addition to excessive consumption of alcohol or other toxic substances. , Nevertheless, despite more than a century of research and speculation, we still do not understand the fundamental mechanisms of AF and have not learned how to treat it effectively.
AF is a progressive disease reflecting gradual molecular changes that, in turn, may lead to an unrelentless electrophysiologic and/or structural remodeling in both atria, making the arrhythmia become more stable and long-lasting. Many conditions, including heart failure (HF), hypertension, and atrial amyloidosis, as well as a history of myocardial infarction (MI), diabetes mellitus, and obesity, predispose myopathy to AF progression. In addition, AF itself somehow leads to electrical remodeling and fibrosis of the atria, but the mechanism(s) remain incompletely understood. Atrial electrical remodeling and changes in subcellular atrial myocyte function in the short term lead to an abbreviation of the atrial action potential duration (APD) and refractory period, which helps promote the stabilization of reentry and AF. Whether and how these changes contribute to human AF perpetuation in the long term, however, has not been fully determined. In addition, the precise mechanisms governing the progression from paroxysmal to persistent and permanent AF are poorly understood, and prevention and treatment remain suboptimal. ,
Oxidative stress, atrial dilation, calcium overload, inflammation, and myofibroblast activation are all thought to be involved in AF-induced atrial remodeling, but it is unknown to what extent and at which points in time such alterations influence the remodeling process that perpetuates AF. This chapter discusses what we have learned to date about mechanisms of AF progression based on research in animal models and humans. The discussion circles around recent advances in our understanding of cellular and molecular mechanisms of atrial remodeling and AF progression based on data obtained in an animal model of tachypacing-induced, long-term, persistent AF. , This chapter also examines results from experiments in the same model aimed at testing the effectiveness of antifibrotic drugs used as upstream therapy toward preventing AF perpetuation. Finally, a study conducted recently in thousands of recordings from implantable devices in patients with demonstrated AF progression is discussed. The results provided initial validation to the idea that the dominant frequency (DF) of atrial electrical activation represents a reliable and objective patient-specific electrophysiologic biomarker of AF progression that could lead to the stratification of AF patients for treatment based on the degree of atrial remodeling.
AF progression frequently starts as paroxysmal AF with spontaneous termination often occurring within 48 hours. , Some patients suffer paroxysmal AF episodes indefinitely, but a significant number (around 40%) progress to persistent AF within 10 years follow-up. AF-mediated remodeling involves changes at the structural, ionic, and mechanical levels that favor the initiation, maintenance, and perpetuation of the arrhythmia. , Electrical remodeling, manifested by shortening of the atrial APD and refractoriness and loss of APD adaptation to changes in frequency, is known to develop within the first few days of AF , and to contribute to progressive prolongation of the duration of AF episodes (the “AF begets AF” phenomenon). Several ion channel modifications underlying such electrical changes have been described in animal models and humans. Nevertheless, the sequence and the way such changes integrate to perpetuate AF has not been elucidated, particularly in humans. Structural remodeling at the cell and tissue levels also contributes to intra-atrial conduction disturbances and increases susceptibility for AF, yet its role in progression from paroxysmal to persistent AF remains unknown. Finally, atrial mechanical function is significantly altered, , with reduced contractility and electromechanical dissociation.
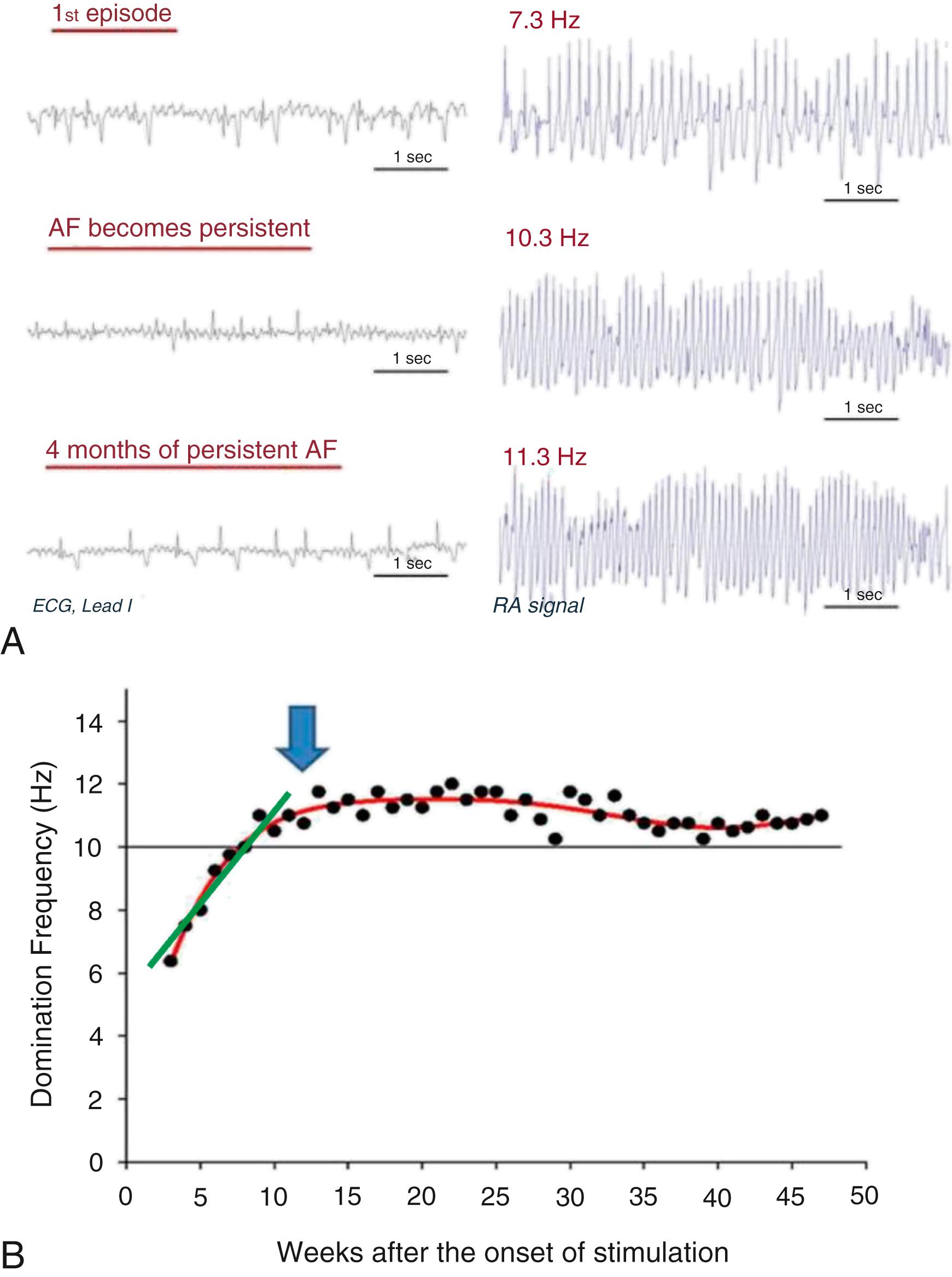
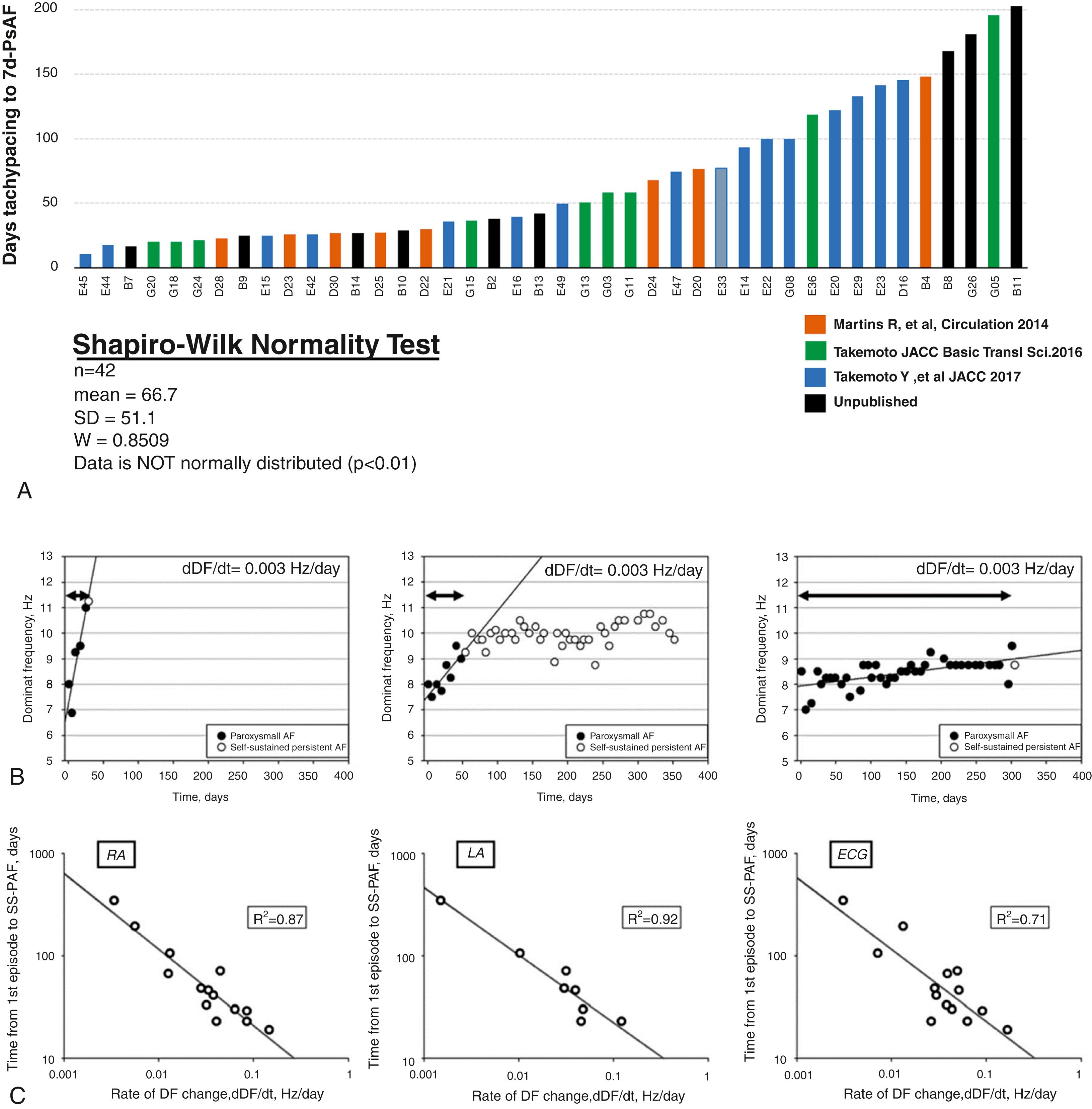
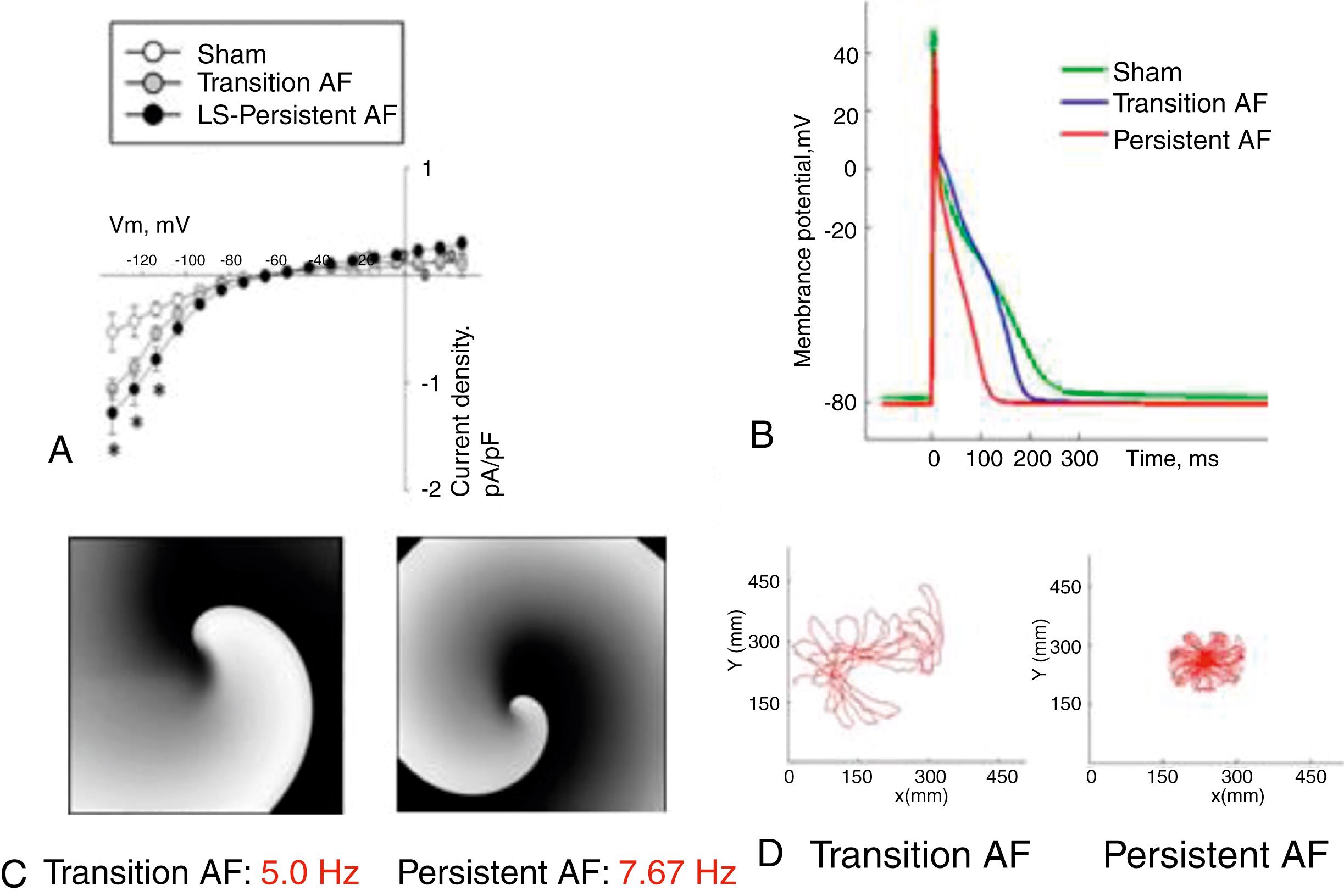
Recent studies using a clinically relevant ovine model have shown that during intermittent right atrial tachypacing, not only did the duration of AF episodes increase as expected, but the DF also increased gradually in both the left and right atria until it stabilized at a time coinciding with the onset of persistent AF ( Fig. 52.1 ). In brief, the model was established as follows: 6- to 8 - month-old sheep (weighing around 40 kg) were implanted with a subcutaneous pacemaker, with an atrial lead inserted transvenously into the right atrial appendage. In some sheep, an implantable loop recorder (ILR) was placed subcutaneously on the left side of the sternum. After 10 days of recovery, sheep were assigned to either sham-operated or atrial tachypacing groups. The pacemaker was programmed to induce AF by burst tachypacing (30-second pacing, 20 Hz, twice diastolic threshold) followed by 10-second sensing. Pacemakers resumed pacing only if AF stopped and sinus rhythm was detected. The pacemaker device was used to record intracardiac RA electrograms to accurately confirm the occurrence of AF, generate histograms, and follow the evolution of AF from the first episode of paroxysmal AF to eventual confirmed establishment of persistent AF (see Fig. 52.1 ), at which time the pacemaker was switched off. In accordance with clinical practice, persistent AF was defined as episodes lasting longer than 7 days without reversal to sinus rhythm. Three recordings were obtained in tachypaced sheep during follow up: (1) RA-lead electrogram, (2) ECG lead I, and (3) single-lead ILR (LA far-field signal). Such recordings enabled us to follow the progression of AF during weekly interrogations of the DF, obtained after fast Fourier transformation of the electrograms and the ECG. As illustrated in Fig. 52.1A–B , DF increased progressively during the paroxysmal-to-persistent AF transition and stabilized when AF became persistent. Nevertheless, the slope (dDF/dt) measuring the increase in dominant frequency with respect to time during transition ( green line in Fig. 52.1B ) was highly variable from animal to animal. Consequently, the number of days needed to maintain tachypacing to achieve 7 days of self-sustaining persistent AF was different for each animal ( Fig. 52.2A ). As illustrated in Fig. 52.2B , sheep that developed self-sustained persistent AF early had a high dDF/dt value, regardless of the initial DF during the first episode. Others progressed at intermediate rates, and still others had the slowest dDF/dt and protracted onset of persistent AF (see Fig. 52.2B ). Thereafter, once persistent AF was established in each animal, the DF remained stable at the highest value during the entire 12-month follow-up period. Thus we hypothesized that dDF/dt during the transition could predict when AF became persistent in each animal. Indeed, a strong nonlinear relationship was found between time to persistent AF onset and dDF/dt, which when plotted on a log-log scale (see Fig. 52.2C ), revealed that the faster the DF increase (i.e., the steeper the dDF/dt), the quicker the animal developed self-sustained persistent AF, regardless of whether DF was determined in the RA, LA, or the surface ECG (R2 = 0.87, 0.92, and 0.71, respectively; Fig. 52.2C ). Furthermore, noninvasive measurement of dDF/dt on the surface ECG lead I correlated strongly with RA and LA dDF/dt. Both the increases in the AF episode duration and the concomitant DF acceleration were associated with I CaL and I Na downregulation, and I K1 upregulation ( Fig. 52.3A ), along with corresponding APD abbreviation (see Fig. 52.3B ), changes in gene expression, and ion-channel protein subunits. Structural remodeling in the form of cellular hypertrophy, atrial dilation and interstitial fibrosis also developed in the course toward AF stabilization.
Consistent with the aforementioned findings, other studies have demonstrated numerous transcriptional changes in ion channel expression, including upregulation of KCNJ2 and KCNJ4 (encoding K ir 2.1 and K ir 2.3 subunits, respectively, which contribute to I K1 ) and downregulation of CACNA1C (encoding the I CaL α-subunit), CACNAB2 (a I CaL β-subunit), , and CACNAC1D, which is atrial specific and its absence in mice leads to impaired calcium homeostasis and increased AF susceptibility. Finally, chronic AF decreases the transient outward current and the ultrarapid component of the delayed rectifier current differentially on each atria and increases the slow component of the delayed rectifier current in both. Nevertheless, the time course of the aforementioned changes, and whether they occur with equal step as dDF/dts during the transition, has not been established.
The foregoing results with the sheep model of persistent AF suggested that, unlike the tachypacing-induced electrical remodeling that can occur over minutes or hours, there existed a protracted, slowly progressing electrical remodeling that occurred secondary to an AF that sustained for days or weeks. , Further, a consistent left-versus-right atrial DF difference in most animals correlated with the presence of faster and more stable rotors (see Fig. 52.3C–D ), DF gradients, and outward propagation from the posterior left atrium during sustained AF in both computer simulations and the explanted Langendorff-perfused sheep hearts. , ,
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here