Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Wilson’s disease is an autosomal recessive disorder due to mutations in the gene ATP7B for copper-transporting ATPase located in the trans -Golgi network of the liver. It is uncommon but important and treatable. Normal hepatic copper transport is disrupted owing to various ATP7B mutations, leading to the accumulation of copper in hepatocytes and liver disease. The large number and diverse mutations identified currently preclude simple genetic testing, in contrast to hereditary haemochromatosis (discussed later). Liver biopsy is important for histological diagnosis and monitoring.
Chemical quantitation of copper concentration in the biopsy sample helps to establish the diagnosis and is sometimes used for determination of the genetic status of a patient’s siblings. Copper determination can be made from specimens obtained by routine liver biopsy or retrieved from paraffin blocks, without special copper-free solutions or instruments. Homozygous individuals have increased liver copper levels from an early age but do not develop symptoms of liver disease in the first few years of life. Increased liver copper levels precede the development of histological abnormalities. Hepatic copper levels are typically greater than 4 μmol/g dry weight (>250 μg/g dry weight).
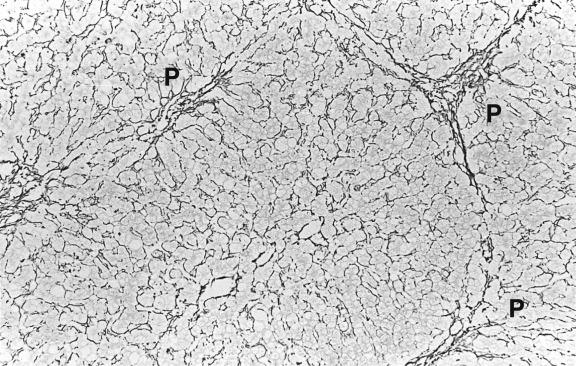
Histological lesions develop before the disease is clinically apparent. In the early, precirrhotic phase there is fatty change, sometimes with the formation of fat granulomas. Slender fibrous septa extend from portal tracts ( Fig. 14.1 ). There may be unusually abundant lipofuscin pigment in hepatocytes and glycogen vacuolation of hepatocyte nuclei, but neither feature is easy to evaluate; both are found in normal individuals, and nuclear vacuolation is particularly common in the young. Lipofuscin granules may be larger and less regular in outline than normal, possibly due to increased numbers of autophagic vacuoles which develop as protection against copper cytotoxicity. Inflammation is absent or mild in the early stages. Kupffer cells are sometimes enlarged and may stain for iron as a result of haemolysis. Electron microscopy helps in the diagnosis of both early and late disease because of characteristic changes in mitochondria and lysosomes ( Ch. 17 ).
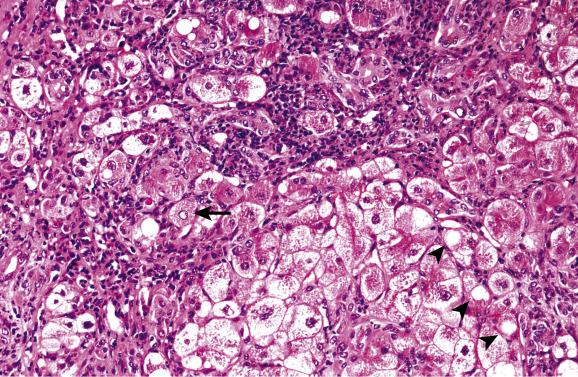
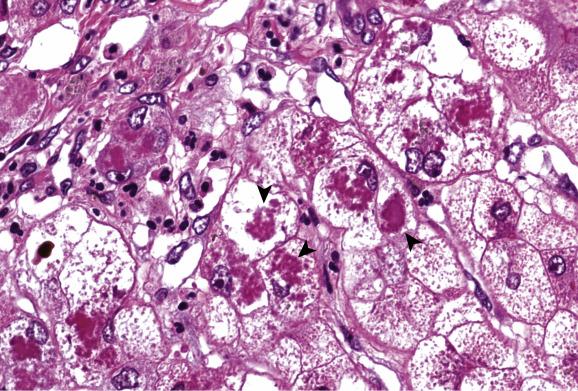
In some patients a phase of chronic hepatitis develops next that is difficult to distinguish histologically from chronic viral hepatitis. Stains for copper and copper-associated protein may be helpful, as will be discussed later. Cirrhosis develops in untreated patients, with or without a recognisable preceding phase of chronic hepatitis. A common though not invariable pattern is of active cirrhosis with fatty change, ballooned hepatocytes, focally dense eosinophilic cytoplasm and glycogen vacuolation of nuclei ( Fig. 14.2 ). Cholestasis may be present. Hepatocytes often contain Mallory–Denk bodies and these are sometimes very abundant. They are associated with an infiltrate rich in neutrophils, as in steatohepatitis ( Fig. 14.3 ). Partial fibrous occlusion of efferent veins has been reported. Hepatocellular carcinoma is a rare sequel of cirrhosis in Wilson’s disease.
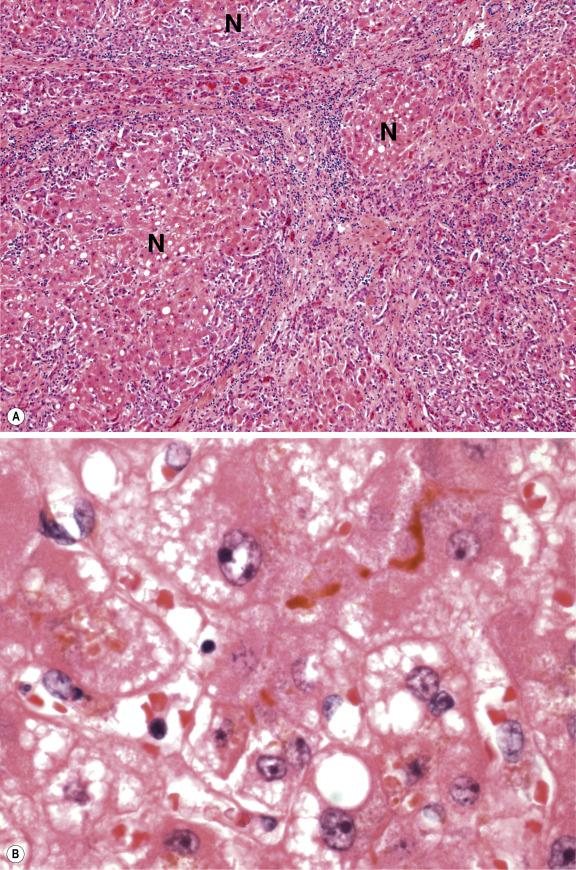
Fulminant hepatic failure may be the first manifestation of Wilson’s disease and is a major indication for liver transplantation. The presence of haemolysis in a young individual with acute liver failure should therefore prompt consideration of Wilson’s disease. Cirrhosis is usually already present in such cases, in contradistinction to acute liver failure, owing to viral or drug hepatitis where recent massive necrosis is evident. The cirrhotic nodules are frequently small and separated by septa containing abundant ductular structures and variable chronic inflammatory cells ( Fig. 14.4 ). The death of hepatocytes in the fulminant disease occurs by both apoptosis and necrosis, resulting in new zones of confluent necrosis superimposed on the underlying cirrhotic architecture. Cholestasis is often striking, and hepatocytes may contain large- or small-droplet fat. The presence of much stainable copper and/or copper-associated protein in hepatocytes and Kupffer cells distinguishes Wilson’s disease from other causes of fulminant hepatic failure.
Staining for copper and copper-associated protein plays a part in the diagnosis of Wilson’s disease, though staining results (as well as the copper concentration) can vary considerably throughout the liver. Failure to stain in either case is common at some stages of the disease and does not therefore exclude the diagnosis. Conversely, both copper and copper-associated protein are found in other liver diseases, usually as a result of failure to secrete copper into the bile. Thus, in a child with liver disease, strong staining for copper might reflect loss of bile ducts rather than Wilson’s disease. Other copper storage disorders have been described, including Indian childhood cirrhosis ( Ch. 13 and Fig. 13.27 ), which is also occasionally seen elsewhere in the world. Furthermore, neonatal liver is normally rich in copper.
Rarely, biopsies with substantial iron overload and haemosiderin deposits may also show positive copper staining, even in the absence of significant quantitative copper overload. This has been attributed to co-localization in lysosomes of several copper-containing proteins (cuproproteins) such as multicopper oxidases and copper/zinc superoxide dismutase that are involved in control of the redox state.
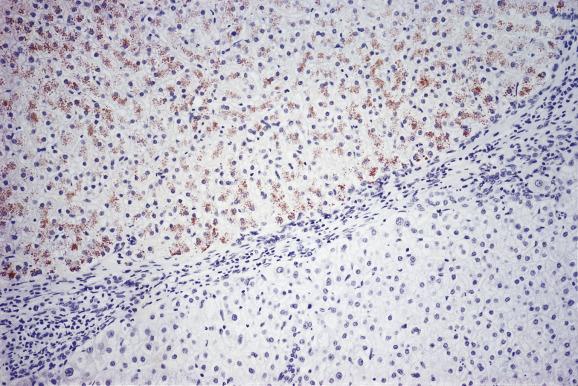
In the early phases of Wilson’s disease, liver copper levels are high, but the copper is difficult to demonstrate histochemically. This is because it is diffusely distributed in hepatocytes and not concentrated in lysosomes. Sensitive histochemical methods (e.g. Timm’s silver method or rhodanine) may show faint cytoplasmic staining. Later in the course of the disease copper begins to accumulate in hepatocyte lysosomes and is then more easily stained. Once cirrhosis has developed, the distribution of copper is typically uneven, some nodules staining strongly while others are negative ( Fig. 14.5 ). Staining for copper and copper–protein may be dissociated, although in most cases both are positive. Timm’s silver stain appears to be the most sensitive staining method for demonstrating copper in this disease.
Because of the great variety of histological lesions in the liver, Wilson’s disease can easily be mistaken for other liver disorders. Clinicians and pathologists should consider Wilson’s disease in the differential diagnosis of hepatocellular disease, especially in the young, but also at all ages, including (uncommonly) older-aged individuals. The disease can be arrested by treatment and its development prevented in siblings. The penalties for missing the diagnosis are therefore very great.
Siderosis (or haemosiderosis) means the presence of demonstrable iron in tissues, irrespective of cause. The main forms of iron in hepatocytes are ferritin, haemosiderin and haem. Stainable iron is mainly haemosiderin, which is principally located in lysosomes and is seen as granules concentrated towards the biliary poles of the cells. Ferritin gives rise to more diffuse staining, imparting a bluish hue to the liver-cell cytoplasm on iron staining. Hepatocellular siderosis almost always shows a diminishing gradient of intensity from the periphery of lobules towards the central (efferent) veins. It is most severe in periportal regions (acinar zones 1) near small portal tracts, and least severe in centrilobular regions (acinar zones 3). The normal adult liver is usually negative on iron staining or at best shows minimal siderosis. This is also true of the neonatal liver, although some cases may show mild periportal liver-cell siderosis (residual iron storage from the active period of hepatic haemopoiesis of the third trimester).
Because iron stains of liver tissue are expected to be negative in most instances, a positive stain requires explanation. In this regard, two major categories of hepatic iron storage disease need to be considered, designated as primary and secondary iron overload disorders ( Box 14.1 ). The primary disorders are predominantly forms of hereditary haemochromatosis in which genetic mutations alter iron homeostasis in the gastrointestinal tract and liver. The secondary disorders are acquired conditions in which increased iron in the liver is due to exogenous sources of iron, abnormal erythrocyte destruction or changes in iron absorption and distribution related to underlying liver disease. The pathologist may be able to suggest the reason for the siderosis, based on the distribution of the stainable iron. For example, in most of the primary iron overload disorders, such as classic HFE -related haemochromatosis, the excess iron is mainly hepatocellular. In thalassaemia both hepatocytes and macrophages are positive, while exogenous iron overload leads to Kupffer-cell storage in the first instance. Various types of underlying liver disease are also associated with siderosis. Cirrhotic livers of varied aetiology may contain much iron, even within macroregenerative nodules. In viral hepatitis and alcoholic liver disease small amounts of stainable iron are often found. Siderosis in the setting of non-alcoholic fatty liver disease ( dysmetabolic iron overload syndrome ) is increasingly recognised. Dense, iron-positive granules are common in endothelial cells in a variety of conditions, including acute hepatitis, chronic hepatitis B and C and alcoholic liver disease, but their significance is not known. As mentioned earlier in the chapter, siderotic livers rarely may also stain positively with rhodanine or other copper stain due to physiologic storage cuproproteins.
Type and standard name of hereditary haemochromatosis (HH)
( Gene mutated --protein product affected)
Type 1A ∗
∗ Most common type of hereditary haemochromatosis
Classical HFE -associated HH
( HFE --HFE : C282Y/C282Y homozygous)
Type 1B HFE compound heterozygote
( HFE --HFE : C282Y/H63D)
Type 1C HFE S65C heterozygote
( HFE --HFE: S65C)
Type 2A Juvenile HH
( HJV —hemojuvelin)
Type 2B Juvenile HH
( HAMP —hepcidin)
Type 3 Transferrin receptor 2 HH
( TFR2 --transferrin receptor 2)
Type 4A Ferroportin disease
( FPN [ SLC40A1 ∗∗
∗∗ The ferroportin gene FPN1 is also known as SLC40A1
]— ferroportin : loss of function)
Type 4B Non-classical FPN disease
( FPN [ SLC40A1 ]—ferroportin: gain of function)
Aceruloplasminemia
Others
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here