Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Appropriate thyroid hormone function is essential for normal neurodevelopment in infancy and childhood. Hypothyroidism in the first year of life can result in significant deleterious effects on growth and neurologic injury.
Delays in treating congenital hypothyroidism (CH) is the most common preventable cause of intellectual disability.
Neonatal screening can provide early diagnosis and can prevent delays in treatment. Newborn screening methods differ and may miss rare forms of congenital hypothyroidism.
In preterm newborns, thyroid hormone levels may fall because of the immaturity of the thyroid gland, but these changes may be exacerbated by complications of prematurity.
Thyroid metabolism can be affected by exogenous sources of iodine, dopamine infusions, blood transfusion, and glucocorticoid treatment.
The clinical manifestations of Graves disease in the newborn include irritability, flushing, diarrhea, vomiting, tachycardia, hypertension, poor weight gain, thyroid enlargement, and exophthalmos.
The hypothalamic-pituitary-thyroid (HPT) axis functions as a typical feedback loop ( Fig. 86.1 ). Triiodothyronine (T 3 ) levels in the pituitary gland direct the secretion of thyroid-stimulating hormone (TSH). Also, an inverse relationship exists between thyroid hormone formation and iodide level in the thyroid. Subsequently, the rate of hormone production is not affected by rapidly changing levels of iodide, and the reservoir of thyroid hormone balances against quick changes in hormone synthesis.
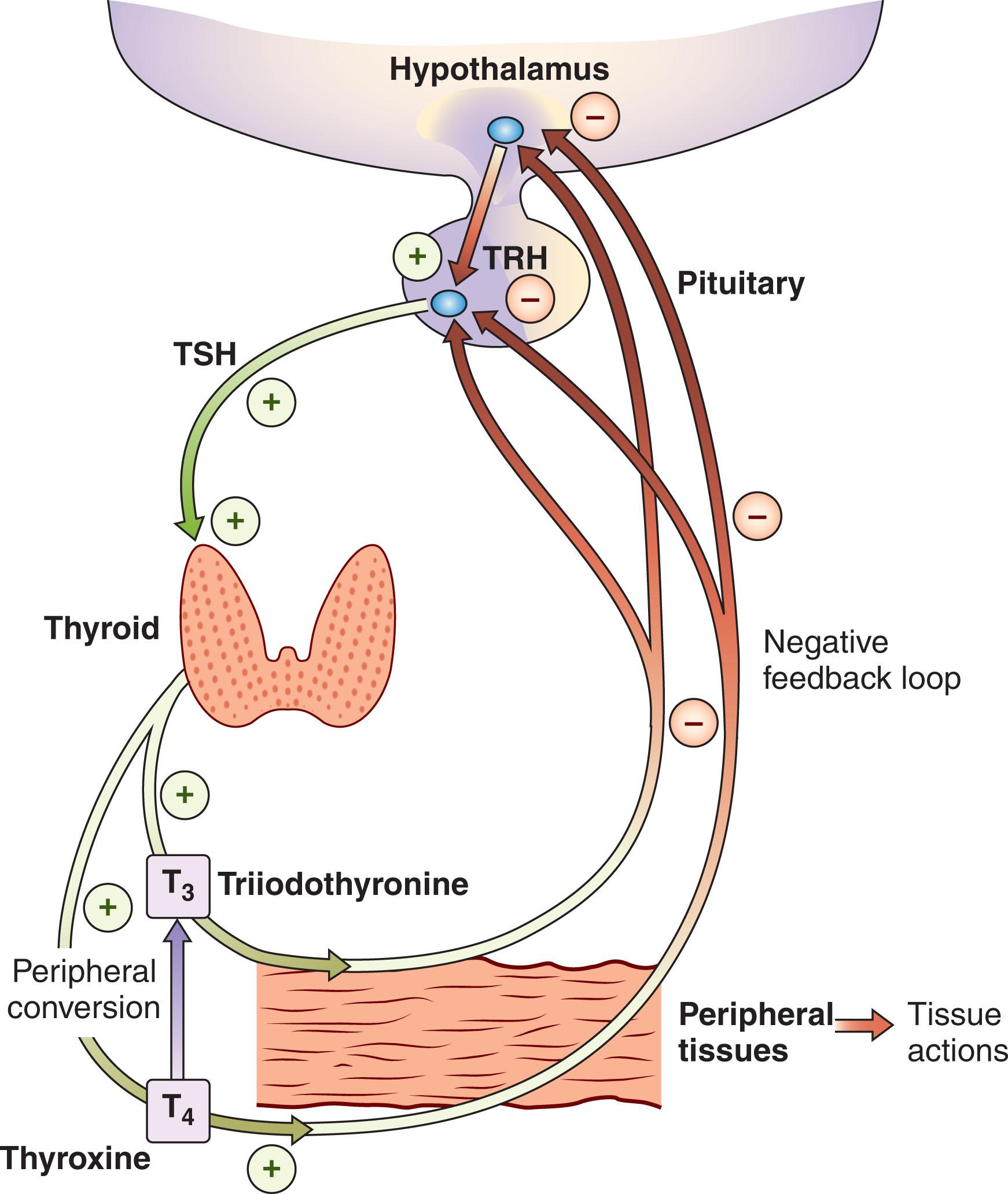
TSH, produced in the anterior pituitary, is the chief player in thyroid gland function and morphology. TSH provides negative feedback by decreasing the synthesis of thyrotropin-releasing hormone (TRH) from the hypothalamus, as well as blocking the action of TRH that stimulates TSH release. Release of TSH fuels uptake of iodide by the thyroid gland and accelerates many steps in thyroid hormone synthesis. It also drives the growth and vascularization of the thyroid gland itself. Both T 3 and thyroxine (T 4 ) provide negative feedback to TSH secretion, while TRH determines the setpoint. Given the logarithmic relationship between free T 4 (fT 4 ) and TSH, TSH levels are a sensitive indicator of thyroid hormone status.
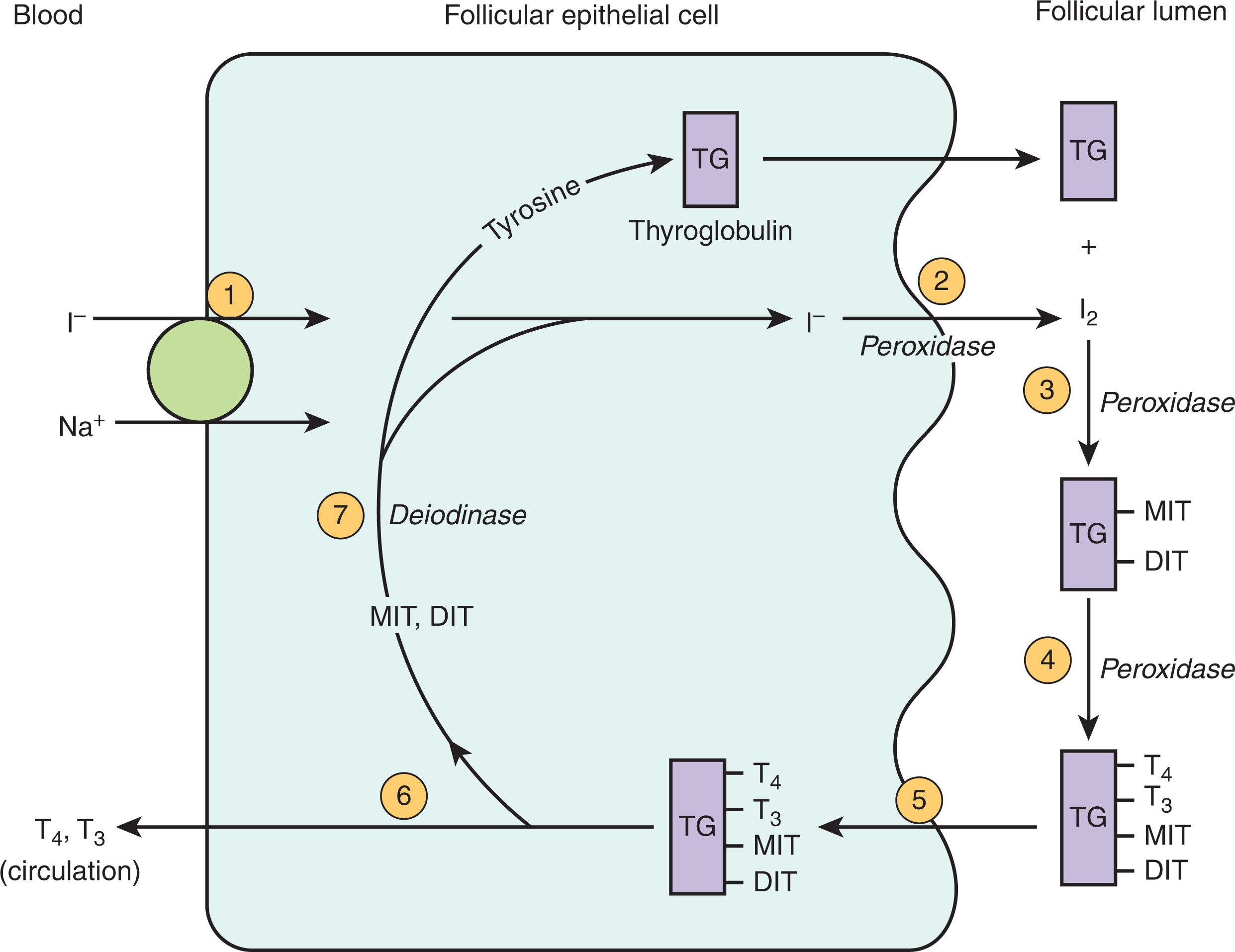
Synthesis of thyroid hormone is initiated by the entry of iodide molecules into the follicular cell of the thyroid gland via the sodium–iodide transporter; these are then transported into the colloid. Once they are in the thyroid gland, oxidization of iodide occurs, after which it is quickly associated with thyroglobulin (TG). Organification of the iodide forms tyrosine residues, which are then coupled to form the two active thyroid hormones: T 4 and T 3 . This complexed TG, which resides in the colloid of the thyroid gland, serves as the reservoir for the production of T 3 and T 4 . Thyroid hormones are then moved via endocytosis into the follicular cell. This vesicle fuses with lysosomes, after which T 4 and T 3 are disassociated from TG via hydrolysis and eventually released into the bloodstream. Residual monoiodothyronine (MIT) and diiodothyronine (DIT) are deiodinated and enter the synthesis cycle again along with iodide (I − ) and tyrosine. Important steps in thyroid hormone synthesis are summarized in Fig. 86.2 .
In the circulation, T 3 and T 4 are transported by attachment to binding proteins that include T 4 -binding globulin (TBG), transthyretin (TTR; formerly termed T 4 -binding prealbumin [TBPA]), and albumin. These binding transport proteins are produced in the liver. Production of these proteins increases through the latter half of gestation and is stimulated by estrogen. Free thyroid hormone levels appear to remain constant despite increasing total hormone concentrations because of an increase in binding protein production.
The serum concentration of T 4 is vastly greater than that of T 3 by a factor of 50 to 100. The primary transport protein (~75% of T 3 and T 4 ) is TBG. The remaining 25% of thyroid hormone is equally distributed between TBPA and albumin. The fT 4 concentration more precisely reflects the metabolic status in comparison with total T 4 or T 3 . This is because only an unbound hormone can enter cells to exert its action.
The thyroid gland is the first endocrine organ to develop in the fetus. The development of the thyroid gland and the HPT axis occurs in two phases. The first phase is characterized by the embryogenesis of the structures involved, and the second phase is characterized by the maturation of the HPT axis, which is described later. By about 3 weeks of gestation, the rudimentary beginnings of the hypothalamus have developed. Subsequently, anlagen from the floor of the forebrain and the Rathke pouch converge to form the pituitary gland. By 14 to 15 weeks of gestation, this embryologic structure has become the mature pituitary gland. Detection of TSH has been observed as early as 10 to 12 weeks of gestation.
A programmed sequence of transcription and homeobox factors (e.g., thyroid transcription factors-1 and -2 [TTF-1 or TiTF-1, now designated as NKX2 homeobox 1 -NKX2.1-; and TTF-2, now Forkhead box E1 -FOXE1-] and paired box gene 8 [PAX8]) directs thyroid embryogenesis. Thyroid gland development begins with convergence of three structures: the median anlage from the floor of the pharyngeal pouch (caudal end of the foregut) and two lateral anlagen from the fourth pharyngobranchial pouch. The median anlage is visible by day 22 after conception. The lateral anlagen develop into a pair of ultimobranchial bodies from which the calcitonin-producing parafollicular C cells arise. Between day 32 and day 48 after conception, this primordial thyroid structure breaks off from the pharyngeal pouch and completes its migration to the pretracheal region. Little is known about the molecular factors involved in this migration; however, FOXE-1 has been implicated in cases of thyroid ectopy.
By 4 weeks after thyroid descent is complete, functional and structural changes are mature, and the thyroid can produce thyroid hormone. Follicles fill with colloid. By 12 weeks of gestation, the thyroid gland is able to take up iodide, synthesize TBG, and produce hydrogen peroxide (H 2 O 2 ) at the apical membrane—all steps required for thyroid hormone production. T 4 has been detected in human fetal blood as early as 11 to 12 weeks of gestation. This accrual of the ability to produce thyroid hormone coincides with the maturation of the hypothalamus and pituitary gland with the production of TRH and TSH.
The placenta is of utmost importance in fetal thyroid physiology as it regulates the passage of maternal thyroid hormone to the fetus ( Fig. 86.3 ). Thyroid hormone is paramount for normal neurodevelopment. Maternal-to-fetal transfer of T 4 is essential, especially in the first trimester when the fetal thyroid axis has yet to mature. With the landmark study by Vulsma et al., it was shown that T 4 does cross the placenta, contradicting previous concepts—placental transfer of T 4 in athyreotic fetuses resulted in levels that were 25% to 50% of those of normal term newborns. There are mixed reports on the effect of hypothyroidism in the mother on the neonate. In case series of women with severe hypothyroidism diagnosed during pregnancy and treated to normalize levels during the third trimester, the offspring were noted to have normal intellectual outcomes. Additionally, similar developmental outcomes were seen in offspring of both T 4 -treated versus placebo-treated women with low T 4 levels. Multiple clinical studies have shown the deleterious effects of inadequate maternal thyroid hormone levels.
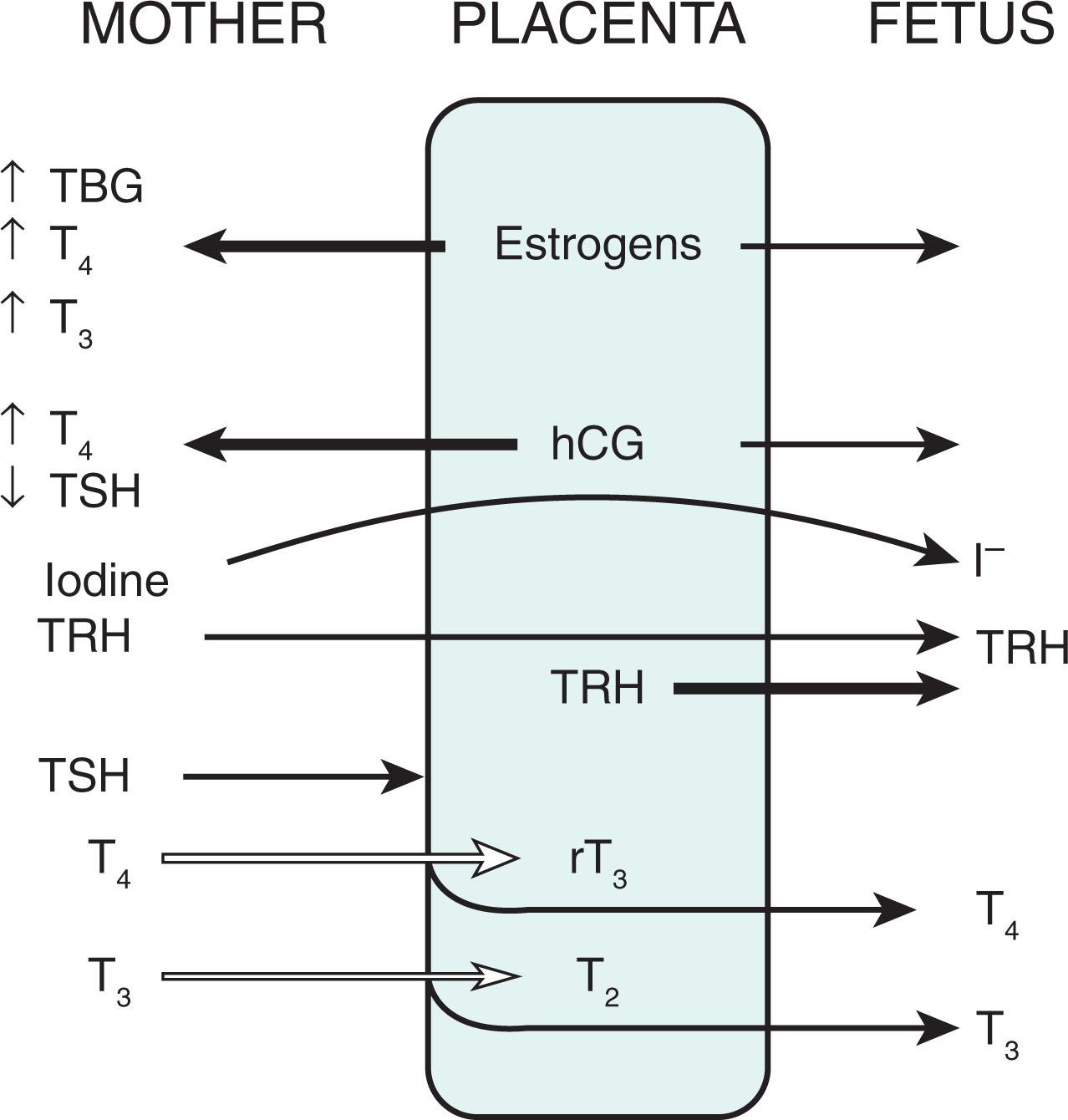
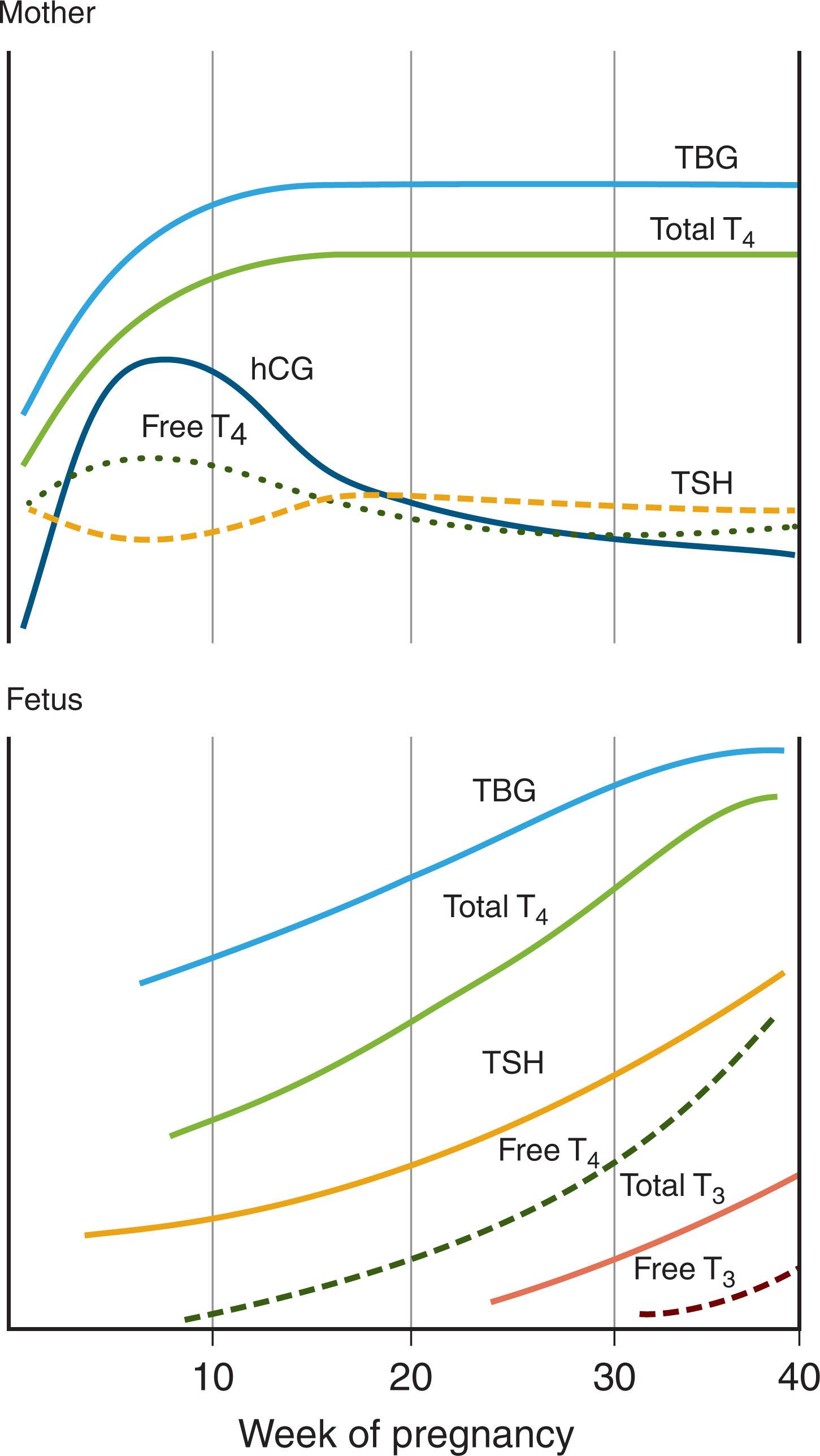
TSH does not cross the placenta; however, TRH does. During pregnancy, maternal thyroid axis changes result in increased fT 4 levels early and maintenance of higher levels until birth ( Fig. 86.4 ). This is a result of human chorionic gonadotropin produced by the placenta, which is homologous to TSH with some TSH-like activity.
Although most of the transfer of the maternal thyroid hormone is dependent on maternal thyroid hormone concentrations, plasma membrane thyroid hormone transporters and metabolism via deiodinases (DIs) also play a role. Thyroid hormone transporters are found in the apical and basolateral membrane of the placenta. Several transporters have been discovered in the placenta (monocarboxylate transporters, L-type amino acid transporters, and organic anion–transporting polypeptides). The placenta also has both type 2 and type 3 DI, which is involved in the metabolism of thyroid hormone in this milieu. The activity level of type 3 DI is 200 times higher than that of type 2 DI. T 3 is thought not to cross the placenta; however, this is not clear.
Iodide is taken up by the thyroid gland for thyroid hormone synthesis. There is free passage of iodides between the mother and the fetus. Large-quantity ingestion of iodine will decrease the synthesis of T 4 transiently. This decreased synthesis is due to decreased uptake via the sodium–iodide symporter. Typically, there is an escape from this phenomenon in about 48 hours. However, cases of goiter and hypothyroidism have been reported with excessive iodine intake. An adequate amount of iodine, around 250 μg/day, is still recommended for pregnant women according to the American Thyroid Association.
Miscellaneous other compounds can cross the placenta, affecting fetal thyroid function. TSH receptor-blocking antibodies (TRBAbs), also known as TSH Binding Inhibitory Immunoglobulins, have been implicated in cases of transient neonatal hypothyroidism. In mothers with a history of Graves disease, thyroid-stimulating immunoglobulin (TSI) can lead to thyrotoxicosis in the neonate. Antithyroid medications ingested by the mother can result in fetal goiter with or without hypothyroidism.
The fetal thyroid gland can synthesize and secrete thyroid hormone after 12 weeks of gestation. As the pregnancy continues, the levels of fT 4 and T 4 continue to rise in step with the gestational age of the fetus. T 3 levels, however, remain low until about 30 weeks of gestation, after which they increase slowly until birth. This delayed rise in T 3 levels is due to low rates of conversion of T 4 to T 3 by type 1 DI and high activity of type 3 DI metabolizing T 3 to diiodothyronine (DIT). With an increased amount of type 1 DI produced by the liver after 30 weeks, there is increased conversion of T 4 to T 3 in the liver and decreased metabolism of T 3 by placental type 3 DI, resulting in the steady rise until delivery. TSH has also been detected at 12 weeks of gestation, with subsequent increases in its level in parallel with increases in fT 4 level. The level of reverse T 3 (rT 3 ) is relatively high early in the third trimester because of placental type 3 DI activity converting T 4 to rT 3 and T 3 to DIT ( Fig. 86.5 ).
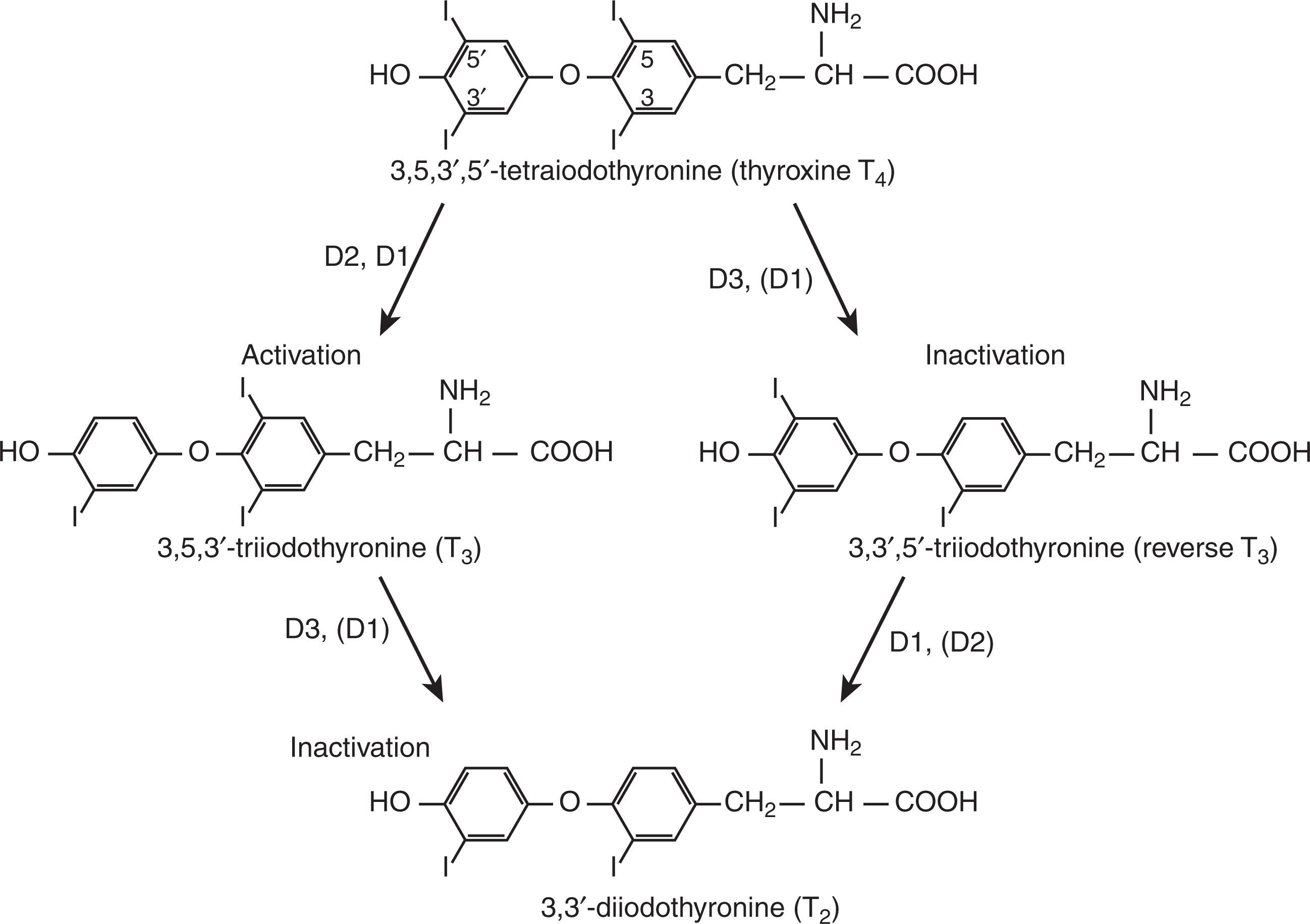
The thyroid gland is the body's only source of T 4 . Conversion of T 4 to T 3 peripherally contributes to most of the circulating T 3 . This conversion is accomplished by a group of three DIs, types 1, 2, and 3 (see Fig. 86.5 ). T 3 , with its greater affinity for the thyroid hormone receptor, is the active form of thyroid hormone. T 3 is derived from the deiodination of the outer ring of T 4 . Conversely, deiodination of T 4 at the inner ring produces the inactive rT 3 .
Type 2 and type 3 DIs are selective in their action, performing deiodination on only the outer ring and inner ring, respectively. Type 1 DI is nonselective, with deiodination at the outer ring converting T 4 to T 3 and deiodination at the inner ring producing rT 3 from T 4 . Type 1 DI is produced in the liver, kidney, and thyroid. Type 1 DI is inhibited by propylthiouracil and rT 3 and stimulated by thyroid hormone. Type 2 DI is primarily found in the brain, pituitary, placenta, skeletal muscle, heart, thyroid, and brown adipose tissue, and is not sensitive to propylthiouracil and is inhibited by thyroid hormone. It converts T 4 to T 3 . Type 3 DI is found primarily in fetal tissues, including the placenta, brain, and skin. Type 3 DI converts T 4 to rT 3 and T 3 to DIT.
During pregnancy, fetal DI levels vary with time and are reflective of thyroid hormone concentrations. Type 1 DI in hepatic tissues remains quiescent until late in gestation, resulting in a late rise of fetal T 3 levels. Type 3 DI activity is responsible for high rT 3 levels in the fetus. The interplay of these enzymes is critical to normal brain development. Given that both type 1 and type 2 DI are sensitive to thyroid hormone levels, a hypothyroid fetus will have increasing type 2 DI activity in the brain and decreasing type 1 hepatic activity, resulting in more T 4 in the brain, at which point it is converted to T 3 by type 2 DI.
At birth, fT 4 levels in the newborn are slightly below that of the maternal concentration, and the T 3 level is relatively low in umbilical cord blood. Also, at this time, partly in response to cold and stress, there is a TSH surge that peaks at about 2 to 4 hours of life and then returns to its initial value within about 48 hours ( Fig. 86.6 ). T 3 and T 4 levels also rise within a few hours of birth and reach their peak by 24 hours. TSH, as well as extrathyroidal conversion of T 3 to T 4 by type 1 and type 2 DIs, contributes to this surge of thyroid hormone. During the next 4 to 5 weeks of life, T 4 and T 3 levels progressively decrease to levels just above the normal range of children. By 1 month of age, the TSH level has declined to about 5 mU/L and declines further to a near-adult range of 0.5 to 4 mU/L by the second month.
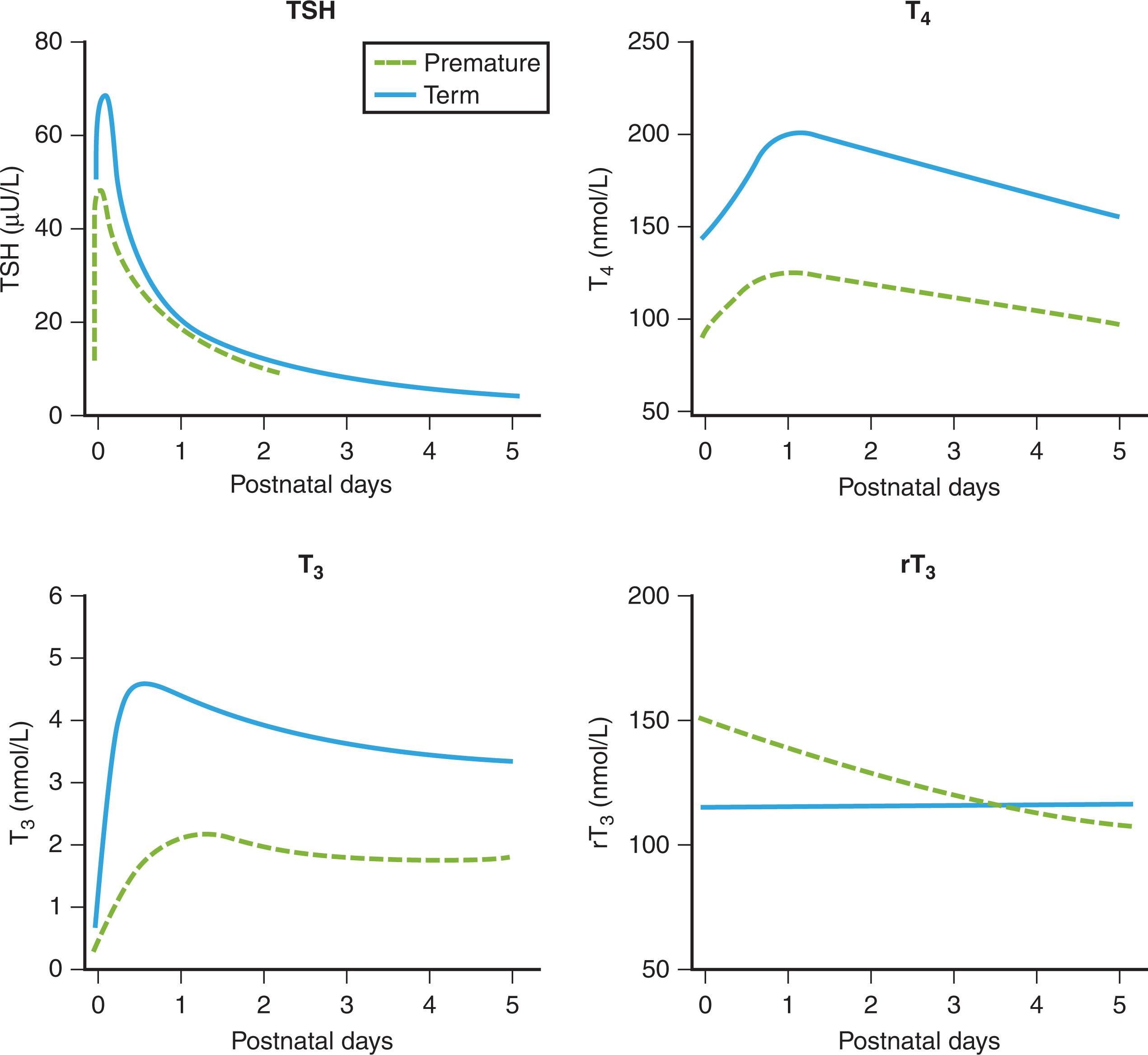
Infants born prematurely appear to have an attenuated TSH surge. This is due to an immature HPT axis. T 3 and T 4 levels also have a blunted rise in comparison with the levels in term newborns. The TSH level can peak at about 40 mU/L by 30 minutes. Many factors need to be taken into account when one is assessing thyroid function in preterm infants. As many preterm newborns have other complications, such as respiratory distress syndrome or nutritional problems, serum T 4 , and especially T 3 , concentration may fall to low levels as a result of the immaturity of the thyroid gland, as well as reduced TBG production. Illness also causes suppression of the hypothalamic-pituitary-thyroid axis, impairs the conversion of T 4 to T 3 , and increases type 3 DI activity.
Congenital hypothyroidism (CH) is defined as variable dysfunction of the hypothalamic-pituitary-thyroid axis present at birth, resulting in insufficient thyroid hormone production and thyroid hormone deficiency. CH is caused by abnormal development or function of the thyroid gland, pituitary, hypothalamus, or thyroid hormone function. The overall incidence is 1 in 2500 to 1 in 3000 newborns. Higher incidence rates have been reported in certain populations. The prevalence rates of CH can differ with sex, ethnicity, and birthweight. Twice as many female as male infants are affected. Compared with whites, the prevalence of CH is higher in Asians and Hispanics and lower in Blacks. A study noted an increased risk of CH in macrosomic (>4500 g) and low birthweight (<2000 g) infants. There is an increased risk in infants with Down syndrome. The reported incidence of central CH is 1 in 80,000 to 1 in 100,000.
In most cases, CH is permanent and results from an abnormality in thyroid gland development (dysgenesis or agenesis) or a defect in thyroid hormonogenesis. Less commonly, the altered thyroid function is transient, attributable to transplacental passage of maternal medication, maternal blocking antibodies, or iodine deficiency or excess. In rare cases, CH may result from a pituitary or hypothalamic abnormality. Potential causes are listed in Table 86.1 . Regardless of the duration of the thyroid dysfunction, immediate treatment with levothyroxine (LT 4 ) in the newborn period is needed to prevent cognitive or neurodevelopmental decline. The developing fetal brain is protected in utero by an adequate source of T 3 , supplied by local deiodination of maternal T 4 in the fetal brain.
| Disorder | Incidence |
|---|---|
| Thyroid Dysgenesis | 1 in 2500 to 1 in 4000 |
| Agenesis | |
| Hypogenesis | |
| Ectopia | |
| Thyroid Dyshormonogenesis | 1 in 30,000 |
| TSH receptor defect | |
| Iodide-trapping defect | |
| Thyroid peroxidase defect | |
| H 2 O 2 generation defect | |
| Defect in thyroglobulin | |
| Pendred syndrome | |
| Dehalogenase defect | |
| TSH signaling defect: Albright hereditary osteodystrophy | |
| Transient Hypothyroidism | 1 in 12,000 to 1 in 30,000 |
| Drug induced | |
| Maternal antibody induced | |
| Idiopathic | |
| Hypothalamic-Pituitary Hypothyroidism | 1 in 80,000 to 1 in 100,000 |
| Hypothalamic-pituitary anomaly | |
| Panhypopituitarism | |
| Isolated TSH deficiency |
The diagnosis of hypothyroidism should be considered in any infant with prolonged jaundice, transient hypothermia, an enlarged (greater than 5 mm) posterior fontanel, failure to feed properly, or respiratory distress with feeding. Approximately one-third of maternal T 4 crosses to the fetus at term. With a half-life of 6 days, the maternal T 4 will be metabolized and excreted by 3 to 4 weeks of age. The classic signs evolve during the first few weeks after birth. Fetal growth is normal; however, there is a delay in bone maturation and a rapid reduction in growth rate after birth with progressively worsening myxedema in subcutaneous tissues and tongue. The thickened tongue becomes protuberant, and increasing difficulty in nursing and handling salivary secretions is noted. The cry is hoarse because of myxedema of the vocal cords. Additional signs and symptoms include marked muscular hypotonia; constipation; thick, dry, cold skin; long, abundant, and coarse hair; abdominal distention; umbilical hernia; hyporeflexia; bradycardia; hypotension with narrow pulse pressure; anemia; and widely patent cranial sutures. Goiter can be present. The typical facies are characterized by a depressed nasal bridge, a relatively narrow forehead, and puffy eyelids. The cardiac silhouette may be enlarged, and the electrocardiogram shows low voltage and a prolonged conduction time. Some of the signs and symptoms are present by 6 to 12 weeks postnatally, especially lethargy, constipation, and umbilical hernia. The characteristic facies and growth retardation become increasingly obvious during the first several months of life.
Nonspecific symptoms and signs associated with hypothyroidism are listed in Table 86.2 . Because clinical manifestations of hypothyroidism may not appear until weeks after birth, even in athyreotic infants, newborn screening has enabled pediatricians to identify newborns with low thyroid hormone production and to initiate therapy within the first 2 weeks of life before the development of signs and symptoms.
| Age/Manifestation | Frequency (%) |
|---|---|
| 0–7 Days | |
| Prolonged jaundice >3 days | 73 |
| Birthweight >4 kg | 40 |
| Poor feeding | 40 |
| Transient hypothermia | 38 |
| Large posterior fontanel (>5 mm) | 32 |
| 1–4 Weeks | |
| Failure to gain weight | 45 |
| Constipation | 35 |
| Hypoactivity | 33 |
| 1–3 Months | |
| Failure to thrive | 90 |
| Umbilical hernia | 49 |
| Macroglossia | 43 |
| Myxedema | 40 |
| Hoarse cry | 30 |
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here