Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The extraocular muscles (EOMs) have many anatomic, physiologic, and molecular characteristics distinct from those of other striated muscles. These unique characteristics, which likely developed in response to the specialized demands placed on EOMs, including tonic position, maintaining contractures, conjugate smooth pursuit and saccades, and dysconjugate vergence movements, may account for the often predictable involvement or sparing of extraocular muscle in specific pediatric neuromuscular disorders. This selective involvement of the EOM can assist the clinician in formulating differential diagnoses for neuromuscular conditions.
Extraocular muscle dysfunction in neuromuscular disease typically presents with ptosis, ophthalmoplegia, or incomitant strabismus, or a combination of these symptoms. Ptosis, or blepharoptosis, is drooping of the upper eyelid as a result of dysfunction of the levator palpebrae superioris muscle. Ophthalmoplegia is the inability to move the globe into one or more fields of gaze, and may or may not be accompanied by strabismus. Strabismus is pathologic misalignment of the eyes, resulting in loss of binocular vision. With paralytic (incomitant) strabismus, the angle of deviation of the eyes varies with the direction of gaze. This is the form of strabismus typically associated with neuromuscular disease.
In this chapter, we review isolated ocular motor neuropathies, congenital ptosis, congenital cranial dysinnervation syndromes, and pediatric neuromuscular disorders associated with abnormalities of eye movement. Strabismus syndromes associated with pediatric neuromuscular conditions are summarized in Table 46.1 .
| Disorder | Ocular Misalignment | Other Visual Abnormalities |
|---|---|---|
| Ataxia-telangiectasia | Erratic vertical EOMs | Ocular motor apraxia |
| Nystagmus | ||
| Cockayne syndrome | Esotropia, exotropia | Nystagmus, pigmented retinal dystrophy, enophthalmos, cataracts, corneal opacities |
| Mitochondrial Disorders | ||
| MELAS | External ophthalmoplegia | Ptosis |
| Leigh disease | Horizontal gaze palsy, tonic downgaze deviation, external ophthalmoplegia | Internuclear ophthalmoplegia, dorsal midbrain (Parinaud’s) syndrome, ptosis, optic atrophy |
| Kearns-Sayre syndrome | External ophthalmoplegia | Pigmented retinopathy, ptosis |
| CPEO | External ophthalmoplegia | Ptosis |
| Nutritional/Metabolic | ||
| Abetalipoproteinemia with vitamin E deficiency | Convergence insufficiency, upgaze limitation | Internuclear ophthalmoplegia, pigmented retinopathy |
| Other Disorders | ||
| CIDP | CN III palsy | |
| Joubert syndrome | Congenital fibrosis syndromes, skew deviation, horizontal tonic gaze deviation, supranuclear EOM deficits | Torsional/pendular nystagmus, ocular motor apraxia, retinal dystrophy, ptosis, colobomata |
Four recti and two oblique muscles move the globe, and the levator palpebrae superioris raises the eyelid. These muscles are innervated by the oculomotor (CN III), trochlear (CN IV), and abducens (CN VI) cranial nerves. Axons of the paired oculomotor nuclei (nIII), each composed of contiguous midbrain subnuclei, form two branches. The inferior branch projects uncrossed axons to the medial rectus, inferior rectus, inferior oblique, and pupillary constrictor muscles. The superior branch projects crossed axons to the superior rectus and both crossed and uncrossed axons to the levator palpebrae superioris (LPS) muscle. Notably, the axons innervating the LPS arise from a single midline subnucleus located at the caudal aspect of the oculomotor complex. The paired trochlear nuclei (nIV) in the caudal midbrain send axons across the tectum to exit the brainstem and innervate the contralateral superior oblique muscle. The axons of the paired abducens nuclei (nVI) in the pons innervate the ipsilateral lateral rectus. The anatomy of the ocular cranial nerves and nuclei is depicted schematically in Figures 46.1–46.3 and described in greater detail in the following discussion.
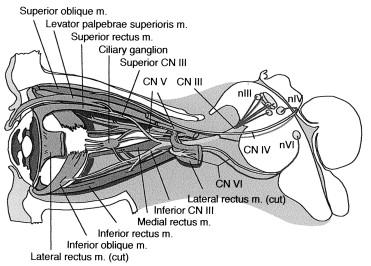
![Figure 46.3, Schematic anterior view of the human brainstem showing details of the cranial nerve nuclei that innervate the extraocular muscles. (nIII=oculomotor nucleus; nIV=trochlear nucleus; nVI=abducens nucleus.) The nIII subnuclei are shown as follows, with the muscle they innervate in brackets: 1=ventral lateral [medial rectus]; 2=medial [superior rectus]; 3=intermediate lateral [inferior oblique]; 4=dorsal lateral [inferior rectus]; 5=central caudal [levator palpebrae superioris]; 6=Edinger-Westphal [visceral motor]. Figure 46.3, Schematic anterior view of the human brainstem showing details of the cranial nerve nuclei that innervate the extraocular muscles. (nIII=oculomotor nucleus; nIV=trochlear nucleus; nVI=abducens nucleus.) The nIII subnuclei are shown as follows, with the muscle they innervate in brackets: 1=ventral lateral [medial rectus]; 2=medial [superior rectus]; 3=intermediate lateral [inferior oblique]; 4=dorsal lateral [inferior rectus]; 5=central caudal [levator palpebrae superioris]; 6=Edinger-Westphal [visceral motor].](https://storage.googleapis.com/dl.dentistrykey.com/clinical/DisordersoftheOcularMotorCranialNervesandExtraocularMuscles/2_3s20B9780124170445000469.jpg)
The globe is suspended in the bony orbit by the EOMs, connective tissue fascia, and fat. Because the two orbits point outward at approximately 25 degrees in the anterior-posterior plane, the vertical recti and oblique muscles are not aligned with the primary visual axis. The action of each muscle therefore depends somewhat on the position of the globe at the time of the action. The lateral and medial recti are antagonists in the horizontal plane, abducting and adducting the globe, respectively. The superior and inferior recti are partial antagonists in the vertical plane. The superior rectus primarily elevates and secondarily intorts and adducts the globe, and the inferior rectus primarily depresses and secondarily extorts and adducts the globe. The two obliques are partial antagonists and have greatest effect when the globe is adducted. The superior oblique intorts, depresses, and abducts the globe, and the inferior oblique extorts, elevates, and abducts the globe. The extraocular muscles are reciprocally innervated such that when an agonist muscle contracts, its antagonist relaxes, and they are yoked in pairs (i.e. the right lateral rectus and left medial rectus) so that the eyes move together.
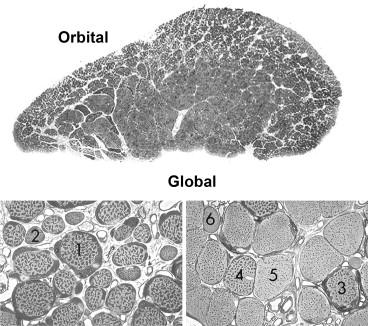
The extraocular lower motor unit has a number of unique features allowing a range of pathologic responses that differs from those of other skeletal muscles (reviewed by Porter and Baker, 1996). The histology of EOM is significantly different from that of the skeletal striated muscles, and it appears to be more resistant to pathologic stressors. The architecture of EOM differs from that of limb muscle in at least three ways. EOMs have longer fibrous tendinous insertions than limb muscle. Biopsy specimens may inadvertently be taken from these elongated tendons, resulting in a misdiagnosis of pathologic fibrosis. Anatomic and neuroimaging studies have revealed the existence of connective tissue pulleys in the orbit that serve as functional mechanical origins of the four recti muscles. Anterior to these pulleys, the paths of the recti shift with gaze to follow the scleral insertions, whereas posterior to the pulleys, these paths are stable in the orbit. The recti and oblique muscles are divided into global and orbital layers ( Figure 46.2 ). The global layer, adjacent to the optic nerve and globe, extends over the entire length of each EOM, while the orbital layer, adjacent to the orbital walls, is absent in the most anterior aspect of each EOM. The two layers differ from one another in their distribution of fiber types and the richness of their vascular supply, and may have variable insertion points upon the EOM.
The morphology of EOM fibers differs from those of limb skeletal muscle in at least two ways. Normal EOM myofibers are rounder and smaller, with greater variability in size, and are surrounded by a greater amount of perimysial and endomysial connective tissue than is limb skeletal muscle. EOM fiber typing is different from that of limb skeletal muscle; the traditionally recognized fiber classification schemes cannot be applied to EOM. Typing of EOM fibers is based on (1) distribution into global and orbital layers; (2) single versus multiple nerve contacts per fiber; and (3) mitochondrial/oxidative enzyme content. This fiber type scheme identifies six fiber types: orbital singly, orbital multiply, global red singly, global intermediate singly, global pale singly, and global multiply innervated fibers (see Figure 46.2 ). The levator palpebrae superioris differs from other EOMs. This muscle appears to use a ligament as a fulcrum to translate its anterior-posterior line of force into upward movement of the eyelid, has no differentiation of orbital and global layers, and lacks multiply innervated fiber types.
The gene and protein expression profiles of EOM myofibers differ from skeletal limb muscle both during development and in maturity. While EOMs contain the normal components of the dystrophin-glycoprotein complex, including dystrophin, dystroglycans, sarcoglycans, syntrophins, dystrobrevin, and merosin, they also retain embryonic myosin, fetal acetylcholine receptors, and sarcolemmal-wide expression of polysialated neural cell adhesion molecules. These isoforms are more typically associated with developing or regenerating muscle fibers but may be used by EOM as an adaptation for these muscles’ normal functional demands.
The molecular and cellular biology of the oculomotor motor neuron is distinct from that of spinal motor neurons. For example, the homeobox gene PHOX2A , which is mutated in congenital fibrosis of the extraocular muscles (CFEOM2) (see the following), is essential for development of oculomotor and trochlear motor neurons, but not spinal motor neurons. The alpha motor neurons of nIII, nIV, and nVI form very small motor units, often innervating only three to ten muscle fibers. In addition, a single EOM fiber can be innervated by axons from more than one motor unit (multiply innervated fibers). These motor neurons have an impressively high firing rate, often an order of magnitude higher than that of spinal motor neurons.
When interpreting abnormal eye movements or ptosis, or both, several basic approaches may be helpful. Priorities in examination are to:
Determine if the abnormality is attributable to a single cranial nerve or to multiple nerves.
Determine if the abnormality exists in isolation (e.g. congenital strabismus) or occurs in association with dysfunction of other skeletal muscle groups, syndromic malformations, or features of systemic disease.
Determine if the disorder is congenital or acquired. This distinction cannot be made simply by asking whether eye movements appeared normal at birth. The normal development of volitional eye movements such as fixation, visual following, and binocular alignment in the first few months of life may delay recognition of a congenital eye movement disorder. Conversely, acquired deficits may develop insidiously and thus be confused with congenital deficits. Serial examinations over time (including observation of old photographs) and attention to compensatory features (e.g. a large fusion angle suggesting congenital strabismus) may help.
Determine whether the dysfunction is static, progressive, recurrent, or resolving.
Ptosis can be quantified using the following methods:
Measure the distance between the upper lid margin and the midcorneal reflex when the globe is in normal primary position. Ptosis is present when this distance is <2 mm or varies by more than 2 mm between the eyes.
Measure the amount of the superior portion of the cornea covered by the upper lid when the globe is in primary position; normal is approximately 2 mm. With ptosis, more than 4 mm of cornea is covered.
Measure the vertical width of the palpebral fissure; it is normally approximately 9–15 mm.
Assess pupil size and reactivity. If anisocoria is present, it should be observed under varied illumination; in Horner syndrome, the anisocoria is more apparent in darkness and a lag in pupillary dilation may be observed, whereas with pupillary constrictor paresis, the difference is more evident under bright light. A relative afferent pupillary defect, indicating optic nerve dysfunction, is detected by swinging a bright light source from one pupil to the other, watching for dilation in the affected eye. Slit lamp examination may identify sectoral irregularities in the iris, an indication of reinnervation.
Assess ocular alignment by the alternate cover test or Maddox rod testing. A patient with a phoria prefers to fixate with one eye and will shift eye position only when the preferred eye is covered; if a tropia is present, fixation will shift when either eye is occluded. The degree of horizontal or vertical misalignment can be quantified using prisms. The Bielschowsky three-step test is valuable for identification of trochlear nerve palsies (see “Trochlear Palsy (CN IV)” section). Information as to whether ocular misalignment is long-standing may be obtained via stereoacuity tests.
Assess ductions by having the patient follow a hand-held target to all cardinal positions of gaze; observe the range, speed, and smoothness of these movements in each eye, as well as whether the two eyes move conjugately. The examiner can best observe both eyes simultaneously by fixating on the patient’s nose. When there is limitation of movement to a given position of gaze, it may be difficult to distinguish between weakness of an EOM (e.g. lateral rectus) and restriction or overaction (or both) of its antagonist (medial rectus). The speed and smoothness of movement may serve as a clue; sudden slowing toward the end of an excursion suggests restriction of the antagonist. The definitive tests, however, are those of forced duction and active force generation. Forced duction testing is performed by grasping the anesthetized extraocular muscle, tendon, or globe itself, and pulling it through its range of motion; the examiner directly feels any mechanical restriction. In young children, this procedure often requires general anesthesia. Active force generation requires the alert patient’s cooperation to attempt eye movements while the examiner grasps muscle, tendon, or globe and senses muscle force.
Assess horizontal and vertical saccades by asking the patient to rapidly switch fixation between the examiner’s nose and targets held in each extreme of the visual field. Slow saccades suggest muscle weakness or, in the case of the medially directed saccades, an internuclear ophthalmoplegia; saccades that begin rapidly but slow toward the end of their excursion suggest restriction of the opposing EOM. Repeated saccades may reveal the fatigability associated with myasthenic syndromes. Hyper- or hypometric saccades suggest cerebellar disease. Occasionally, disturbances in smooth pursuit suggest a particular pattern of EOM involvement—for example, the upshoot seen during medial pursuit in Duane’s syndrome (see below). Both horizontal and vertical head thrusts take advantage of vestibular input to indicate the true range of motion of the eyes where voluntary maneuvers fail to do so; in addition, they provide helpful information about central control of eye movements. Identification of nystagmus indicates central dysfunction but may also provide an indirect indication of limitation, such as with the abducting nystagmus seen when medial deviation of the opposite eye is limited.
Finally, additional general ophthalmologic testing assists in identifying visual pathway dysfunction that may affect ocular motility. This testing may include assessment of visual acuity at far and at near, color perception, the visual fields, and the funduscopic appearance of the optic nerves and the central and peripheral retina. The latter also may be particularly helpful to identify complex neurologic syndromes of which abnormal EOMs are a single component.
The oculomotor nucleus consists of a series of closely associated subnuclei arranged along the dorsal-ventral and rostral-caudal dimensions of the tectum of the midbrain (see Figure 46.3 ). A midline dorsal and caudal subnucleus provides bilateral innervation to the levator palpebri. Together with the contralateral axons from the superior rectus subnucleus, these axons form the superior division of the nerve. All EOMs other than the superior recti are innervated ipsilaterally via the inferior division of the nerve. Pupillary fibers emerge from the single midline Edinger-Westphal nucleus in the complex, and run superficially within the nerve fascicle to the ipsilateral ciliary muscle and iris sphincter.
The anatomy of the oculomotor nuclei and nerve produces clinical features helpful in the localization of oculomotor palsies. Third nerve palsies can be localized to the nucleus if they include contralateral involvement of the superior rectus and bilateral involvement of the levator palpebri (and probably also of the medial recti). They may localize to the nerve if ptosis and ophthalmoplegia are ipsilateral, and to only the superior division of the nerve if the ophthalmoplegia is limited to vertical limitations as seen in CFEOM (see the following). Given the close proximity of the oculomotor nucleus to other brainstem structures, acquired nuclear lesions are often associated with other neurologic signs such as somnolence and hemiplegia. Lesions affecting the oculomotor nerve fascicle cause ipsilateral signs because the axons serving the superior recti have already decussated within the nuclear complex. Because the oculomotor nerve fibers pass through the reticular formation, red nucleus, and substantia nigra, a lesion along their course may lead to ipsilateral ataxia (dentatorubrothalamic tract) or contralateral hemitremor, hemichorea, or hemiballismus (red nucleus and substantia nigra). In the interpeduncular cistern, CN III may be subject to compression from aneurysms, although this is very rare in childhood. Early involvement of the pupil in oculomotor palsies points to compressive lesions, as the dorsomedial placement of the pupillary fibers in the nerve fascicle renders them susceptible to compression.
Just before entering the cavernous sinus, the third nerve is susceptible to compression at the free edge of the tentorium and the clivus. Uncal herniation generally causes an ipsilateral, and rarely a contralateral, oculomotor palsy in which the pupil is involved early. Ipsilateral involvement of CN III, CN IV, and CN VI localizes to the cavernous sinus or orbital apex. Involvement of the trigeminal nerve is often more extensive with cavernous sinus lesions, whereas the presence of proptosis or visual loss from optic neuropathy, or both, defines the orbital apex syndrome. In the cavernous sinus, the nerve lies dorsally and deep in the lateral wall, superior to the trochlear nerve; as it enters the superior orbital fissure, it divides into superior and inferior divisions. The superior division innervates the superior rectus and levator palpebrae superioris, whereas the inferior division innervates the inferior and medial recti, inferior oblique, and ciliary ganglion. Thus, an isolated superior division palsy causes globe depression and ptosis, whereas an isolated inferior division palsy results in abduction, elevation, and mydriasis. Lesions at the apex or within the superior orbital fissure typically produce divisional palsies, but because the fibers are segregated within the nerve along much of the fascicular course, more proximal lesions can also produce these findings.
Oculomotor palsies classically present with ptosis, mydriasis, and external ophthalmoplegia. With a complete third nerve palsy, the pupil is large and does not constrict to light or vergence efforts, and near accommodation is impaired. The affected eye is depressed and abducted, and limited in adduction and elevation. Ptosis is severe. Correspondingly, patients complain of ptosis, blurred vision especially at near (due to inability to accommodate sufficiently), and diplopia.
Although the major clinical features are the same in congenital and acquired forms of oculomotor palsy, subtle differences in presentation (e.g. presence of amblyopia, synkinesis, pupillary involvement, or fluctuating symptomatology) may help distinguish the two forms.
Third nerve palsies are rare, and less common than fourth or sixth nerve palsies in childhood. About ⅔ are partial, and ⅓ complete. As many as 47% of all pediatric oculomotor palsies are congenital. Acquired causes include trauma (12–37%), infection and other inflammation (6–21%), tumor (3–17%), and migraine (3–9%). Aneurysms and other vascular events account for 3% to 11%. The cause remains undetermined less often in children (2–14%) than in adults (25–32%).
Congenital oculomotor palsies in otherwise normal children are thought to relate to perinatal trauma, possibly due to molding of the skull during labor or with forceps use. Under these circumstances, the nerve may be compressed against the tentorium by displacement of the temporal lobe or by a diffuse increase in intracranial pressure. In other cases, the nucleus or nerve, or both, fails to develop (see later discussion of CFEOM). Congenital oculomotor palsies may be associated with contralateral hemiplegia, or developmental anomalies of the midbrain or cerebellum. A frequent clue to the congenital nature of an oculomotor palsy is the presence of aberrant regeneration with synkinesis, which presumably occurs in response to aplasia of the nucleus or disruption of the nerve. Such regeneration may lead to miosis, rather than dilation, of the affected pupil. Another common finding is amblyopia, usually in the paretic eye but occasionally in the nonparetic eye.
Partial congenital oculomotor palsy may manifest as a divisional palsy or as isolated dysfunction of CN III-innervated muscles, in particular the levator, inferior rectus, inferior oblique, or pupillary constrictor. These disorders likely represent a spectrum of developmental anomalies of the subnuclei and muscles.
Acquired oculomotor palsies are more often partial than complete, and may be isolated or associated with more generalized neuromuscular processes ( Table 46.2 ). The most common causes of oculomotor palsies are trauma (up to 72%), neoplasm (5–31%), inflammation or infection (9–25%), migraine (7–19%), and other vascular pathologies (3–13%). As many as 20% of cases are cryptogenic. Painful ophthalmoplegia often involves more than one cranial nerve, and suggests acquired conditions such as infection, tumor, orbital pseudotumor, hemorrhage, thrombosis, aneurysm, or Tolosa-Hunt syndrome. In contrast, synkinesis or sparing of the pupil suggests a more benign cause. Diplopia in acquired oculomotor palsy is usually oblique in primary position, but varies with the relative degree of weakness in each muscle, and may be obscured if ptosis is severe enough to occlude the paretic eye. Although intorsion due to inferior oblique involvement is not often detected clinically, the patient may perceive a tilted sense of vertical (not always in the paretic eye or in the expected direction).
| Etiology | III Cranial Nerve | IV Cranial Nerve | VI Cranial Nerve |
|---|---|---|---|
| TRAUMA | 25–72% | 8–50% | 20–42% |
| Diffuse axonal injury | + | ||
| Ischemic | + | ||
| Shearing forces | + | + | + |
| Subdural hematoma | + | + | |
| Cavernous sinus thrombosis | + | + | + |
| Postoperative | + | + | + |
| NEOPLASIA | 5–31% | Rare | 17–39% |
| Cavernous sinus | |||
| Meningioma | + | + | + |
| Schwannoma | + | + | + |
| Pituitary fossa | |||
| Pituitary adenoma | + | + | + |
| Craniopharyngioma | + | + | + |
| Dermoid/epidermoid | + | + | + |
| Teratoma | + | + | + |
| Sellar germ cell tumor | + | + | + |
| Cerebellar | |||
| Astrocytoma | + | + | |
| Medulloblastoma | + | + | |
| Brainstem glioma | + | + | ++ |
| Other neoplasms | |||
| Pinealoma | + | + | |
| Meningioma | + | + | |
| Schwannoma | + | + | |
| Acoustic neuroma | + | + | |
| Ependymoma | + | + | |
| Nasopharyngeal carcinoma | + | + | |
| Clivus chordoma | |||
| Infiltrating neoplasms | |||
| Leptomeningeal sarcoma | + | + | + |
| Lymphoma | + | + | + |
| Carcinomatous meningitis | + | + | + |
| Mesencephalic cyst | + | ||
| VASCULAR | 3–13% | 1–2% | |
| Subdural hemorrhage | + | + | |
| Aneurysm | + | + | + |
| Cavernous hemangioma | + | + | + |
| Arteriovenous malformation | + | + | |
| Carotid-cavernous fistula | + | ||
| INFECTION | 9–25% | ||
| Meningitis | + | + | + |
| Encephalitis | + | + | + |
| Mastoiditis, Gradenigo’s syndrome | + | ||
| INFLAMMATORY | |||
| Sarcoidosis | + | + | + |
| Tolosa-Hunt syndrome | + | + | + |
| Orbital pseudotumor | + | + | + |
| RHEUMATOLOGIC/AUTO-IMMUNE | |||
| Polyarteritis nodosa | + | ||
| Multiple sclerosis, ADEM | + | + | + |
| Guillain-Barré syndrome | + | + | + |
| Sjögren’s syndrome | + | ||
| OTHER | |||
| Migraine | 7–19% | + | |
| Toxins | + | + | |
| Increased intracranial pressure | + | + | |
| Thyroid eye disease | + | + | + |
| Iatrogenic | + | + | |
| Cryptogenic | <20% | 0–21% | 9–36% |
Acquired partial oculomotor palsies suggest pathology at extremes of the oculomotor nerve: either discrete lesions in the midbrain, involving an oculomotor subnucleus, or focal intraorbital lesions. Nuclear causes include focal metastasis, ischemia, or demyelination, while orbital causes include myasthenia gravis, trauma, tumor, or inflammation.
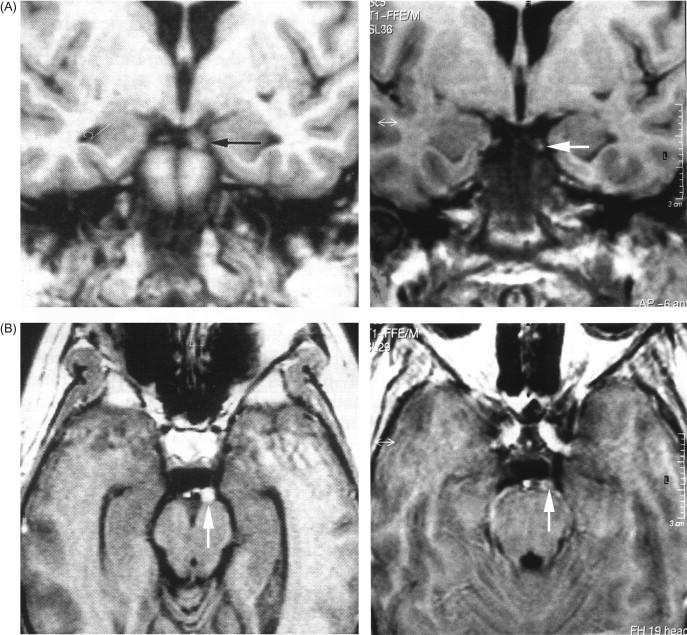
Recurrent transient oculomotor palsies in childhood are most commonly due to ophthalmoplegic migraine. This uncommon inflammatory cranial neuropathy is much more frequent in children than adults. Onset is generally before age 10. Each episode typically begins with a severe ipsilateral hemicranial headache, with ophthalmoplegia developing within hours to days (rarely up to 14 days), and generally resolving after 3 to 4 days. Deficits rarely persist as long as a month, and synkinesis may develop. Although a complete oculomotor palsy is most frequent, isolated pupillary involvement can occur, and involvement of the fourth and sixth cranial nerves, oculomotor divisions, and levator palpebrae are also reported in ophthalmoplegic migraine. Rarely, recurrent ophthalmoplegia may occur in the absence of headache, in what may represent a migraine equivalent. Magnetic resonance imaging (MRI) studies commonly show enlargement and contrast enhancement of the interpeduncular portion of the oculomotor nerve during, and occasionally following, ophthalmoplegic migraines ( Figure 46.4 ), while the CSF examination is normal.
Tolosa-Hunt syndrome is an acute syndrome of orbital pain and ophthalmoplegia caused by granulomatous inflammation within the cavernous sinus or orbit, which responds rapidly and dramatically to oral steroids but may recur. Myasthenia gravis should be suspected whenever ptosis or diplopia fluctuate in severity.
Pediatric CN III palsies occasionally present with isolated pupillary dilatation. This must be distinguished from Adie’s tonic pupil and from a contralateral Horner syndrome. Adie’s pupil may develop cryptogenically or as a parainfectious, posttraumatic, or migrainous phenomenon, and is often associated with depressed deep tendon reflexes. Adie’s tonic pupil is differentiated from an oculomotor palsy by the presence of light-near dissociation (strong constriction when viewing near targets, with poor reaction to light or accommodation). Slit lamp examination frequently reveals asymmetrical segmental constriction of pupillary fibers, often with vermiform iris movements, once reinnervation has had time to occur. Although there is supersensitivity of the pupil to 0.1% pilocarpine in lesions more than 2 weeks old, this may also be found in CN III palsies.
In its complete form, Horner syndrome consists of ipsilateral ptosis of both upper and lower lids, miosis, and decreased sudomotor function. In congenital Horner syndrome, ocular hypotony may occur and iris heterochromia is common. Lesions along the sympathetic pathway may be secondary to birth or surgical trauma, perinatal infection, the Arnold-Chiari malformation, neuroblastoma, and other tumors. Localization may be refined by identification of concurrent involvement of the fourth or sixth nerves (see the following) and by the use of 1% hydroxyamphetamine or 4% cocaine drops to distinguish between involvement of first, second, and third order neurons.
Trochlear palsy is the most common congenital ocular motor palsy, the most common cause of acquired vertical diplopia, and, overall, the most common isolated cranial nerve palsy.
The trochlear is the only cranial nerve to emerge from the dorsal aspect of the brainstem, the only one to cross completely, and the longest and thinnest of the ocular motor nerves. The trochlear nucleus is located ventral and lateral to the Sylvian aqueduct at the level of the inferior colliculus, dorsal to the medial longitudinal fasciculus and caudal to the oculomotor nuclear complex. The nerve fascicle leaves the nucleus, courses along the aqueduct, crossing in its roof inferior to the inferior colliculus, and exits the dorsal midbrain contralateral to the nucleus. The cisternal portion travels laterally around the midbrain to its ventral aspect, then passes along the free edge of the tentorium and along the lateral aspect of the clivus to enter the cavernous sinus. Here, it lies in the lateral wall just inferior to the oculomotor nerve, entering the orbit through the superior orbital fissure and running medially across the superior rectus muscle to the superior oblique muscle.
Associated neurologic signs aid localization of fourth nerve lesions. The dorsal midbrain syndrome, or Parinaud syndrome, may include trochlear palsy with upgaze weakness, downgaze limitation, convergence spasm, convergence-retraction nystagmus, lid retraction, light-near dissociation, or a combination of these symptoms. A contralateral Horner syndrome or intranuclear ophthalmoplegia indicates involvement of the fourth nerve within the midbrain. Ipsilateral limb ataxia or contralateral sensory loss may reflect involvement of the cerebellar peduncle or sensory lemniscus, respectively. Concurrent involvement of the third and fourth nerves may localize to the midbrain, the cavernous sinus or orbital apex, where the trigeminal nerve may also be affected.
In children, fourth nerve palsy often presents with a head tilt away from the affected eye. This helps neutralize the vertical diplopia and rotation of the image from the paretic eye that the patient will perceive with the head erect and gaze in primary position. In addition to the direction of head tilt, the patient’s perception in primary position, as well as version testing, will localize the paretic eye. Viewing a horizontal edge, the patient may perceive two images of the edge tilted with respect to one another, and intersecting as if forming an arrow that points to the paretic side. This is less common in children than adults and is not found in congenital trochlear palsies.
The Bielschowsky Three-Step Test helps to differentiate between trochlear palsy and its mimickers, such as dissociated vertical deviation, skew deviation, and double elevator palsy ( Table 46.3 ).
| Step 1 | Step 2 | Step 3 | ||
|---|---|---|---|---|
| Primary | R gaze | L gaze | R tilt | L tilt |
| R hypertropia | ||||
| RSO | RSO | RSO | ||
| RIR | RIR | RIR | ||
| LIO | LIO | LIO | ||
| LSR | LSR | LSR | ||
| L hypertropia | ||||
| LSO | LSO | LSO | ||
| LIR | LIR | LIR | ||
| RIO | RIO | RIO | ||
| RSR | RSR | RSR | ||
Most trochlear palsies are congenital (refer to Congenital Cranial Dysinnervation Disorders section below) or arise as a result of trauma. Differentiation between congenital and acquired trochlear palsy may be difficult because the congenital form is often well compensated and thus asymptomatic prior to adolescence or adulthood, when it may decompensate and be mistaken for an acute palsy. Review of photographs from infancy may reveal a long-standing head tilt, suggesting a congenital lesion. Facial asymmetry, with retrusion and upslanting of the mouth on the side of the head tilt, is also indicative of chronicity. Children with congenital trochlear palsy do not generally perceive a tilted image, presumably due to a combination of the head tilt and more complex central physiologic mechanisms. Similarly, amblyopia is rare because the compensatory head tilt enables fusion of the images from both eyes. MRI findings can also be helpful in distinguishing congenital and acquired trochlear palsies . Finally, forced duction testing reveals decreased resistance of the superior oblique in congenital palsies, and normal resistance in acquired forms.
Up to 50% of acquired pediatric trochlear nerve palsies are due to trauma or neurosurgery (see Table 46.2 ). Orbital fractures may damage the nerve, tendon, trochlea, or the muscle itself. Trauma may also cause decompensation of a congenital palsy. Acquired bilateral trochlear palsy is most often due to trauma, and is thought to result from injury at the fascicular decussation. Such cases usually present with a chin-down head position, rather than a head tilt, and with a right hypertropia in left gaze and a left hypertropia in right gaze (alternating adducting hypertropia). A V-pattern esotropia is generally present, and is often large.
Inflammatory and infectious causes of trochlear palsies include the Tolosa-Hunt and Guillain-Barré syndromes, Herpes zoster ophthalmicus, and, less commonly, sarcoidosis and even tetanus. Recurrent CN IV weakness may be seen in the Tolosa-Hunt syndrome, sarcoidosis, ophthalmoplegic migraine (see earlier discussion), and a familial syndrome involving multiple recurrent cranial nerve palsies.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here