Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Disorders of skeletal muscle encompass a variety of illnesses that cause weakness, pain, and fatigue in any combination. They vary from the protean symptoms of muscle pain and fatigue that often defy any explanation to the muscular dystrophies, which one recognizes instantly on clinical grounds. Motor neuron disease (e.g., spinal muscular atrophies), neuromuscular junction disorders (myasthenia gravis, Lambert-Eaton syndrome, and congenital myasthenia), and certain polyneuropathies (e.g., chronic inflammatory demyelinating polyneuropathy) can cause similar symptoms and may be difficult to differentiate from muscle disorders on clinical grounds. Some definitions are worth reviewing. Myopathy simply refers to an abnormality of the muscle and has no other connotation. Muscular dystrophies are genetic myopathies usually caused by a disturbance of a structural protein or enzyme, resulting in necrosis of muscle fibers and replacement by adipose and connective tissue. Congenital myopathies are a group of illnesses that usually present in young children; many are relatively nonprogressive. However, rare “congenital myopathies” may manifest initially in adults (e.g., central nuclear myopathy, nemaline myopathy) and can be progressive. With the advent of molecular genetics, we recognize that many are allelic to what others have reported as dystrophies, further blurring their distinction as a separate category from muscular dystrophy. Myositis implies an autoimmune or infectious disorder in which the muscle histology shows an inflammatory response. The myotonias are diseases in which the occurrence of involuntary persistent muscle activity accompanied by abnormal repetitive electrical discharges distorts the normal contractile process. This occurs after percussion or voluntary contraction. Metabolic myopathies refer mainly to disorders of glycogen or lipid metabolism leading to impaired synthesis of adenosine triphosphate (ATP) or cause abnormal accumulation of material in the cell. The term endocrine myopathy refers to myopathies associated with disorders of the thyroid and parathyroid glands and to myopathies associated with corticosteroids.
Within muscle fibers chemical energy is converted into mechanical energy. The component processes include (1) excitation and contraction occurring in the muscle membranes, (2) the contractile mechanism itself, (3) various structural supporting elements that allow the muscle to withstand the mechanical stresses, and (4) the energy system that supports the activity and integrity of the other three systems. The logical categorization of myopathies is according to the part of the system involved. Abnormalities in the membrane ion channels (channelopathies) involved in muscle excitation cause various forms of myotonia and periodic paralysis (see Chapter 98 ). The complex of proteins that include dystrophin, the sarcoglycans, and α-laminin constitute a vital structural mechanism linking the contractile proteins with the extracellular supporting structures. Defects in these proteins are the basis of many forms of muscular dystrophy. Although knowledge remains incomplete, it seems reasonable to modify the classic description of the myopathies to incorporate the new information. For this reason, in the sections that follow, disease descriptions are under the heading of their known molecular defect where possible; the classic appellation appears parenthetically. Before describing the illnesses themselves, we first review the techniques used in the clinical evaluation of patients.
The technique of muscle biopsy is not difficult. Under local anesthesia, a small incision made over the muscle allows, with careful dissection, removal of a small strip of muscle. Needle biopsies are useful in some situations. Histochemical studies of frozen sections are essential for proper interpretation. A transverse section of normal muscle shows fibers that are roughly of equal size and average approximately 60 mm in transverse diameter ( Fig. 109.1 ). The muscle fibers of infants and young children are proportionately smaller. Each fiber consists of hundreds of myofibrils separated by an intermyofibrillar network containing aqueous sarcoplasm, mitochondria, and the sarcoplasmic reticulum with the associated transverse tubular system. Surrounding each muscle fiber is a thin layer of connective tissue (the endomysium). Strands of connective tissue group muscle fibers into a fascicle, separated from each other by the perimysium. Groups of fascicles are collected into muscle bellies surrounded by epimysium.
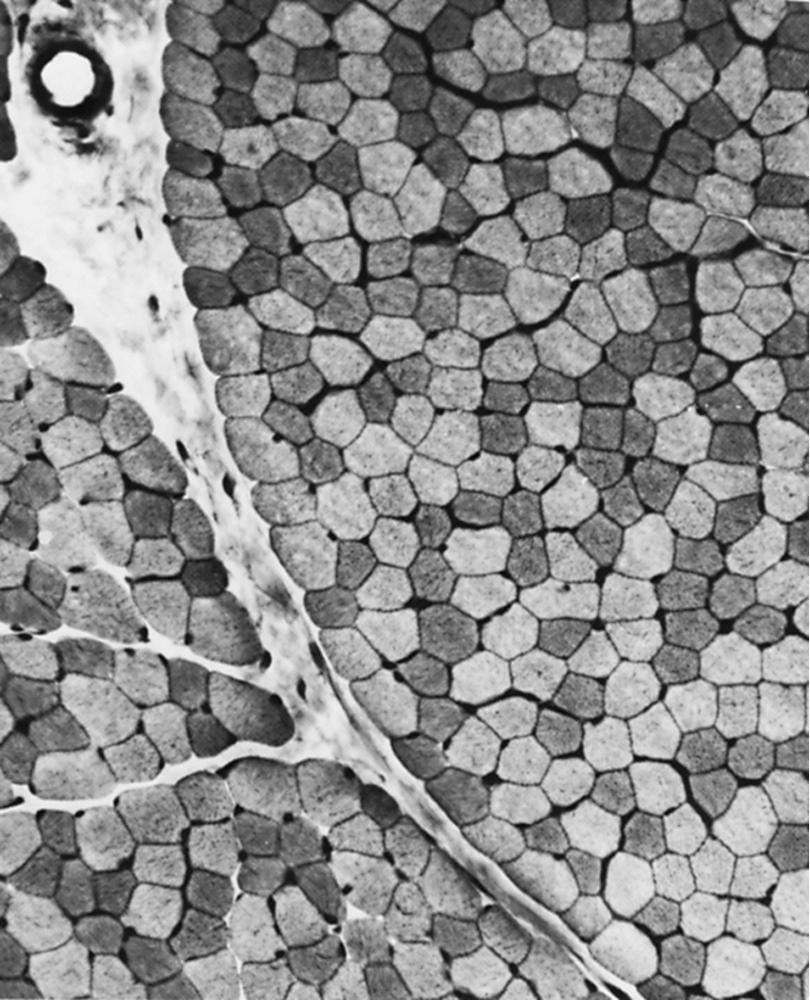
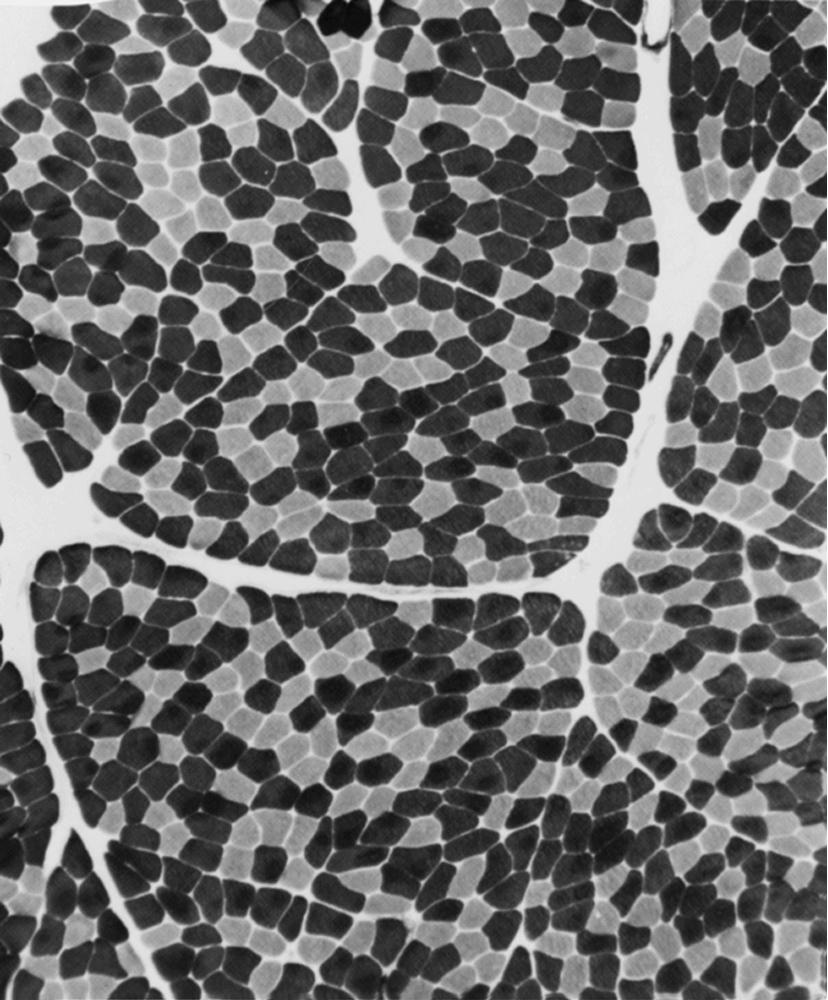
Situated at the periphery within muscle fibers are the sarcolemmal nuclei. The fibers are of different types. The simplest division is into type 1 and type 2 fibers, best demonstrated with the histochemical reaction for myosin adenosine triphosphatase (ATPase; Fig. 109.2 ). The type 1 and type 2 fibers are roughly synonymous with slow and fast fibers or with oxidative and glycolytic fibers in human muscle. Type 2 fibers can be further subdivided based on both staining properties and resistance to fatigue. The best demonstration of the intermyofibrillar network pattern is with the histochemical reactions for oxidative enzymes, such as reduced nicotinamide adenine dinucleotide dehydrogenase (NADH). A regular network extends across the whole fiber. In addition to the routine stains with hematoxylin and eosin, modified Gomori trichrome, myosin ATPase, and NADH, the use of other special stains demonstrates fat (Sudan black or oil red O), complex carbohydrates (periodic acid–Schiff), amyloid (Congo red), or specific enzymes (e.g., phosphorylase, succinic dehydrogenase, cytochrome oxidase [COX]). Immunocytochemical techniques demonstrate the location and integrity of structural proteins such as dystrophin. They also characterize cell types in biopsy samples with inflammatory changes.
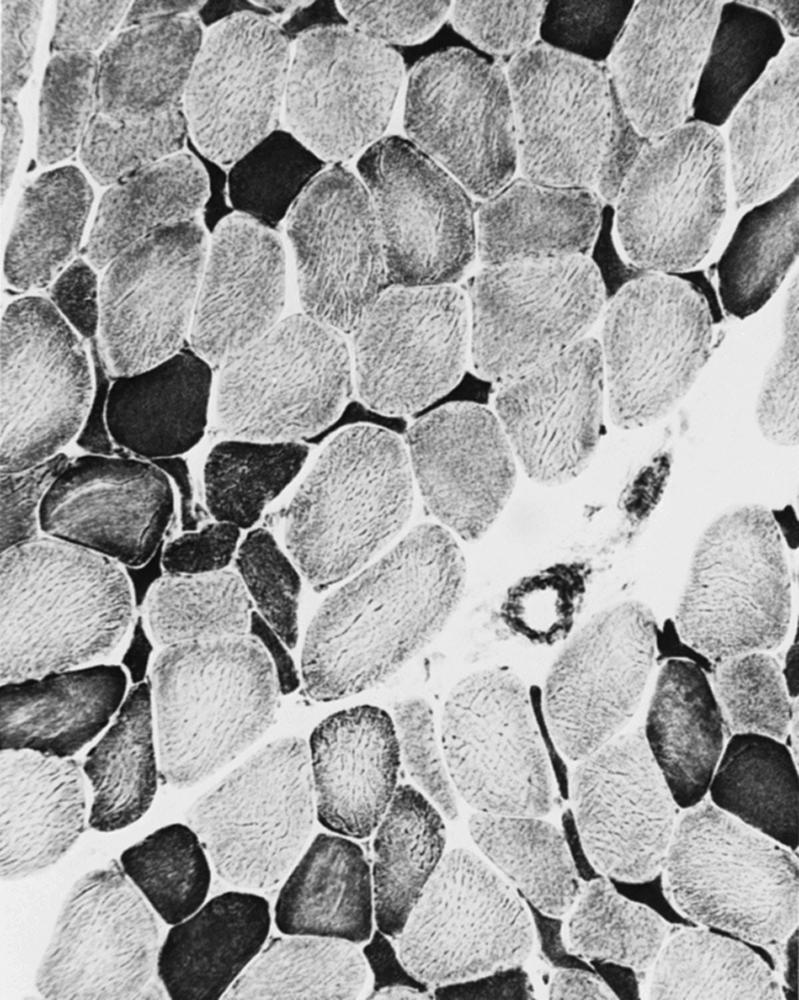
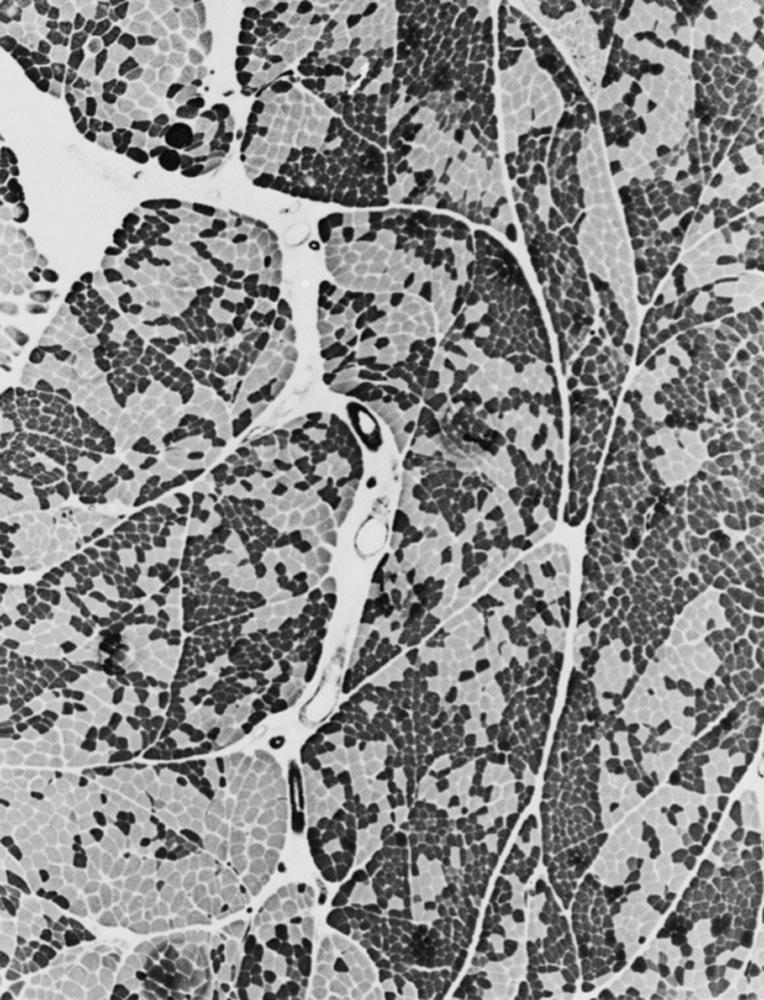
When muscle loses its nerve supply, muscle fibers atrophy, often resulting in fiber squeezing into the spaces between normal fibers and assuming an angulated appearance ( Fig. 109.3 ). Scattered angulated fibers appear early in denervation. Sometimes, picturesque changes in the intermyofibrillar network occur, as in the “target fiber,” which characterizes denervation and reinnervation. This is a three-zone fiber on which the intermediate zone stains more darkly, and the central “bull’s eye” stains much lighter than normal tissue ( Fig. 109.4 ). Often a neighboring nerve twig reinnervates a denervated fiber. This results in the same anterior horn cell supplying two or more contiguous fibers. If that nerve twig then undergoes degeneration, instead of only one small angulated fiber being produced, a small group of atrophic fibers develops. Group atrophy suggests denervation ( Fig. 109.5 ). As the process continues, large groups of geographical atrophy occur in which entire fascicles are atrophic. In addition to the change in size, a redistribution of the fiber types occurs as well. Normally a random distribution of type 1 and 2 muscle fiber types exists, sometimes incorrectly called a checkerboard or mosaic pattern . The same process of denervation and reinnervation results in larger and larger groups of contiguous fibers supplied by the same nerve twig. Because all fibers supplied by the same nerve twig are of the same fiber type, groups of type 1 fibers next to groups of type 2 fibers replace the normal random pattern. This fiber type grouping is pathognomonic of reinnervation ( Fig. 109.6 ). When long-standing denervation is present, the atrophic muscle fibers almost disappear, leaving small clumps of pyknotic nuclei in their place.
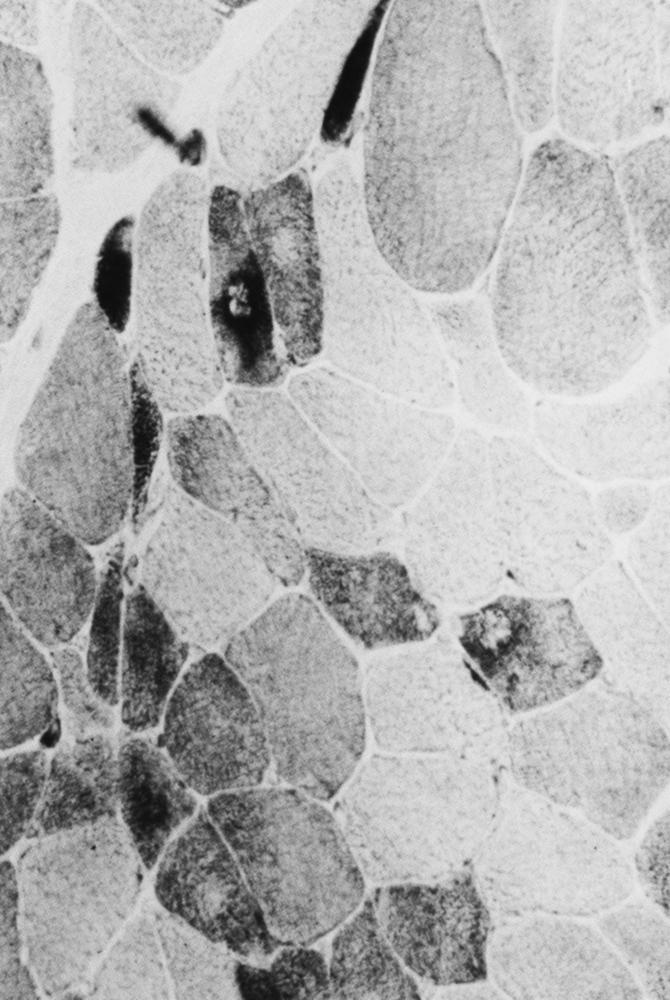
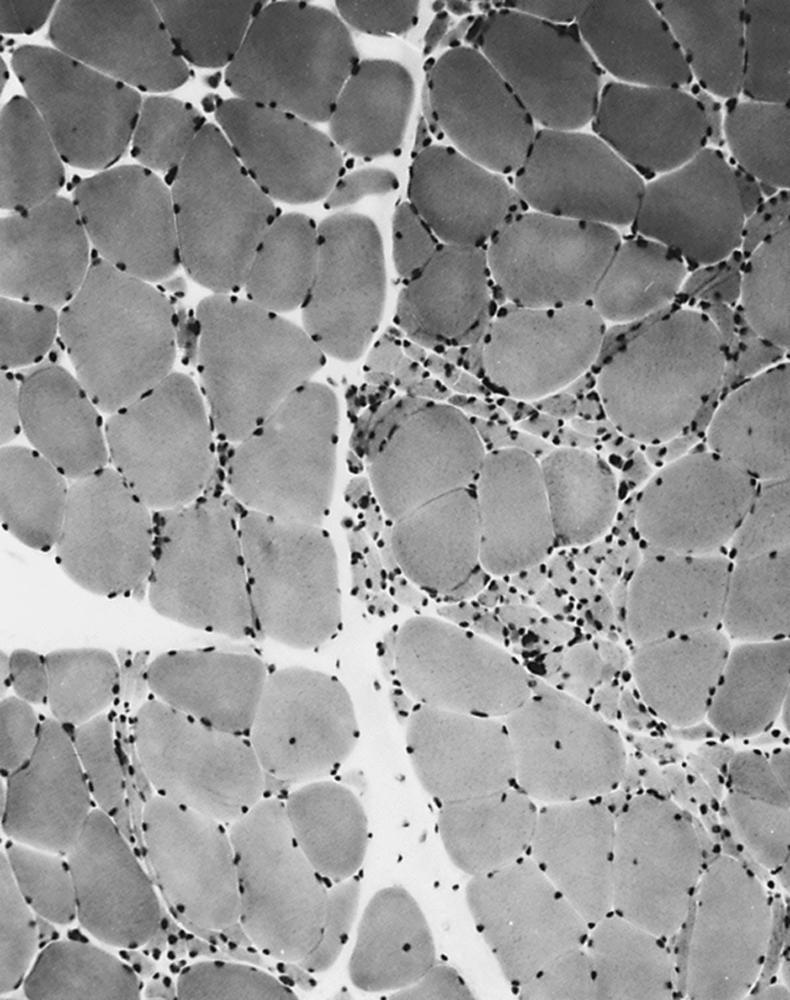
Myopathies are typically associated with greater variation in pathological changes than those that occur with denervation. The type of change depends on the type of muscle disease. The normal peripherally placed nuclei may migrate toward the center of the fiber. Internalized nuclei may be seen in normal muscle (up to 2% of fibers), but when they are numerous, they usually indicate a myopathic process. Numerous internal nuclei are a feature of the myotonic dystrophies and the limb–girdle muscular dystrophies (LGMDs). Occasionally, internal nuclei are seen in certain chronic denervating conditions (e.g., juvenile spinal muscular atrophy). Necrosis of muscle fibers, in which the fiber appears liquefied and later presents as a focus of phagocytosis, occurs in many of the myopathies. These changes usually represent an active degenerative process. They often are a feature of myoglobinuria, toxic myopathies, inflammatory myopathies, and metabolic myopathies, but can also be seen in dystrophies. Fiber-size variation may occur in primary diseases of muscle, with large fibers and small fibers intermingling in a random pattern. This is sometimes the only indication of a pathological process. Fiber splitting often accompanies muscle fiber hypertrophy. In transverse section, recognition of split fibers is by a thin fibrous septum, often associated with a nucleus that crosses partway but not all the way across the fiber. A detailed study of serial transverse sections may reveal more split fibers than in a single section. Fiber splitting is particularly visible in dystrophic conditions such as LGMD.
Degeneration and regeneration of fibers characterize many illnesses. When this occurs, the regenerating fibers often become basophilic, and myonuclei enlarge because of the accumulation of ribonucleic acid (RNA) needed for protein synthesis. Fiber basophilia is a sign of an active myopathy. Cellular responses include frank inflammatory reactions around blood vessels, which characterize the collagen vascular diseases and dermatomyositis (DM). Endomysial inflammation with invasion of non-necrotic muscle fibers occurs in inclusion body myositis (IBM) and polymyositis (PM). Importantly, pronounced inflammatory cellular responses may occur in dystrophies, particularly facioscapulohumeral dystrophy (FSHD) and dysferlinopathies. Even the so-called congenital inflammatory myopathies actually represent forms of congenital muscular dystrophy.
Fibrosis is another reactive change in muscle. Normally a very thin layer of connective tissue separates the muscle fibers. In dystrophic conditions, this layer thickens, and muscle fibrosis may be quite pronounced. In the inflammatory myopathies, there may be a loose edematous separation of fibers, but fibrosis is not usually characteristic in early phases of the disease except when associated with systemic sclerosis or in IBM.
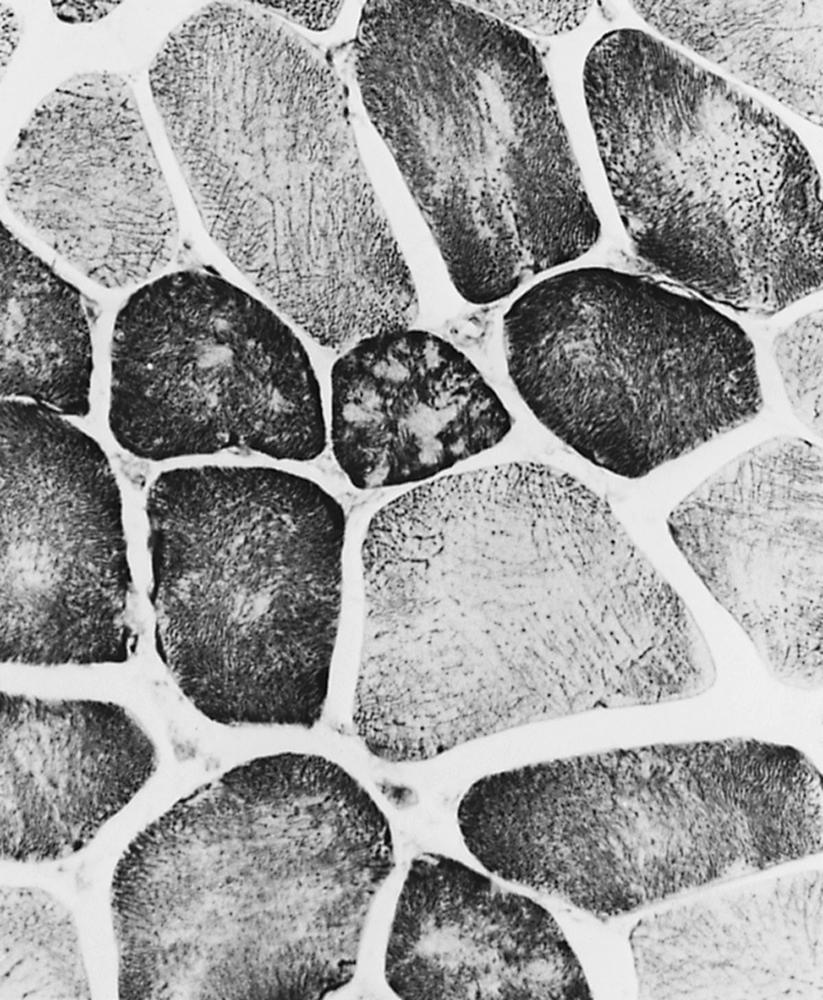
Changes in the intermyofibrillar network pattern are common in myopathic disorders. There is often a moth-eaten, whorled change to the intermyofibrillar network in LGMD and FSHD ( Fig. 109.7 ); the intermyofibrillar network loses its orderly arrangement and swirls, resembling the current in an eddying stream. These changes may be seen in several diseases but tend to be much more common in the myopathies.
Selective changes in fiber types can occur. Type 2 fiber atrophy is one of the most common abnormalities seen in muscle ( Fig. 109.8 ). Type 2 atrophy, particularly if limited to type 2B fibers, is nonspecific and indicates muscle disuse. If a limb is casted and the muscle examined some weeks later, selective atrophy of type 2 fibers is noted. Any chronic systemic illness tends to produce type 2 atrophy. It occurs in rheumatoid arthritis, nonspecific collagen vascular diseases, cancer (hence the name cachectic atrophy ), intellectual disability in children, and pyramidal tract disease. Type 2B fiber atrophy can also result from chronic corticosteroid administration. Therefore type 2 fiber atrophy should probably be regarded as a nonspecific result of anything less than robust good health.
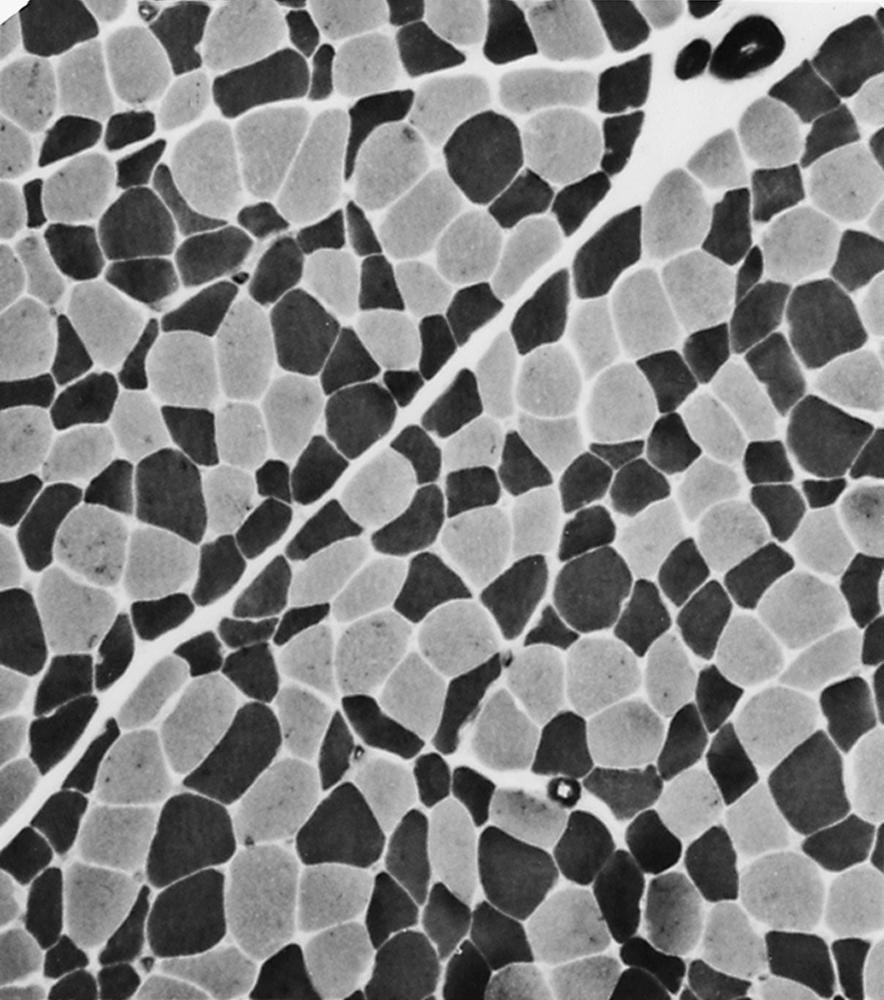
Type 1 fiber atrophy is more specific. It occurs in some of the congenital myopathies and dystrophies, congenital myasthenia, and is characteristic of myotonic dystrophy type 1 ( ![]() ).
). ![]() Changes in the proportion of fiber types are quite separate from changes in the fiber size. The name fiber type predominance refers to a change in the relative numbers of a particular fiber type. Type 1 fiber predominance is a normal finding in the gastrocnemius and deltoid muscles. When widespread, it is also the hallmark of congenital myopathies and many of the early dystrophies. Type 2 fiber predominance is seen in the lateral head of the quadriceps muscle. Type 2 predominance occurs occasionally in juvenile spinal muscular atrophy and motor neuron disease but is not firmly associated with any particular disease condition.
Changes in the proportion of fiber types are quite separate from changes in the fiber size. The name fiber type predominance refers to a change in the relative numbers of a particular fiber type. Type 1 fiber predominance is a normal finding in the gastrocnemius and deltoid muscles. When widespread, it is also the hallmark of congenital myopathies and many of the early dystrophies. Type 2 fiber predominance is seen in the lateral head of the quadriceps muscle. Type 2 predominance occurs occasionally in juvenile spinal muscular atrophy and motor neuron disease but is not firmly associated with any particular disease condition.
Myotonic Dystrophy . Myotonic discharges are seen in a muscle at rest on needle electromyography in a patient with type 1 myotonic dystrophy.
Some changes in muscle biopsy results are pathognomonic of a particular disease. Perifascicular atrophy , in which the atrophic fibers are more numerous around the edge of muscle fascicles, is the hallmark of DM. The presence of lipid vacuoles or abnormal pockets of glycogen characterizes the metabolic myopathies. Enzyme defects including myophosphorylase deficiency and phosphofructokinase (PFK) deficiency are detectable with appropriate histochemical stains. Interpretation of a muscle biopsy usually includes the description of a constellation of changes and the subsequent association of these changes with a particular diagnosis when possible. Illnesses that have characteristic biopsies include infantile spinal muscular atrophy, DM, IBM, the congenital myopathies, lipid storage myopathies, and glycogen storage diseases (e.g., Pompe disease, myophosphorylase deficiency). Immunocytochemical staining can also distinguish many forms of muscular dystrophy from each other. Although not disease specific, characteristic biopsy changes differentiate chronic denervation from acute simple denervation.
The use of biopsy material to identify missing proteins is increasing with the greater availability of commercial antibodies to the proteins of interest. Although genetic testing is becoming easier, cheaper, and more commonly utilized, immunohistochemical testing remains useful. Identification of an absent muscle protein may allow for diagnosis when genetic testing is either unremarkable or reveals a variant of uncertain significance in a gene of interest. Immunohistochemical findings may also allow narrowing of the panel of genetic tests to be sent. For example, if a patient with suspected Duchenne muscular dystrophy (DMD) undergoes genetic testing but no pathogenic mutation is discovered, confirmation of the diagnosis may rest on demonstrating absent or abnormal dystrophin in the muscle tissue. All the sarcoglycans are demonstrated using similar techniques. Deficiency of any of the sarcoglycans causes a muscular dystrophy, and because they comprise a complex, when one is missing, all or some of the others may be absent in the biopsy. The α-sarcoglycan is particularly prone to be missing, which makes it a suitable and economical screening tool. Absence or reduction of laminin-α 2 chain (merosin) or α-dystroglycan occurs in some forms of congenital muscular dystrophy. The lack of nuclear membrane staining with anti-emerin antibodies occurs in X-linked Emery-Dreifuss muscular dystrophy (EDMD).
Immunohistochemical techniques may give information about the type of inflammatory cell present via affinity for various markers such as CD68 (macrophages and dendritic cells), CD20 (B cells), CD3 (activated T cells), CD8 (cytotoxic T cells), and CD4 (T-helper and dendritic cells). These identify cells involved in cytotoxic, humoral, and innate immune mechanisms. Antibodies to the major histocompatibility antigen 1 (MHC1) are used to demonstrate overexpression on muscle fibers in inflammatory myopathy, while membrane attack complex (MAC) deposits may be found on the capillary endothelium in DM. Immunohistochemistry can be used to demonstrate inclusions (e.g., p62, TDP43) within muscle fibers to assist in diagnosis of IBM.
Immunoblot or Western blot of muscle biopsy is more sensitive than immunohistochemistry, particularly when dealing with an enzyme deficiency or nonstructural protein in evaluation of dystrophies. Patients with Becker muscular dystrophy may have normal-appearing immunostaining for dystrophin, because the commercial antibodies may react to that part of the dystrophin protein that is normally made. However, immunoblot reveals abnormal size or amount of dystrophin in such cases. Immunoblotting is valuable in assessing for calpainopathy (LGMD2A), dysferlinopathy (LGMD2B), and in the secondary α-dystroglycanopathies. Reductions of proteins in these disorders may be secondary, so a primary deficiency must be confirmed by genetic testing.
The muscular dystrophies are a group of hereditary muscle disorders that occur at all ages and with varying degrees of severity. The traditional classification is on clinical grounds. Increasing information about the molecular basis of these disorders provides both reassurance and puzzlement to clinicians ( Table 109.1 ). Different dystrophies are due to distinct molecular abnormalities; however, patients with similar molecular defects may show a wide variability in phenotype not always easily explained. Disorders traditionally classified as congenital myopathies on clinical grounds have been found to result from mutations in myofiber structural proteins, similar to those affected in muscular dystrophies.
| Disease | Inheritance | Chromosome | Affected Protein |
|---|---|---|---|
| X-Linked Dystrophies | |||
| Duchenne/Becker | XR | Xp21 | Dystrophin |
| Emery-Dreifuss | XR | Xq28 | Emerin |
| Scapuloperoneal/reducing body myopathy | XR | Xq26.3 | Four-and-a-half LIM domain 1 (FHL1) protein |
| Limb-Girdle Muscular Dystrophies (LGMD) (Classical Classification/Proposed New Classification Where Appropriate) | |||
| LGMD1A/myofibrillar myopathy | AD | 5q22.3-31.3 | Myotilin |
| LGMD1B/EDMD | AD | 1q11-21 | Lamin A and C |
| LGMD1C/rippling muscle disease | AD | 3p25 | Caveolin-3 |
| LGMD1D/LGMD D1 | AD | 6q23 | DNAJB6 |
| LGMD1E/myofibrillar myopathy | AD | 2q35 | Desmin |
| LGMD1F/LGMD D2 | AD | 7q32 | Transportin 3 |
| LGMD1G/LGMD D3 | AD | HNRNPDL | |
| LGMD1I/LGMD D4 | AD | 15q15.1-21.1 Calpain-3 |
15q15.1-21.1 Calpain-3 |
| LGMD2A/LGMD R1 | AR | 15q15.1-21.1 | Calpain-3 |
| LGMD2B/LGMD R2 ∗ | AR | 2p13 | Dysferlin |
| LGMD2C/LGMD R5 | AR | 13q12 | γ-Sarcoglycan |
| LGMD2D/LGMD R3 | AR | 17q12-21.3 | α-Sarcoglycan |
| LGMD2E/LGMD R4 | AR | 4q12 | β-Sarcoglycan |
| LGMD2F/LGMD R6 | AR | 5q33-34 | δ-Sarcoglycan |
| LGMD2G/LGMD R7 | AR | 17q11-12 | Telethonin |
| LGMD2H/LGMD R8 | AR | 9q31-33 | E3-ubiquitin-ligase (TRIM 32) |
| LGMD2I/LGMD R9 | AR | 19q13 | Fukutin-related protein (FKRP) |
| LGMD2J/LGMD R10 | AR | 2q31 | Titin |
| LGMD2K/LGMD R11 | AR | 9q31 | POMT1 |
| LGMD2L/LGMD R12 | AR | 11p14.3 | Anoctamin 5 |
| LGMD2M/LGMD R13 | AR | 9q31-33 | Fukutin |
| LGMD2N/LGMD R14 | AR | 14q24 | POMT2 |
| LGMD2O/LGMD R15 | AR | 1p32 | POMGnT1 |
| LGMD2P/LGMD R16 | AR | 3p21 | α-Dystroglycan |
| LGMD2Q/LGMD R17 | AR | 8q24 | Plectin 1 |
| LGMD2R/myofibrillar myopathy | AR | 2q35 | Desmin |
| LGMD2S/LGMD R18 | AR | 4q35.1 | TRAPPC11 |
| LGMD2T/LGMD R19 | AR | 3p11 | GDP-mannose pyrophosphorylase B |
| LGMD2U/LGMD R20 | AR | 7p21 | Isoprenoid synthase domain containing protein |
| LGMD2V | AR | 17q25.31 | α-1,4-Glucosidase |
| LGMD2W | AR | 2p14 | LIM and senescent cell antigen like domains 2 |
| LGMD2X | AR | 6q21 | Popeye domain-containing protein 1 |
| LGMD2Y | AR | 1q25.1 | Torsin-A interacting protein 1 or lamin-associated protein 1 |
| LGMD2Z/LGMD R21 | AR | 3q13.33 | Protein O-glucosyltransferase 1 |
| LGMD R23 | AR | 6q22-23 | Laminin-α 2 |
| LGMD R24 | AR | 3p22.1 | POMGNT2 |
| Congenital Muscular Dystrophies (MDC) | |||
| Bethlem myopathy/LGMD R22 | AR | 21q22.3 and 2q37 | Collagens 6A1, 6A2, and 6A3 |
| Bethlem myopathy/LGMD D5 | AD | 21q22.3 and 2q37 | Collagens 6A1, 6A2, and 6A3 |
| MDC1A | AR | 6q22-23 | Laminin-α 2 |
| α 7 -Integrin-related MDC | AR | 12q13 | α 7 -Integrin |
| MDC1C | AR | 19q13 | Fukutin-related protein (FKRP) |
| Fukuyama | AR | 9q31-33 | Fukutin |
| WWS | AR | 9q31 | POMT1 |
| MEB disease | AR | 1p32 | POMGnT1 |
| Rigid spine syndrome | AR | 1p35-36 | Selenoprotein N1 |
| Ullrich | AR | 21q22.3 and 2q37 | Collagens 6A1, 6A2, and 6A3 |
| Distal Dystrophies/Myopathies | |||
| Welander | AD | 2p13 | TIA1 |
| Udd | AD | 2q31 | Titin |
| Markesbery-Griggs | AD | 10q22.3-23.2 | ZASP |
| Nonaka or hIBM/GNE myopathy | AR | 9p1-q1 | GNE |
| Miyoshi 1∗ | AR | 2p13 | Dysferlin |
| Miyoshi 2 | AR | 11p14.3 | Anoctamin 5 |
| Laing (MPD1) | AD | 14q11 | MyHC 7 |
| Williams | AD | 7q32 | Filamin C |
| Distal myopathy with vocal cord and pharyngeal weakness (VCPDM or MPD2) | AD | 5q31 | Matrin 3 |
| Other Dystrophies | |||
| Facioscapulohumeral type 1 | AD | 4q35 | Deletion in D4Z4 region with secondary increase in DUX4 |
| Facioscapulohumeral type 2 | AD | 18p11.32 | SMCHD1 with secondary increase in DUX4 |
| Facioscapulohumeral type 3 | AD | 20q11.21 | DNMT3B (DNA methyltransferase 3B) |
| Scapuloperoneal dystrophy | AD | 2q35 | Desmin |
| AD | 14q11 | MyHC 7 | |
| XR | Xq26.3 | Four-and-a-half LIM domain 1 (FHL1) protein | |
| Emery-Dreifuss type 3 | AD | 6q24 | Nesprin-1 |
| Emery-Dreifuss type 4 | AD | 14q23 | Nesprin-2 |
| Emery-Dreifuss type 5 | AD | 3p25.1 | TMEM43 |
| Oculopharyngeal | AD | 14q11.2-13 | PABP2 |
| Myotonic dystrophy 1 | AD | 19q13.3 | DMPK |
| Myotonic dystrophy 2 | AD | 3q21 | ZNF9 |
| Myofibrillar myopathy | AD | 5q22.3-31.3 | Myotilin |
| AD | 10q22.3-23.2 | ZASP | |
| AD | 7q32.1 | Filamin-c | |
| AD | 11q21-23 | αB-crystallin | |
| AD/AR | 2q35 | Desmin | |
| AR | 1p36 | Selenoprotein N1 | |
| AD | 10q25-26 | BAG-3 | |
| AD | 2q31 | Titin | |
| Hereditary Inclusion Body Myopathies | |||
| AR hIBM | AR | GNE | |
| hIBM with FTD and Paget disease | AD | VCP | |
| hIBM 3 | AD | MyHC IIa | |
∗ LGMD 2B and Miyoshi distal dystrophy are the same condition.
For the most part, the underlying molecular abnormalities in the dystrophies involve structural proteins. Therefore it is useful to review these proteins as they occur in normal muscle. The contractile proteins, actin and myosin, are arrayed with other proteins such as troponin to form the familiar thick and thin filaments of the sarcomere. The reaction between actin and myosin results in realignment between the two molecules. In the sliding filament model, the thick and thin filaments form an array that slides back and forth.
The contractile proteins connect to the “outside” of the cell by means of a complex of proteins that ultimately links up with the basal lamina of the extracellular matrix. The first step in this connection is the protein dystrophin, located on the cytoplasmic face of the muscle membrane. This large protein (427 kD) is coded by a gene on the short arm of the X chromosome. Dystrophin consists of two ends separated by a long, flexible rod-like region. The amino terminus binds to actin, and the carboxyl terminus links dystrophin to a complex of glycoproteins in the sarcolemma. Two of these, the dystroglycans, form a direct link between dystrophin and laminin, a protein within the basal lamina. α-Dystroglycan is located extracellularly and connects to laminin. The α 2 chain of laminin (also called merosin) provides the anchor into the extracellular matrix because it provides the attachment for α-dystroglycan. β-Dystroglycan spans the sarcolemmal membrane, linking dystrophin and α-dystroglycan. Merosin also binds to α 7 β 1 D integrin, a protein complex located on the sarcolemma membrane. The sarcoglycan complex, composed of α-, β-, γ-, and δ-sarcoglycans, also spans the sarcolemmal membrane and link to the dystrophin-dystroglycan complex. Dystrophin, the sarcoglycans, the dystroglycans, and merosin appear to function as a unit in stabilizing the muscle membrane. Together these proteins make up the dystrophin-glycoprotein complex.
Other sarcolemmal proteins not directly linked to the dystrophin-glycoprotein complex are affected in other forms of muscular dystrophies (e.g., dysferlin, caveolin-3). Sarcomeric proteins (e.g., myosin, actin, tropomyosin, myotilin, Z-band alternatively spliced PDZ motif-containing protein [ZASP], filamin-c, desmin, titin, and telethonin, etc.), important in stabilizing the contractile apparatus, are mutated in certain dystrophies and congenital myopathies, highlighting the pathophysiological overlap of these historically clinically defined classifications. Mutations of the muscle-specific calcium-dependent protease, calpain-3 gene, are responsible for the majority of nondystrophin-related LGMDs in patients of Italian and Spanish ancestry. In addition, secretory enzymes (e.g., O -mannose-β-1,2- N -acetylglucosaminyl transferase, fukutin, and fukutin-related protein [FKRP]), which probably play a role in glucosylation of α-dystroglycan and other import proteins, are responsible for some forms of congenital muscular dystrophy, but may be associated with milder LGMD. Furthermore, mutations encoding for the nuclear envelope proteins, emerin, lamin A/C, and nesprin 1 and 2 cause EDMD.
An absence or deficiency of dystrophin is responsible for two disorders that cause progressive destruction of muscle. Absence of dystrophin impairs the integrity of the sarcolemmal membrane, rendering the membrane susceptible to mechanical damage. Molecules such as calcium can gain access to the fiber, initiating a chain of destructive processes ultimately leading to necrosis of the muscle fiber. Eventually, this process leads to severe loss of muscle and replacement of the muscle fibers with fibrous tissue.
The responsible gene is located on the short arm of the X chromosome at locus Xp21. The gene is extremely large, comprising more than 2.5 million base pairs and 79 exons or coding regions. Approximately 65%–75% of cases are associated with a deletion or duplication of one or more exons in the gene. Genetic testing assays designed to detect deletions and duplications should be used first, such as multiplex ligation-dependent probe amplification (MLPA) and microarray-based comparative genomic hybridization (CGH) ( ; ). “Hot spots” for these gene deletions exist, notably between exons 43 and 52 and particularly 44 and 49 ( ). The remaining 25%–35% of cases are due to point mutations (many of which introduce a premature stop codon), smaller deletions, or small insertions or duplications, which are best detected using next-generation sequencing. Whether a deletion is in frame or out of frame (see Chapter 48 ) determines whether dystrophin is absent from the muscle or present in a reduced altered form. This has clinical significance because the former is usually associated with the severe DMD, whereas the latter may cause the milder Becker variant (BMD). In BMD, the abnormal dystrophin preserves enough function to slow down the progress of the illness. Reading of the DNA code is triplet by triplet. Maintenance of this reading frame throughout the length of the gene is required for dystrophin production. If a deletion removes a multiple of three base pairs, the reading frame may be intact upstream and downstream and may make limited sense, as if the sentence “You cannot eat the cat” were changed to “You not eat the cat,” and some modified dystrophin may be formed. This is often the situation in the mild form of dystrophin deficiency (BMD). In the severe form, the reading frame is destroyed, as if a deletion resulted in the sentence “Yoc ann ote att hec at.” Exceptions to this rule exist, as frameshift deletions have been associated with the milder form of the disease, particularly at the 5′ end of the gene in exons 3–7. The prevalence of DMD in the general population is approximately 3 per 100,000, and the incidence among live-born males is 1 per 3500. BMD is approximately one-tenth as common. Although the inheritance is clearly X-linked recessive, almost a third of cases are sporadic. Presumably this is due to a spontaneous mutation occurring either in the child or in the mother’s ova.
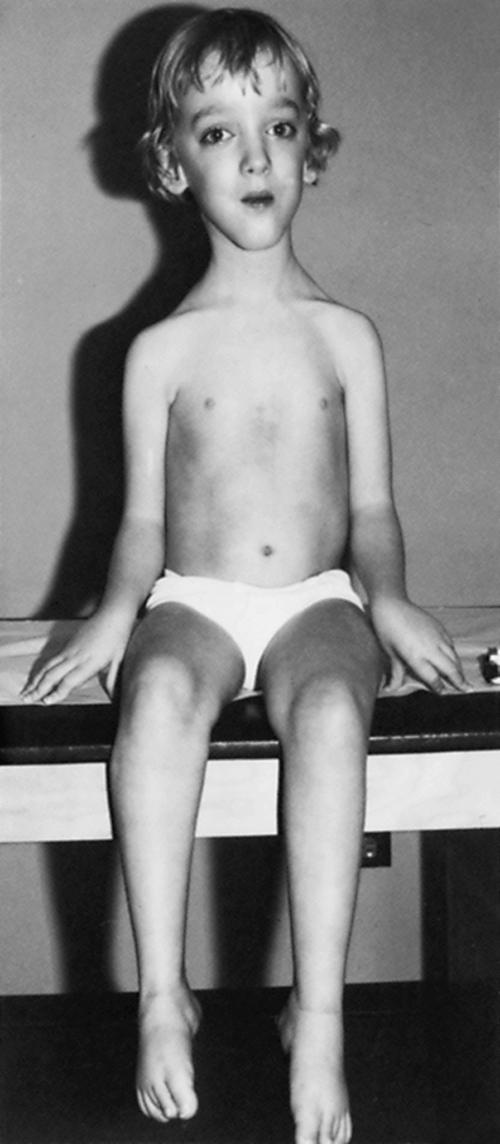
Affected children are typically normal at birth. After affected boys begin walking, however, the clumsiness seen in all toddlers persists. Progressive leg weakness, proximal greater than distal, develops through early childhood. Affected boys must place one hand on the knee to assume an upright position when rising from the floor (Gower maneuver) . Parents notice that the child runs improperly and is unable to jump clear of the floor with both feet. Toe-walking and a waddling gait are common. Often at this stage, the calf muscles are rather firm and rubbery (pseudohypertrophy) ( Fig. 109.9 ). In the absence of therapy, tightness across several joints in the legs develops. The iliotibial bands and the heel cords are usually the first to tighten. This is particularly noticeable in boys who habitually walk on their toes. Apparent improvement may occur between ages 2 and 6 as the child gains motor skills. This is illusory because it simply represents the child’s natural development, which muscle weakness has not yet outpaced.
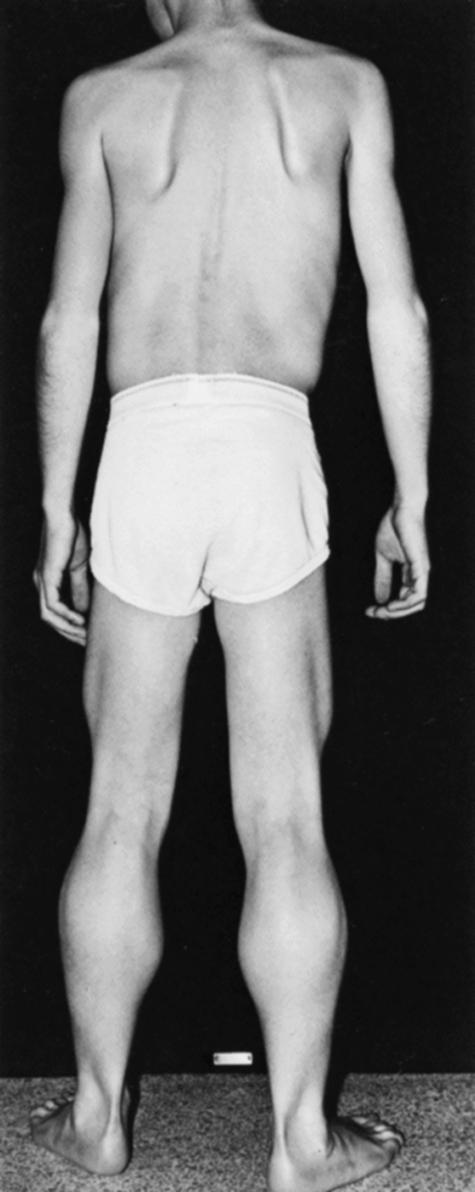
By 5 or 6 years of age, climbing upstairs becomes difficult, requiring the use of the railing. By the age of 6 or 7, the boys often complain of sudden spontaneous falls. At first, these falls occur when the child is in a hurry or knocked off balance by playmates. The fall is quite spectacular to the onlooker; the knees collapse abruptly, and the child drops like a stone to the ground. At approximately 8–10 years of age, affected children cease to be able to climb stairs or stand up from the floor, and upper limb weakness becomes more apparent. This is typically when they begin using a wheelchair for locomotion. Earlier studies suggested that the ability to walk was lost at about age 9, but with appropriate bracing, reconstructive surgery, and physiotherapy, confinement to a wheelchair is about 12 years of age. With the increasing use of corticosteroids, many affected boys maintain ambulation even beyond 12 ( ).
Contractures of the hips, knees, and ankles become severe when the relatively untreated child spends much of the day in a wheelchair. The hips and knees lock at 90 degrees, and the feet turn downward and inward in an exaggerated position of equinovarus. It is very difficult to get normal shoes to fit them, and it is impossible for them to sleep, except in one position: usually with the knees propped up with pillows and slightly turned on one side. Handling the children at this stage becomes very difficult, and back pain and limb pain almost inevitably accompany this severe stage of muscular dystrophy. Development of a severe scoliosis compromises respiratory function.
Cardiac muscle is also affected, and clinically evident cardiomyopathy develops in one-third of patients by age 14 and virtually all patients over age 18. Screening with an electrocardiogram (ECG) and either echocardiogram or cardiac Magnetic Resonance Imaging (MRI) should be performed at diagnosis, then annually while patients are asymptomatic. Co-management with a cardiologist is essential to aid in surveillance and initiation of heart failure therapies. Progressive ventilatory muscle weakness is also uniform. Respiratory screening with once-yearly measurement of forced vital capacity (FVC) should be performed annually while boys are still ambulatory. Affected boys usually die of either cardiac or respiratory complications.
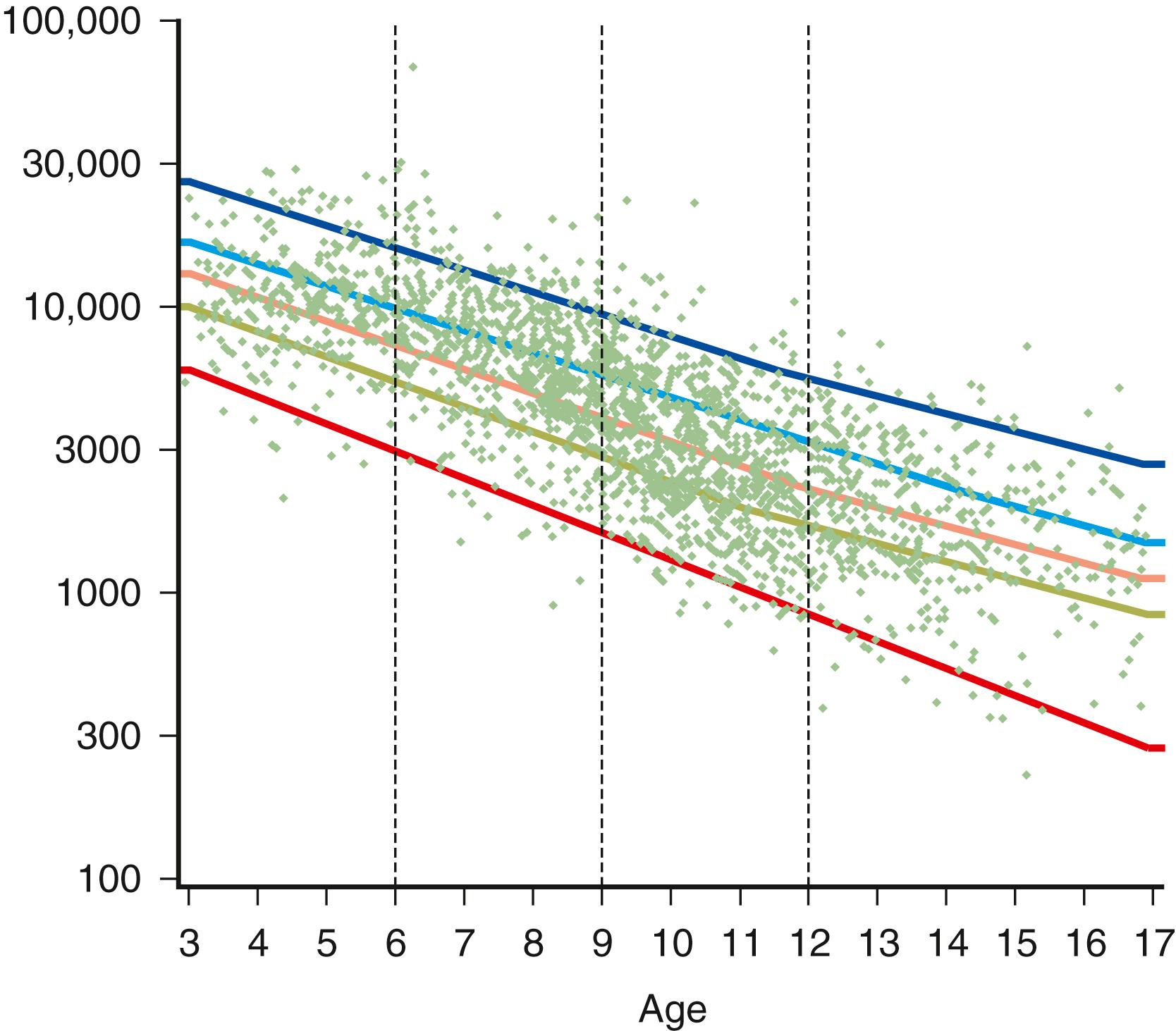
The serum concentration of creatine kinase (CK) is typically markedly elevated; levels greater than 10,000 mU/mL are common ( Fig. 109.10 ). Electromyography (EMG) shows myopathic changes (see Chapter 36 ). Genetic testing has largely replaced muscle biopsy to diagnose DMD as it is less invasive and has become more widely available, but a biopsy is essential if genetic testing is unrevealing. A diagnosis may be made when immunohistochemistry reveals reduction of dystrophin on the sarcolemma or when Western blot demonstrates absent or marked reduced quantity and size of dystrophin. Three antibodies are available against the ends (Dys-2 for the carboxyl terminus and Dys-3 for the amino terminus) and the rod region (Dys-1) of the molecule. In DMD, immunostaining is absent or the stain is irregular and fragmented. Absence of the amino terminus, the end that binds with actin, appears to be associated with more severe symptoms. Variation in myofiber size, fibrosis, groups of basophilic fibers, and opaque or hypercontracted fibers (hyaline fibers) are typically seen ( Figs. 109.11 and 109.12 ).
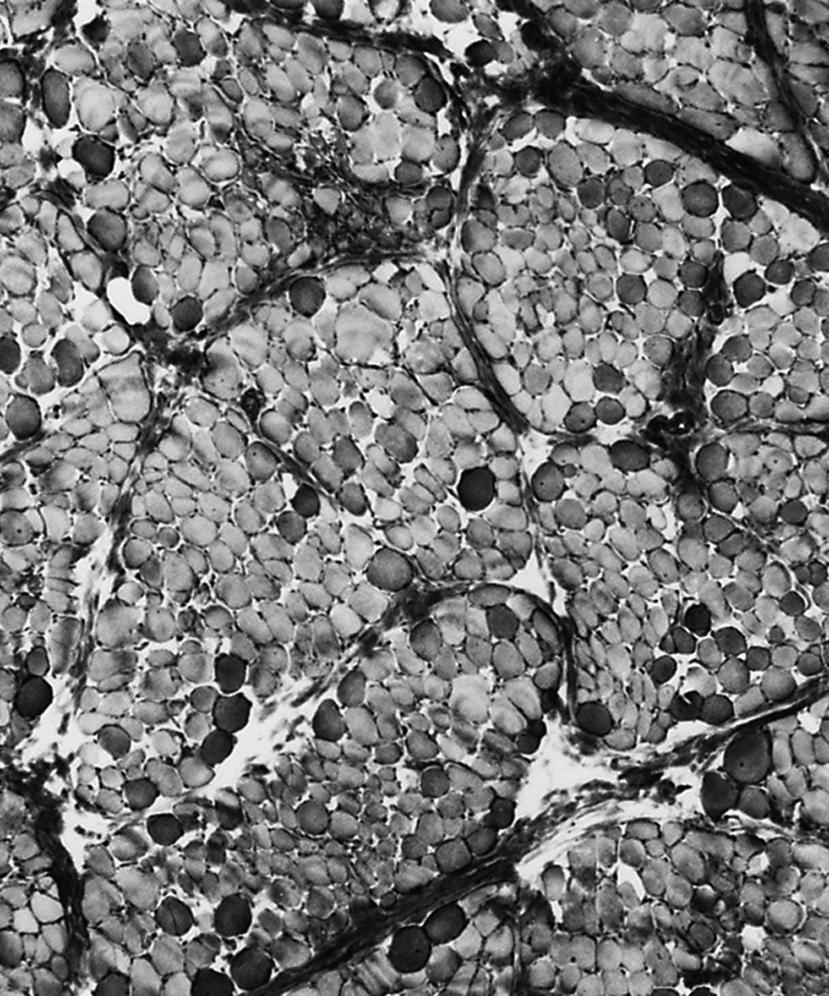
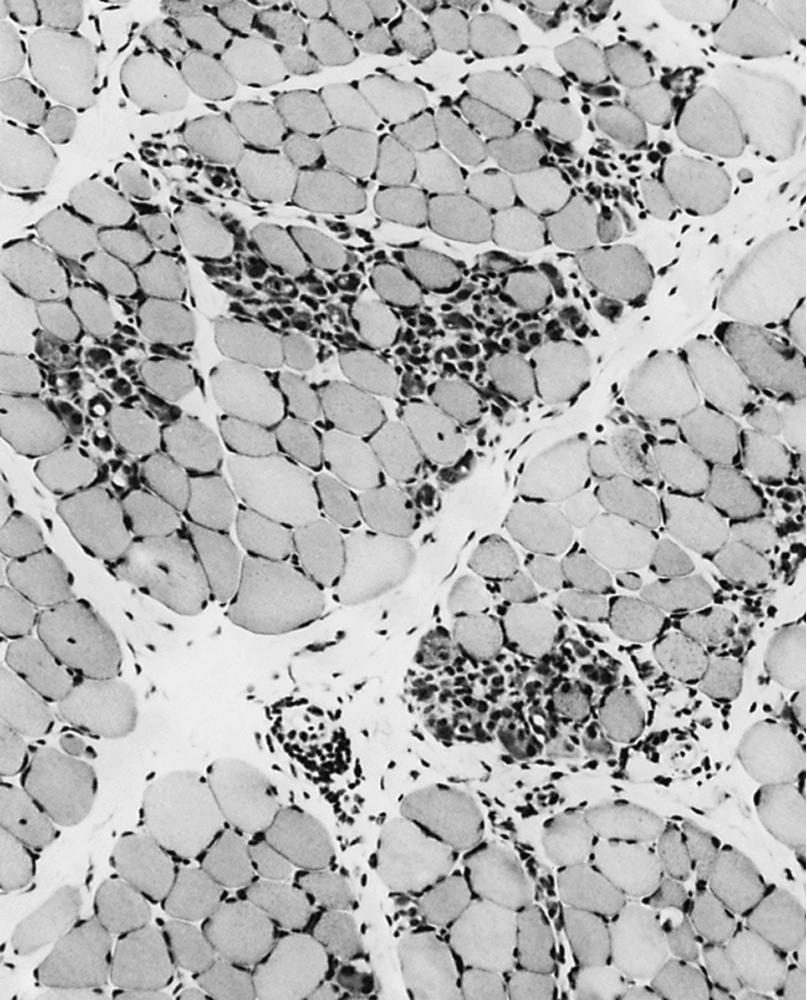
The primary aim of physical therapy is to keep the joints as loose as possible, avoiding contractures. Early on, the iliotibial bands and the heel cords give the greatest problems. Late in the course, elbow, wrist, and finger contractures add to functional disability. Physical therapy generally commences at 3–4 years of age, when parents learn to stretch the child’s heel cords, hip flexors, and iliotibial bands daily. Passive stretching of joints is directed not at increasing the range of motion but rather at preventing any further development of contractures. This requires careful explanation to parents because they can be disheartened to see no improvement in the tightness even after many months of therapy. Night splints molded around the lower part of the legs to maintain the feet at right angles to the legs should be used starting at an early age. Ankle contractures rarely occur in patients who use these splints conscientiously. Unfortunately, some patients, particularly those 6 or 7 years or older, cannot tolerate the splints. Parents often ask about an active exercise program; such a program is largely unnecessary in a young child who runs around to the best of his ability anyway. By the time a child is having difficulty walking or is in a wheelchair, muscle weakness is severe, and exercise does not increase muscle strength.
The appropriate use of bracing may delay the child’s progression to a wheelchair by approximately 2 years. A major factor responsible for inability to stand or walk is weakness of the quadriceps. Such weakness causes the knee to collapse when even slightly flexed; the only stable position is in hyperextension. The addition of a long-leg brace (knee-foot orthosis) can help solve this problem. Such a device stabilizes the knee and prevents the knee from flexing. The children walk stiff legged but do not have the same problem with falling they had previously. Generally, children are ready for bracing when they have ceased to climb stairs, are having great difficulty arising from the floor, and are having frequent daily falls. On examination, inability to straighten the knee against gravity is also an indication for bracing. Because the brace functions as a pendulum, slight elevation of the hip is sufficient to bring the leg forward so the weight of the brace is rarely a problem. There may be some advantage to a lightweight plastic knee-foot orthosis, but it may be more difficult to keep the leg straight with such a device.
Reconstructive surgery of the leg often accompanies bracing. The purpose of leg surgery is to keep the leg extended and prevent contractures of the iliotibial bands and hip flexors. Contractures of the iliotibial bands are associated with a stance in which the boy’s legs are widely abducted. As noted before, the long-leg brace acts like a pendulum. If the leg is widely abducted, the child cannot swing the leg forward. Lifting the hip causes the leg to try to swing inward toward the midline, but resistance from the iliotibial band contractures renders this impossible. A simple way to maintain function in the leg is to perform percutaneous tenotomies of the iliotibial bands, knee flexors, hip flexors, and Achilles tendons. This procedure often allows a child who is becoming increasingly dependent on a wheelchair to resume walking with the aid of bracing.
Spinal stabilization is also an important option for DMD patients. Because of the extreme discomfort of severe scoliosis and the respiratory problems associated with it, spinal surgery is an acceptable procedure for managing the late stages of the disease. It is considered in patients with 35 degrees or more of scoliosis and significant discomfort. To reduce the risks associated with surgery, FVC ideally should be greater than 35% of predicted.
Accumulating evidence suggests that corticosteroids not only improve muscle strength and pulmonary function but also delay time to disease progression milestones such as loss of ambulation ( ; ). There is a possible but less proven beneficial effect for the associated scoliosis and cardiomyopathy, as well. Prednisone 0.75 mg/kg/daily CTD is typically initiated by the age of 5—after motor function plateaus and before substantial decline has begun; 10 mg/kg/weekend CTD is an alternative for those with side effects. The synthetic steroid deflazacort has a similar therapeutic effect and may be associated with less weight gain ( ). The usual dosing is 0.9 mg/kg/daily CTD.
Improved cardiorespiratory intervention has extended median survival dramatically from the late teens into the late 20s and early 30s ( ). Consensus recommendations suggest initiating an angiotensin-converting enzyme inhibitor or angiotensin receptor blocker by the age of 10. Additional treatments such as beta-blockers are used for symptomatic heart failure. Assisted ventilatory support is typically required by the age of 18.
Therapies currently in development aim to circumvent deficient dystrophin protein production. Eteplirsen is a phosphorodiamidate morpholino oligomer that binds to dystrophin gene pre-mRNA and induces exon 51 skipping ( ). Skipping this exon restores the open reading frame that is disrupted by certain dystrophin mutations. Although this actually increases the size of the deletion, it preserves translatable mRNA after the deletion, leading to production of a dystrophin protein more similar to what is seen in BMD mutations rather than DMD mutations. Thus the treatment is not curative but instead aims to convert DMD into a BMD phenotype. Approximately 13% of DMD boys are amenable to exon 51 skipping, based on which specific mutation they harbor. Eteplirsen was granted accelerated approval by the US Food and Drug Administration (FDA) in 2016 based primarily on small trials demonstrating increased dystrophin protein production in the muscle on biopsy specimens. The FDA mandated an additional clinical trial to ensure the efficacy of the drug, which is ongoing. Eteplirsen is not yet approved in Europe. Additional exon skipping therapies are in development. Ataluren, by contrast, was recently approved by the European Commission but is not yet available in the United States. Despite promising early trials, a phase III placebo-controlled trial showed no benefit for its primary endpoint, change in the 6-minute walk test ( ). It targets the estimated 11% of boys with a nonsense mutation, promoting ribosomal read-through of the stop codon.
BMD shares the clinical characteristics of DMD but has a milder course ( ). The disease usually begins in the first decade, although parents may notice the first signs of weakness later because of the milder symptoms. Occasionally, symptom onset is delayed until the fourth decade or later. The muscular hypertrophy, contractures, and pattern of weakness are similar to those seen in DMD. These boys, however, continue to walk independently past the age of 15 years and may not use a wheelchair until they are in their 20s or even later ( ). Teenagers with BMD often complain of leg cramps and muscle pain, often associated with exercise and often more severe than in DMD. A significant proportion of these patients have a cardiomyopathy that can be more disabling than the weakness. Cardiac transplantation has been successful in some patients with this form of the illness.
As with DMD, serum CK levels are elevated but typically not as high. EMG demonstrates myopathic features. Diagnosis requires demonstration of a mutation in the dystrophin gene or reduced quantity or size of dystrophin on muscle biopsy. In BMD, staining may be reduced but can be normal appearing. Therefore immunoblotting is required to show a decreased amount of dystrophin.
Because boys do not have much trouble in the first few years, there is a reduced need for aggressive physiotherapy, surgical reconstruction, and night splints. Patients with BMD are less prone to develop kyphoscoliosis, perhaps because they lack wheelchair confinement until after the spine has become fully mature. We have used corticosteroids only occasionally in patients with BMD. The stabilizing effect of steroids is less noticeable when the disease is already slowly progressive. In every other respect, including bracing and genetic counseling, disease treatment is the same as that for the severe form.
With the development of genetic testing and dystrophin analysis, it is becoming clear that dystrophin deficiency is not always associated with the BMD or DMD phenotype. Some patients have only symptoms of exercise intolerance, muscle pain, and myoglobinuria ( ). Others manifest only a cardiomyopathy or are asymptomatic despite elevated serum concentrations of CK. Most female carriers of dystrophin gene mutations are asymptomatic, but approximately 8% manifest weakness and have a clinical phenotype similar to BMD or LGMD. Manifesting carriers will usually have an elevated serum CK level and myopathic EMG. Muscle biopsy will usually demonstrate a mosaic pattern or patchy staining of dystrophin on the sarcolemma.
It is impractical to perform genetic testing on all patients with neuromuscular complaints. However, the presence of muscle pain, mild weakness, an elevated serum CK, and muscle hypertrophy warrants consideration of analyzing dystrophin. Attempts to correlate the genetic abnormality with the clinical picture are inexact, but abnormalities in the amino terminus and at the carboxyl terminal domains of dystrophin are associated with the more severe form of disease. Alterations in the rod domain are more variable and may be associated with a mild phenotype. In-frame deletions and insertions are associated with a much milder phenotype than out-of-frame alterations.
Because DMD and BMD are X-linked recessive disorders, we recommend testing the carrier state of all women related to an affected person by maternal linkage. Routine laboratory testing (i.e., CK elevation) and muscle biopsy are insensitive, so genetic testing is required. Up to 30% of cases may be sporadic and due to new mutations or deletions. In the experience of many clinicians, an even higher percentage of new patients arriving in the clinic are sporadic cases, perhaps because genetic counseling is widely available and the women who carry the abnormal gene decide not to have children.
Genetic analysis of all potential carriers is advisable. In a family in which the disease is associated with a deletion, there is little problem in determining whether the woman is carrying the affected X chromosome, using techniques that are presently available. Genetics laboratories can identify the presence of a mutant gene over the background contributed by the normal allele through analysis of the gene “dosage.” Two normal alleles that have a double dose are compared against a deleted allele and a normal allele that have a single dose ( ). Unfortunately, situations exist in which a mutation is identified in a boy with “sporadic” DMD but not in the mother, and yet the mother is still a carrier. This occurrence is secondary to germline mosaicism in which the mutation in the mother lies only in a percentage of her oocytes. The estimated recurrence rate of DMD is as high as 14% even in such cases. If desired, prenatal diagnosis using amniotic cells or chorionic villus biopsies can identify whether or not the fetus is affected.
There is a broad diversity in the clinical presentations of patients with forms of LGMD ( ). The traditional diagnosis and classification of LGMDs was accordingly challenging. Many patients present predominantly with proximal weakness, but in others the pattern of weakness is more disparate. In some, hip weakness is greater than shoulder weakness, others the reverse. Some cases are dominantly inherited, others recessively. Onset may be late in life with mild symptoms, but in others severe and early in life.
Beginning with the discovery that a defect in one of the sarcoglycans caused a severe form of LGMD occurring in North Africa, the delineation of several other entities characterized by defects in structural proteins or enzymes followed. These include the sarcoglycans, the α2-chain of laminin (merosin), calcium-activated protease, calpain-3, and others. Those forms with autosomal dominant inheritance have been designated type 1 , and those with autosomal recessive inheritance type 2 . Subclassification with an alphabetical letter has traditionally characterized distinct genetic forms of LGMD1 and LGMD2 (see Table 109.1 ; ). As more and more novel disease genes have been identified, however, this classification scheme has run out of letters for LGMD2. An updated classification schema was recently proposed which replaces 1 and 2 with A and R and the alphabetical letter with a number designating order of gene discovery as well as the gene itself. As an example, LGMD2A would be reclassified as LGMD R1 calpain-3–related ( ). In this text, we will refer to both the traditional and newly proposed nomenclature.
Certain forms of LGMD have been shown to be associated with respiratory muscle weakness and/or cardiomyopathy, so identification of a specific gene can be very helpful in guiding management and informing prognosis. For some culprit genes, certain mutations have been shown to result in a LGMD phenotype while others result in other phenotypes historically classified as distinct from LGMD, such as forms of distal myopathy or congenital muscular dystrophy, autosomal dominant EDMD, exercise intolerance, or even asymptomatic hyperCKemia. Adding to the confusion, a limb–girdle pattern of weakness can also be seen in disorders not strictly classified as a form of LGMD. The following sections outline the known genetic abnormalities and then comment on the more amorphous forms of LGMD. The prevalence of these diseases as a group ranges from 1 to 2.27/100,000 ( ; ). The autosomal recessive LGMDs are more common than the autosomal dominant LGMDs. Although there is heterogeneity between patients and specific disorders, as a general rule the autosomal recessive LGMDs are also more severe.
Patients with LGMD1A can present with proximal arm and leg weakness in their teens to late adult life or with distal leg weakness (e.g., foot drop) later in life. Some patients have early pharyngeal weakness as well ( ). Serum CK concentrations can be normal or moderately elevated. Muscle biopsies may demonstrate rimmed vacuoles within muscle fibers and features of myofibrillar myopathy (MFM).
LGMD1A is allelic to one subtype of MFM and is caused by mutations in the myotilin gene located on chromosome 5q22.3-31.3 ( ). Myotilin is a sarcomeric protein that is present at the Z-disk. The protein is likely important in myofibrillogenesis and stabilization of the Z-disk and sarcomere.
This myopathy is as also known as autosomal dominant EDMD ( ; ; ). Some patients manifest with a limb–girdle pattern of weakness, while others present with a humeral-peroneal weakness. Cardiomyopathy with severe conduction defects and arrhythmias may also occur, with or without skeletal muscle involvement. Sudden death secondary to fatal arrhythmias is common, so early diagnosis is desirable and pacemaker insertion and/or implantable cardiac defibrillator (ICD) is often necessary ( ). Early elbow and ankle contractures as well as spine rigidity are features seen in some patients, but not universal. Serum CK levels may be normal or elevated up to 25-fold. Muscle biopsies demonstrate dystrophic features with the rare occurrence of rimmed vacuoles.
LGMD1B is localized to mutations in the lamin A/C gene located on chromosome 1q11-21 ( ; ). Alternative splicing of the lamin A/C messenger (m)RNA transcript produces lamins A and C. Lamin A/C is an intermediate-size filament located on the nucleoplasmic surface of the inner nuclear membrane, where it interacts with various lamin-associated proteins including emerin, the abnormal protein associated with X-linked EDMD. Lamin A/C may also bind to heterochromatin. Immunostaining of the nuclear membrane with anti-emerin antibodies is normal, helping distinguish this myopathy from X-linked EDMD. Electron microscopy reveals alterations in myonuclei, including the loss of peripheral heterochromatin, altered interchromatin texture, and fewer than normal nuclear pores ( ).
This rare myopathy usually presents in childhood with proximal leg weakness greater than arm weakness and exertional myalgias ( ; ; ). Progression of weakness is variable. The clinical phenotype associated with caveolin-3 mutations is quite heterogeneous. Some patients manifest with mainly distal weakness, involving thenar, hypothenar, and intrinsic hand muscles predominantly. Others have familial hypertrophic cardiomyopathy or autosomal dominant rippling muscle disease ( ; ). Serum CK levels are increased 3–25 times normal. In fact, some patients manifest with asymptomatic hyperCKemia.
LGMD1C is caused by mutations in the gene encoding for caveolin-3 on chromosome 3p25 ( ; ). Caveolins are scaffolding proteins that interact with lipids and other proteins in caveoli , which are flask-shaped invaginations of the sarcolemmal membrane. Immunostaining of muscle biopsies demonstrates a reduction of caveolin-3 along the sarcolemma. Electron microscopy EM reveals a decreased density of caveoli on the muscle membrane as well.
This recently described myopathy presents in the second to sixth decade with proximal muscle or distal weakness greater in the lower extremities ( ; ; ; ). Cardiorespiratory involvement was notably absent. Serum CK levels are typically slightly elevated. Muscle biopsy reveal rimmed vacuoles and features suggestive of a MFM.
This is described in the section on MFM ( ).
This recently described myopathy presents in the first to sixth decade with proximal greater than distal weakness, legs more than arms ( ). Anticipation may be seen, with increasing severity in subsequent generations. CK level is normal to moderately elevated. Muscle biopsy can show rimmed vacuoles and features of MFM. It is caused by mutation in the transportin 3 ( TNP03 ) gene, which encodes a nuclear import receptor for precursor-mRNA splicing factors.
This myopathy was initially described in two kindreds, one Brazilian and one Uruguayan ( ). Onset of weakness is in the second to sixth decade, with proximal lower limb weakness usually preceding upper limb weakness. Finger flexion and toe flexion weakness are typical, and early-onset cataracts may occur. It is caused by mutations in the HNRPDL gene, which encodes a ribonucleoprotein important in mRNA biogenesis and metabolism, mediating alternative splicing.
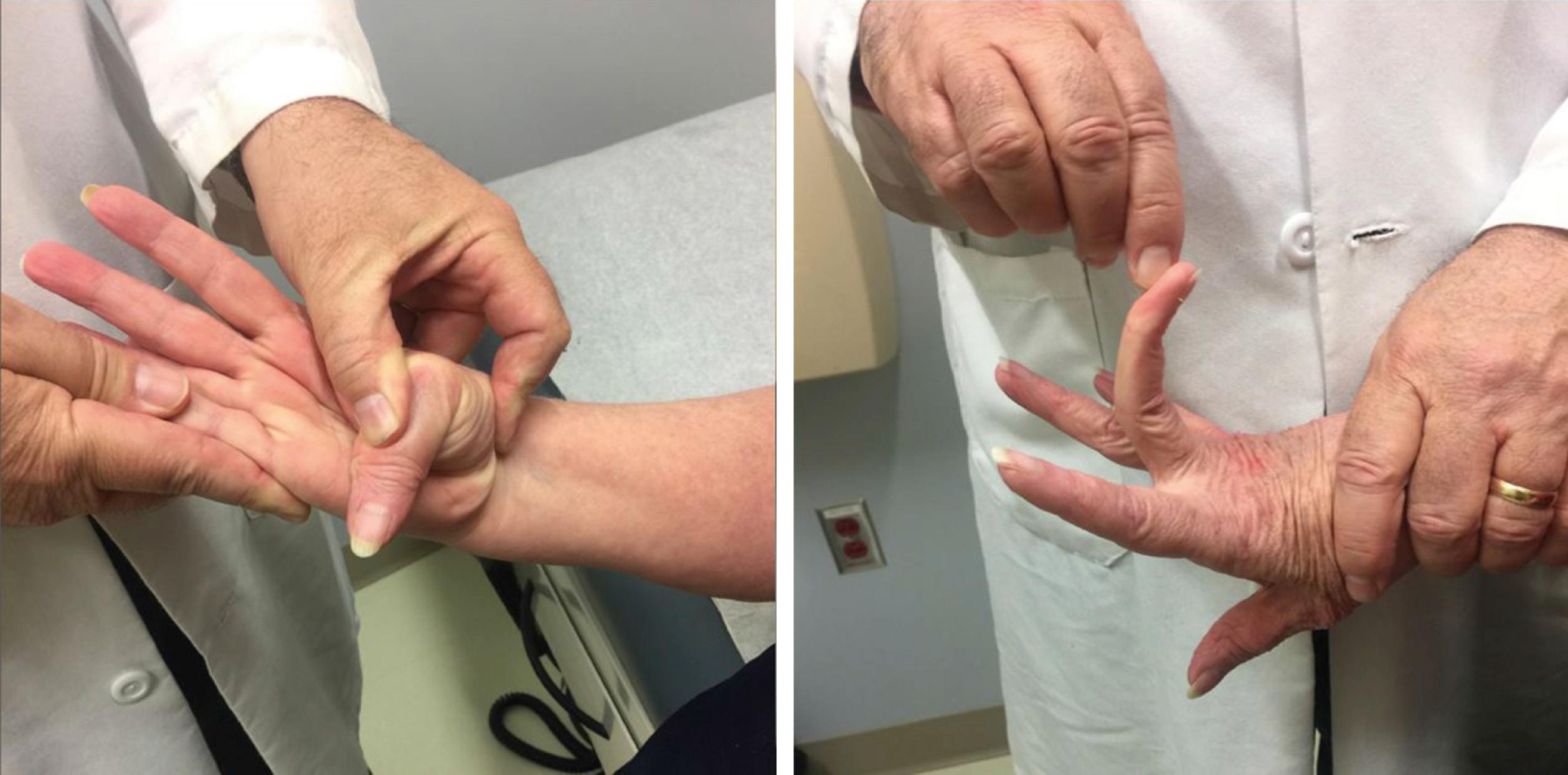
Bethlem myopathy is allelic to the more severe Ullrich congenital muscular dystrophy (UCMD, see below) ( ; ). Both are due to mutations encoding collagen VI (COL6A1, COL6A2, COL6A3). Onset is typically by early childhood. Joint laxity, especially distally, is common early in this disorder ( Fig. 109.13 ). Contractures are also very common, especially at the elbows and ankles, and can lead to confusion with EDMD. Contractures can involve the wrists and fingers over time. Restrictive ventilatory deficits can be seen, presumably from a combination of weakness and contractures. Some patients, however, have a limb–girdle pattern of weakness without any contractures.
LGMD2A was first reported in an inbred population on Reunion Island in the Indian Ocean ( ). It is caused by mutations in the gene for muscle-specific calcium-activated neutral protease ( CANP-3 or calpain-3) ( ). Since the initial description, the disorder has shown a worldwide distribution and is the most common LGMD ( ; ). The underlying pathophysiology of the illness is uncertain. CANP-3 is not a structural protein but an enzyme. It has been suggested that the enzyme has a regulatory role in the modulation and control of transcription factors and thus of gene expression. CANP-3 also binds to titin and can cleave filamin-C; thus it may have a role in stabilizing the sarcomere. Although most commonly autosomal recessive inheritance is seen, autosomal dominant inheritance has recently been reported in a series of 37 patients with a single 21-base-pair, in-frame deletion. These patients had a similar phenotype to traditional LGMD2A, but with milder weakness ( ).
The disease is progressive and typically begins in early adolescence but can begin in early or middle adulthood for some. Most cases have been mild to moderately progressive, with loss of ambulation in adult life. Severe forms occur. Weakness occurs in the hips first and then in the shoulders. Facial strength is preserved, and the neck flexors and extensors are strong. Scapular winging occurs, different from that seen in FSHD, with the whole of the medial scapular border jutting backward. Posterior thigh muscles and adductors are more severely affected than the knee extensors. The rectus abdominis muscles are affected early which can lead to abdominal hernias. Early contractures may develop. Mild to moderate respiratory muscle weakness can develop, commensurate with the severity of skeletal muscle weakness, but cardiac muscle is spared. The serum concentration of CK is markedly elevated early in the course and then decreases to normal concentrations later in the illness. Muscle biopsies may demonstrate endomysial inflammatory cell infiltrate with prominent eosinophils that may lead to misdiagnosis as eosinophilic myositis ( ; ).
Mutations in the gene encoding for dysferlin, located on chromosome 2p13, lead to clinically heterogeneous myopathies. Some patients show a limb–girdle pattern of weakness (LGMD2B), while others present with weakness and atrophy of the calf muscles (Miyoshi myopathy) ( ). In both cases, early preferential involvement of the gastrocnemius and thigh adductors is common ( ). Dysferlinopathies account for only about 1% of LGMDs but about 60% of distal myopathies ( ). Looking at this another way, 80% of patients with dysferlinopathy manifest a distal myopathy, 8% have an LGMD pattern of weakness, and 6% have asymptomatic elevation of serum CK. Less common presentations include progressive foot drop, axial muscle weakness with bent spine syndrome/camptocormia, and rigid spine syndrome ( ; ; ; ). The dysferlinopathies typically present in adolescence or early adult life. Progression is usually slow, but some patients lose ambulation in their 20s while others can walk late in life. Interestingly, intrafamilial variability exists in the pattern of weakness and disease progression.
Serum CK concentrations are markedly elevated, usually 35–200 times normal. Muscle biopsies demonstrate dystrophic features in severely affected muscles but nonspecific myopathic features in less affected muscles. Occasionally a striking endomysial or perivascular inflammatory process is appreciated, leading to an incorrect diagnosis of PM. However, unlike PM, the inflammatory cells usually do not invade non-necrotic muscle fibers. Amyloid deposition may also be evident in some cases ( ). Immunostaining and immunoblot confirm the diagnosis. Dysferlin localizes to the sarcolemmal membrane but does not directly interact with dystrophin or the sarcoglycans. Dysferlin is thought important in membrane repair ( ; ; ).
Four known sarcoglycans expressed in muscle are associated with different forms of autosomal recessive LGMD2. The genetic abnormality underlying LGMD2C, 2D, 2E, and 2F are mutations in the genes for γ-sarcoglycan, α-sarcoglycan, β-sarcoglycan, and δ-sarcoglycan, respectively. The sarcoglycans are a tightly knit family, and when one is absent, the others may also be missing. This is particularly true of α-sarcoglycan, making it both a useful screening tool and a misleading one on occasion. An absence of α-sarcoglycan is an indication to search for the abnormal gene, be it an α-, β-, γ-, or δ-sarcoglycanopathy.
Sarcoglycanopathies may account for more than 10% of patients with a limb–girdle pattern and normal dystrophin ( ; ). Of these, α-sarcoglycanopathies (LGMD2D) are most common, accounting for approximately 6% of cases. The most common type varies considerably between geographic areas. Sarcoglycanopathies may account for up to 50% of patients with muscular dystrophy in North Africa.
The four sarcoglycanopathies are very similar clinically and may be confused with DMD; the lack of cognitive impairment can help distinguish. Childhood-onset is common, with trunk and proximal leg weakness and a serum CK concentration of 1000 units and higher ( ). Facial weakness is absent, but scapular winging and calf hypertrophy occur. Weakness is progressive and many patients require a wheelchair. Many patients experience respiratory muscle weakness and assisted ventilation may be required ( ). Cardiac dysfunction occurs in a minority of affected patients, more commonly in those with LGMD2C and 2E.
Patients with this dystrophy may have either proximal or distal weakness ( ; ). Mean age of onset is approximately 12.5 years. Legs are affected more than arms; the quadriceps and anterior tibial muscles are affected early. Some may have a cardiomyopathy. Serum CK levels are 3–17 times normal. Muscle pathology shows dystrophic features in addition to the frequent occurrence of rimmed vacuoles within muscle fibers.
LGMD2G links to mutations in the gene that encodes for telethonin on chromosome 17q11-12 ( ). Telethonin is one of the most abundant muscle proteins, where it localizes to the sarcomere. Telethonin may interact with the large sarcomeric proteins, titin and myosin. Abnormal telethonin may disrupt normal myofibrillogenesis.
This LGMD was initially reported in families of Manitoba Hutterite origin ( ) and is allelic with sarcotubular myopathy. Most affected individuals have a mild limb–girdle pattern of weakness, with onset from birth to the seventh decade ( ). Patients may note exertional myalgias; scapular winging and facial weakness may be variably noted. Serum CKs range from 250 to over 3000 IU/L, and EMGs reveal myopathic features. Muscle biopsy features demonstrate small vacuoles that represent focal dilations of the sarcoplasmic reticulum. This myopathy is caused by mutation in the gene that encodes for E3-ubiquitin ligase (also known as TRIM 32 ) ( ). This ligase may be important for ubiquinating proteins targeted for destruction by the proteasomes ( ). TRIM32 mutations may lead to dysregulation of myofibrillar protein turnover.
This dystrophy was initially reported in a large consanguineous Tunisian family ( ). Subsequently the dystrophy has shown a worldwide distribution and is the most common type of LGMD in patients of northern European ancestry ( ). The clinical phenotype is variable, with age of onset from the first to the sixth decade. The course resembles a dystrophinopathy in some, including calf hypertrophy ( ). Difficulty running, difficulty climbing stairs, and cramps/myalgias are all common presenting symptoms. A severe cardiomyopathy can develop, and respiratory muscle weakness occurs as the disease progresses. Serum CKs are elevated 10–30 times normal in some affected younger patients but are normal in some older individuals ( , ; ; ).
LGMD2I is caused by mutations in the gene that encodes for FKRP, a glycosyltransferase, and its deficiency is associated with abnormal glycosylation of α-dystroglycan ( ). It is unique among secondary α-dystroglycanopathies in that it more commonly causes an adult-onset LGMD rather than a form of congenital muscular dystrophy, but mutations in FKRP do also cause congenital muscular dystrophy with normal merosin (MDC1C).
TTN is an enormous gene, producing the largest protein in humans. Titin is a protein that spans the entire sarcomere and serves as a ligand for calpain-3. Numerous clinical phenotypes have been described in relation to mutations throughout the gene, with varying forms of skeletal muscle involvement and/or cardiomyopathy. Autosomal dominant disorders include hereditary myopathy with early respiratory failure (HMERF), Udd distal myopathy (discussed in the Distal Myopathies section), and isolated dilated cardiomyopathy; autosomal recessive disorders include LGMD2J, adult-onset distal myopathy, proximal adult-onset rimmed vacuolar myopathy, and a congenital titinopathy (discussed in the Congenital Myopathies section) ( ; ; ). Because of its massive size, it is common to uncover novel or rare variants of uncertain significance (VUS) in the TTN gene when genetic testing is pursued; many of these will not be related to the patient’s condition. By converse, pathogenic mutations can also be missed due to the gene’s large size and complex structure.
LGMD2J is characterized by childhood-onset weakness that is more severe in the proximal muscles of the arms and legs, with distal weakness often present but mild ( ). Some patients develop a cardiomyopathy. CKs are usually mildly elevated. Muscle biopsies reveal dystrophic features, and rimmed vacuoles are usually absent or rare. As the name implies, proximal adult-onset rimmed vacuolar myopathy includes rimmed vacuoles on biopsy, with a unique pattern of weakness affecting the quadriceps and soleus but sparing the tibialis anterior.
HMERF resembles Udd distal myopathy; it is autosomal dominant and associated with progressive foot drop ( ; ; ; ). However, it tends to affect patients earlier in adulthood, may also affect the proximal muscles (legs greater than arms), and is associated with early respiratory failure. Muscle biopsies reveal rimmed vacuoles and features of MFM.
This disorder was initially described in 14 French Canadian patients from eight different families, but has since been shown to be among the most common LGMDs in Northern Europe ( ). Subsequently, patients have been reported with a Miyoshi myopathy-like phenotype with involvement of the calves in the second decade of life, exertional myalgias, hyperCKemia, or calf hypertrophy ( ; ; ; ). Females are more likely than males to have mild phenotypes without weakness ( ). Symptoms may begin from age 20 to 70. Those with the LGMD phenotype usually have associated quadriceps atrophy and myalgias, and weakness may be asymmetric. There is usually no cardiac or respiratory muscle involvement. Serum CK concentrations can be markedly increased. Muscle biopsies demonstrate nonspecific dystrophic features with increased endomysial connective tissue associated with basal lamina duplication and collagen disorganization infiltration. The disorder is caused by mutations in the ANO5 gene that encodes for anoctamin 5, a transmembrane protein that may be a calcium-activated chloride channel.
These are all secondary α -dystroglycanopathies , which usually present in infancy or early childhood as congenital muscular dystrophies (discussed in detail in the Congenital Muscular Dystrophy section) ( ). Rarely they present later in childhood or early adult life with a limb–girdle pattern of weakness with or without CNS involvement ( ).
LGMD2K, or LGMD R11 POMT1-related, is caused by mutations in protein O -mannosyltransferase 1 (POMT1). This is usually associated with Walker-Warburg syndrome, but can also cause childhood onset of a milder LGMD phenotype associated with severe cognitive impairment ( ).
LGMD2M, or LGMD R13 fukutin-related, is caused by mutations in the gene that encodes for fukutin, which usually causes Fukuyama congenital muscular dystrophy (FCMD). However, mutations in the fukutin gene have also been associated with a milder adult-onset myopathy (LGMD2M), particularly outside of Japan ( ; ). Affected individuals can have normal intelligence and brain structure, have a mild limb–girdle weakness, present with only a cardiomyopathy, or have asymptomatic elevation of serum CK concentrations. Some patients have remarkable steroid responsiveness ( ).
LGMD2N, or LGMD R13 POMT2-related, is caused by mutations in the gene encoding protein O -mannosyltransferase 2 (POMT2). Mutations in this gene can also cause Walker-Warburg syndrome and rarely are associated with a milder limb–girdle syndrome affecting hamstrings, gluteal, and paraspinal muscles with universal cognitive impairment ( ).
LGMD2O, or LGMD R15 POMGnT1-related, is caused by mutations in POMGnT1 , which encodes protein O -mannose-β-1,2- N -acetylglucosaminyl transferase. Mutations in this enzyme also cause muscle-eye-brain (MEB) disease. Mutations in POMGnT1 can be associated with milder allelic variants of muscular dystrophy and normal intelligence (LGMD2N) ( ; ).
LGMD2T, or LGMD R19 GMPPB-related, is caused by mutations in GMPPB , encoding guanosine diphosphate mannose pyrophosphorylase B. Those with a LGMD phenotype present from very early childhood to the fourth decade; cognitive impairment, seizures, and cataracts are common with early-onset cases ( ; ).
LGMD2U, or LGMD R20 ISPD-related, is caused by mutations in a gene encoding isoprenoid synthetase domain-containing protein ( ISPD ), more commonly associated with Walker-Warburg syndrome. A childhood-onset LGMD phenotype with normal intelligence has been recently reported ( ).
This is a newly reported rare dystrophy that has presented with onset in the first decade, severe cognitive impairment, and with reduced α-dystroglycan expression on muscle biopsy ( ; ; ). This dystrophy caused by a mutation in the gene encoding for α-dystroglycan.
LGMD2Q has also been considered a form of congenital myasthenia and is associated with epidermolysis bullosa, in which patients develop blistering of the skin and mucous membranes, typically in infancy or early childhood ( ). Affected individuals may present with congenital hypotonia or slowly progressive, proximal weakness in late childhood or adulthood. Ptosis and ophthalmoplegia may be evident. CK levels are usually elevated.
Unlike most cases of primary desminopathy, this recently reported LGMD is inherited in an autosomal recessive as opposed to autosomal dominant fashion ( ; ). It may present in early childhood or adulthood with slowly progressive, predominantly proximal muscle fatigue and weakness and ventilatory failure. CK levels are mildly elevated. Unlike the more common autosomal dominant primary desminopathies (e.g., LGMD1E), biopsies in the reported cases did not reveal features of MFM.
This is another recently reported LGMD in Syrians and Hutterites ( ) that is characterized by an infantile onset of choreiform, athetoid or dystonic movements, seizures, truncal ataxia, and mental intellectual disability. Proximal weakness is apparent in childhood along with scoliosis and hip dysplasia; CK is mild to moderately elevated. It is caused by mutations in the transport (trafficking) protein particle complex, subunit 11 ( TRAPPC11 ), which is important in trafficking proteins between endoplasmic reticulum and the Golgi complex.
LGMD2W, attributed to a mutation in LIMS2 (also called PINCH2), was recently reported in two Northern European siblings, both of whom developed proximal weakness, calf hypertrophy, and an enlarged triangular tongue in early childhood and later developed cardiomyopathy ( ). LGMD2X, attributed to a mutation in BVES , was reported in three members of an Albanian family. Those affected had second-degree heart block and an elevated CK, with the grandfather developing proximal muscle weakness in his 40s ( ). LGMD2Y, attributed to a mutation in TOR1AIP1 , was recently reported in three patients from a consanguineous Turkish family. Weakness began in the first or second decade, along with joint contractures and rigid spine ( ). LGMD2Z, or LGMD R21, is attributed to mutation in POGLUT1 and was recently reported in four patients from a consanguineous Spanish family. Proximal leg weakness began in the third decade, progressing to include upper limb weakness and loss of ambulation ( ).
MFM likely results from disruption of the Z-disc. The characteristic pathological finding in MFM is myofibrillary disruption on EM and excessive sarcoplasmic accumulation of desmin and other proteins on immunostains ( ; ; ; , ; ). Desmin is a cytoskeletal protein linking the Z-disc to the sarcolemma and nucleus. This myopathy has been reported as desmin storage myopathy, desmin myopathy, familial desminopathy, spheroid body myopathy, cytoplasmic body myopathy, Mallory body myopathy, reducing body myopathy, familial cardiomyopathy with subsarcolemmal vermiform deposits, myopathy with intrasarcoplasmic accumulation of dense granulofilamentous material, Markesbery-Griggs myopathy, and hereditary inclusion body myopathy (hIBM) with early respiratory failure ( ). The original classification of many such disorders was as forms of congenital myopathy, but it is now clear that MFM is a muscular dystrophy and many are allelic with forms of LGMD and distal muscular dystrophy/myopathy ( ).
There is a spectrum of clinical phenotypes associated with MFM ( ; ). Most patients develop weakness between 25 and 45 years of age, but onset can occur from infancy to late adulthood. Either cardiac or skeletal muscles can be involved and dominate the clinical picture. Limb weakness can be predominantly distal and affect either the arms or the legs, but in others, proximal muscles are involved more than distal muscles. Facial and pharyngeal muscles are also affected. Some patients have a facioscapulohumeral or scapuloperoneal distribution of weakness.
The cardiomyopathy may manifest as arrhythmias or conduction defects as well as congestive heart failure. Pacemaker insertion or cardiac transplantation may be required. Severe respiratory muscle weakness can also complicate MFM. In addition, there are rare reports of smooth muscle involvement leading to intestinal pseudo-obstruction.
Muscle histology demonstrates variability in fiber size, increased central nuclei, and occasionally type 1 fiber predominance ( ; ; , ; ). Muscle fibers with rimmed vacuoles may also be evident. Two major types of lesions are evident on light and electron microscopy: hyaline structures and nonhyaline lesions. The hyaline structures are cytoplasmic granular inclusions that are typically eosinophilic on hematoxylin and eosin, and dark blue-green or occasionally red on modified Gomori trichrome stains. They appear as cytoplasmic bodies, spheroid bodies, or Mallory bodies on EM. The nonhyaline lesions appear as dark green areas of amorphous material on Gomori trichrome stains. On EM, these nonhyaline lesions correspond to foci of myofibrillar destruction and consist of disrupted myofilaments, Z-disk–derived bodies, dappled dense structures of Z-disk origin, and streaming of the Z-disk. Immunohistochemistry reveals that both the hyaline and nonhyaline lesions contain desmin, myotilin, and numerous other proteins ( ; ; , ). Interestingly, abnormal muscle fibers also abnormally express several cyclin-dependent kinases in the cytoplasm, including CDC2, CDK2, CDK4, and CDK7 ( ).
The pathogenesis of MFM is multifactorial ( ; ). As alluded to previously, MFM can be caused by mutations in the desmin gene, allelic with LGMD1E, and mutations in the myotilin gene, allelic with LGMD1A; the recently proposed updated classification schema of LGMDs, in fact, characterizes both disorders simply as myofibrillar myopathies and not LGMDs at all ( ). Markesbery-Griggs distal myopathy, caused by mutations in the ZASP gene, also demonstrates MFM on pathology ( ). Less common mutations have been identified in the FLNC , CRYAB , BAG3 , FHL1 , TTN , PLEC , ACTA1 , HSPB8 , and DNAJB6 genes, many of which encode proteins important in the formation and stabilization of the Z-disk ( ). Autosomal dominant inheritance is most common, but autosomal recessive and X-linked inheritance have been reported.
The congenital muscular dystrophies, abbreviated MDC by convention, are a group of diseases typically evident at birth or soon thereafter with hypotonia and severe trunk and limb weakness ( ). All are typically autosomal recessive in their inheritance and are distinguished in part by greater or lesser central nervous system (CNS) and eye involvement ( ). Contractures of the joints are prominent, particularly at the ankles, knees, and hips. Intellectual disability may be present, and MRI of the head shows strikingly increased white matter signal in many patients.
Several distinct forms of MDC are recognizable by clinical and genetic feature, and can be stratified based on the defective protein responsible. MDCs related to genes encoding structural proteins of the basal lamina and sarcolemma include MDC type 1 and UCMD. MDCs related to impaired glycosylation of α-dystroglycan are referred to as dystroglycanopathies (DG). Finally, mutations in selenoprotein N1 produce a unique phenotype of rigid spine syndrome.
MDC type 1 is the most common type of MDC in the Western Hemisphere. It is genetically heterogeneous, with approximately 50% of cases associated with primary merosin or α 2 -laminin deficiency (MDC1A). Laminin-α 2 , also known as merosin, is one of a large family of glycosylated proteins found in the basement membrane and attaches to the dystroglycan complex. The hallmark of merosinopathy includes severe weakness of the trunk and limbs and hypotonia at birth, sparing the extraocular muscles and face ( ). Prominent contractures of the feet and hips are present. Although intelligence is often normal, the incidence of epilepsy is 12% to 20%. MRI and computed tomography (CT) of the brain often reveal white-matter abnormalities. For the most part, these children are severely disabled, and many remain dependent on their caregivers for their whole lives. In milder forms of the disease, caused by partial merosin deficiency, delayed onset of symptoms and mild weakness occur ( ). This situation is similar to dystrophin deficiency.
CK concentrations are elevated. EMG, in addition to demonstrating abnormalities in the muscle, shows slowed nerve conduction velocities; laminin-α 2 is also expressed in nerve tissue. Diagnosis is established by identifying a mutation in the laminin-α 2 gene. Demonstration of altered merosin in muscle can be helpful to guide this testing ( ). As in dystrophin deficiency, it may be advantageous to use at least two different antibodies. Skin biopsy may also reveal merosin deficiency.
There are patients with identical symptoms who do not have a mutation in the laminin-α 2 gene ( Fig. 109.14 ). MDC1B, for example, is caused by mutations in the gene encoding for the α 7 subunit of α 7 β 1 D integrin, a sarcolemmal protein that binds to merosin ( ). Frequently the muscle symptoms are milder and progress more slowly; children may gain function as they grow older and may walk independently. Despite a shortened life span, many survive to adulthood. Certain α-dystroglycanopathies produce an identical clinical phenotype, most importantly MDC1C, which results from mutations in FKRP ( ).
UCMD, also known as atonic-sclerotic dystrophy and recently reclassified as LGMD R22 collagen 6-related, is associated with neonatal weakness, multiple contractures, and distal hyperlaxity (Bertini E, 2002). Affected children often have marked protrusion of the calcanei in their feet. The clinical course is static or slowly progressive. Serum CKs are normal or only slightly elevated. Mutations in subunits of collagen type VI cause the disorder. UCMD is allelic to the more benign Bethlem myopathy, or LGMD D5 (see above) ( ). Skeletal muscle MRI scans may reveal early involvement of the thigh muscles that preferentially involve the periphery of each muscle and relatively spare the central regions along with a peculiar involvement of the rectus femoris with a central area of abnormal signal within the muscle ( ; ).
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here